Published online Sep 26, 2019. doi: 10.12998/wjcc.v7.i18.2871
Peer-review started: May 10, 2019
First decision: August 1, 2019
Revised: August 11, 2019
Accepted: August 26, 2019
Article in press: August 26, 2019
Published online: September 26, 2019
Processing time: 141 Days and 1 Hours
In recent years, there have been reports of a new histological type of gastric cancer, termed gastric adenocarcinoma of the fundic gland (GA-FG). This disease entity presents differentiation towards the fundic gland, especially chief cell-predominant differentiation (GA-FG-CCP). GA-FG-CCP easily invades into the submucosa but rarely shows metastasis. The reports mostly describe primarily single lesions. Herein, we report a case with multiple lesions, and summarize the clinicopathologic characteristics of multiple cases.
A 55-year-old woman underwent upper gastrointestinal endoscopy screening. Two whitish lesions on the anterior wall of the gastric corpus and the gastric fundus were detected. The patient had previously received Helicobacter pylori eradication therapy. The mucosa was characterized as grade C-2 atrophic gastritis. We diagnosed the patient with multiple GA-FG (GA-FG-CCP) by hematoxylin and eosin (HE) staining and immunohistochemical staining of the endoscopic biopsy. Upon performing endoscopic submucosal dissection (ESD), one lesion was not found, but the scar from the biopsy was visible; the mucularis mucosa of the biopsy and ESD-resected specimen were intact. The two lesions showed no lymphatic nor venous invasion. The resection performed appeared to be relatively curative.
Cases of multiple GA-FG-CCP are very rare in clinical practice. Most of its clinicopathologic characteristics are similar to those of a single lesion. Our case provides diagnostic and therapeutic information about GA-FG-CCP with multiple lesions.
Core tip: Multiple gastric adenocarcinoma of the fundic gland (chief cell-predominant type, GA-FG-CCP) is rare in clinical practice. Our case provides diagnostic and therapeutic information about multiple GA-FG-CCP. We also summarize characteristics of three multiple cases with GA-FG-CCP, and most of its endoscopic and clinicopathological features are similar to those of single lesions. More data are needed to advance our understanding.
- Citation: Chen O, Shao ZY, Qiu X, Zhang GP. Multiple gastric adenocarcinoma of fundic gland type: A case report. World J Clin Cases 2019; 7(18): 2871-2878
- URL: https://www.wjgnet.com/2307-8960/full/v7/i18/2871.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i18.2871
In 2007, Tsukamoto et al[1] reported the first case of the novel entity adenocarcinoma with chief cell differentiation within the fundic gland of the stomach. In 2010, Ueyama et al[2] proposed a novel disease concept, termed gastric adenocarcinoma of the fundic gland (chief cell-predominant type, GA-FG-CCP) according to the endoscopic features and histopathological features of ten cases. Most of the GA-FG-CCP lesions typically are small. Nevertheless, these small lesions show invasion to the submucosa but rarely show lymphatic and venous invasion. They commonly derive from the deep layer of the normal oxyntic mucosa without atrophy and are not associated with Helicobacter pylori infection. Therefore, GA-FG-CCP is generally considered to have a low potential for malignancy[3-6].
Thus far, most reports regarding this tumor type are about single lesions and rarely present multiple GA-FG-CCP lesions. We herein report our experience with two lesions of gastric adenocarcinoma of the fundic gland that developed on the anterior wall of the gastric corpus and the gastric fundus in one patient who had received Helicobacter pylori eradication therapy. The gastric mucosa was atrophic.
A 55-year-old woman underwent esophagogastroduodenoscopy (EGD) screening, and two whitish lesions on the anterior wall of the gastric corpus and gastric fundus were detected in the gastric mucosa.
She felt burping and abdominal distension in the upper abdomen for approximately 1 mo, without emaciation, loss of appetite, or abdominal pain. About one year ago, she received Helicobacter pylori eradication therapy.
There was no significant past personal history or family history. Her physical examination was unremarkable.
Her laboratory tests only revealed an elevated blood platelet count of 370 × 109/L (normal reference range: 100-300 × 109/L). Blood coagulation, liver function, renal function, electrolyte, tumor marker, cholesterol, blood sugar, anti-autoantibody, urine, and conventional stool test results were all within normal limits.
A computed tomography scan of the chest and abdomen showed no abnormality.
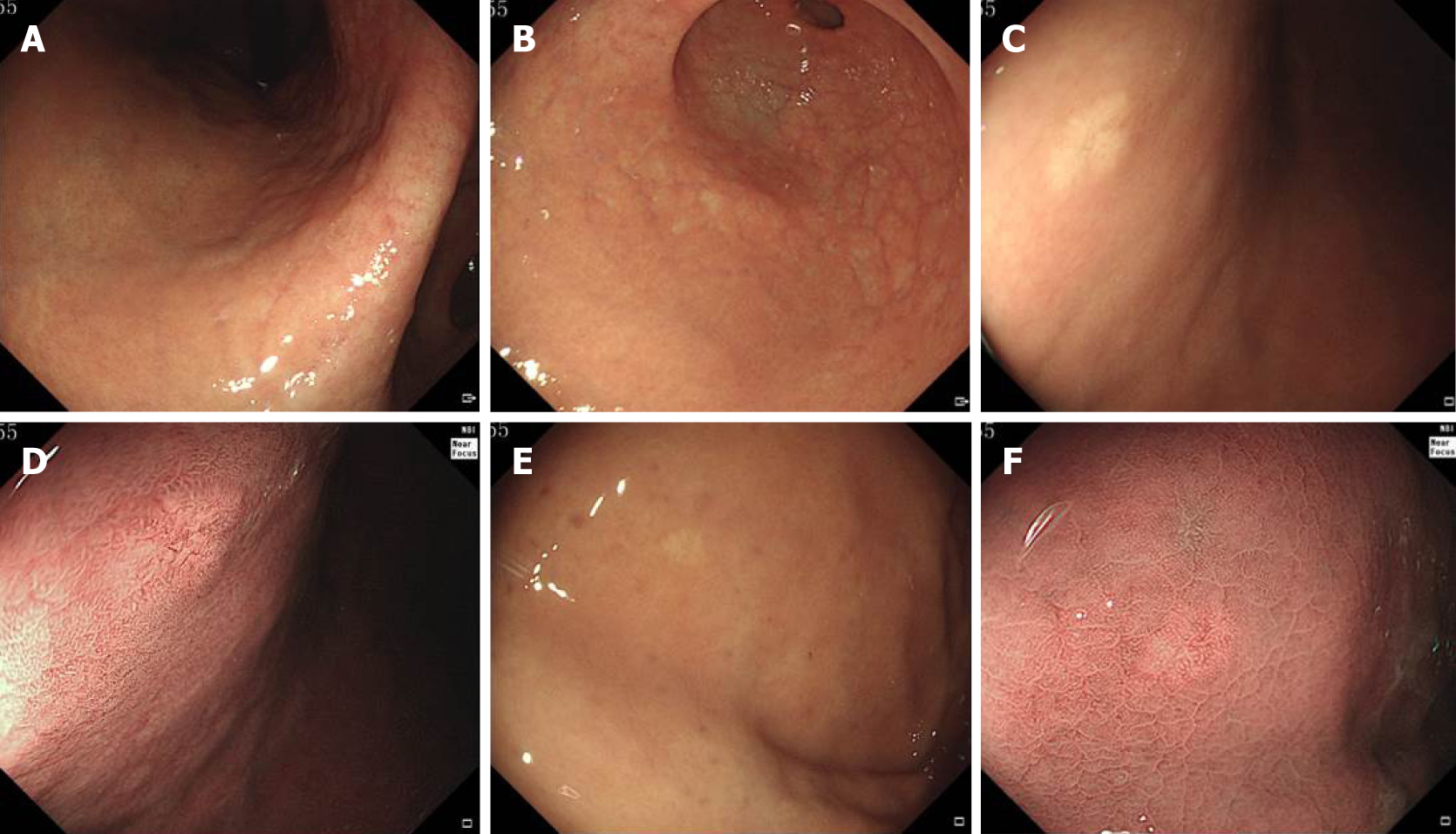
Endoscopic findings of all lesions are shown in Figure 1. The gastric mucosa showed grade C-2 atrophic gastritis according to the Kimura-Takemoto classification[7] (Figure 1A and B). A small, type 0-IIa (superficial elevated) submucosal tumor-like lesion, approximately 6 mm in diameter and with a whitish mucosal surface, was found on the anterior wall of the gastric corpus. The mucosa surrounding the lesion did not show atrophy. Narrow-band imaging showed an irregular microvascular pattern and dilatation of microvessels with branching architecture (Figure 1C and D). The second lesion was on the gastric fundus and was approximately 4 mm in diameter. White light endoscopy revealed a flat, 0-IIb (superficial flat) lesion with a whitish mucosal surface. The mucosa surrounding the lesion did not show atrophy. Narrow-band imaging showed regular and dilated microvessels (Figure 1E and F).
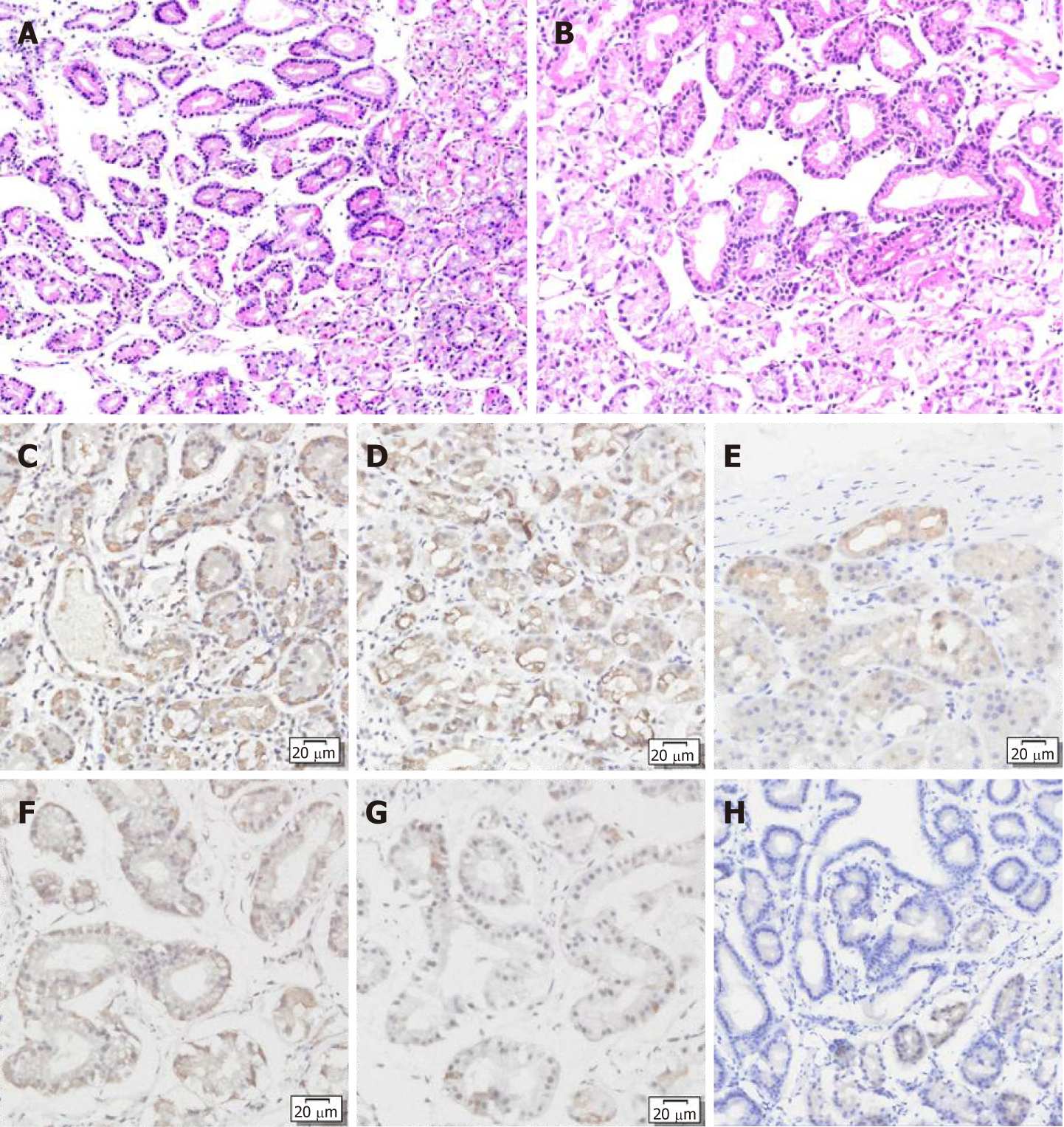
The patient was diagnosed with GA-FG-CCP by histopathological examination and immunohistochemical staining of the endoscopic biopsy (Figure 2). Following the report that pepsinogen I, MUC6, and H+/K+-ATPase are almost always positive on immunohistochemical staining, H+/K+-ATPase is focally positive, and MUC2, MUC5AC, chromogranin A, and CD10 are almost always negative in GA-FG-CCP, the same immunohistochemical staining was performed on the patient’s biopsy specimen[3]. Hematoxylin and eosin (HE) staining showed that the tumors had clear demarcation from the surrounding fundus glands and had an irregular gland structure. The tumors were composed of chief cell-like cells with mild nuclear atypia (Figure 2A and B). Immunohistochemical staining showed that both lesions were positive for pepsinogen I (Figure 2C and F) and MUC6 (Figure 2D and G), partially positive for H+/K+-ATPase (Figure 2E and H), and negative for MUC5AC, CEA, and CA199.
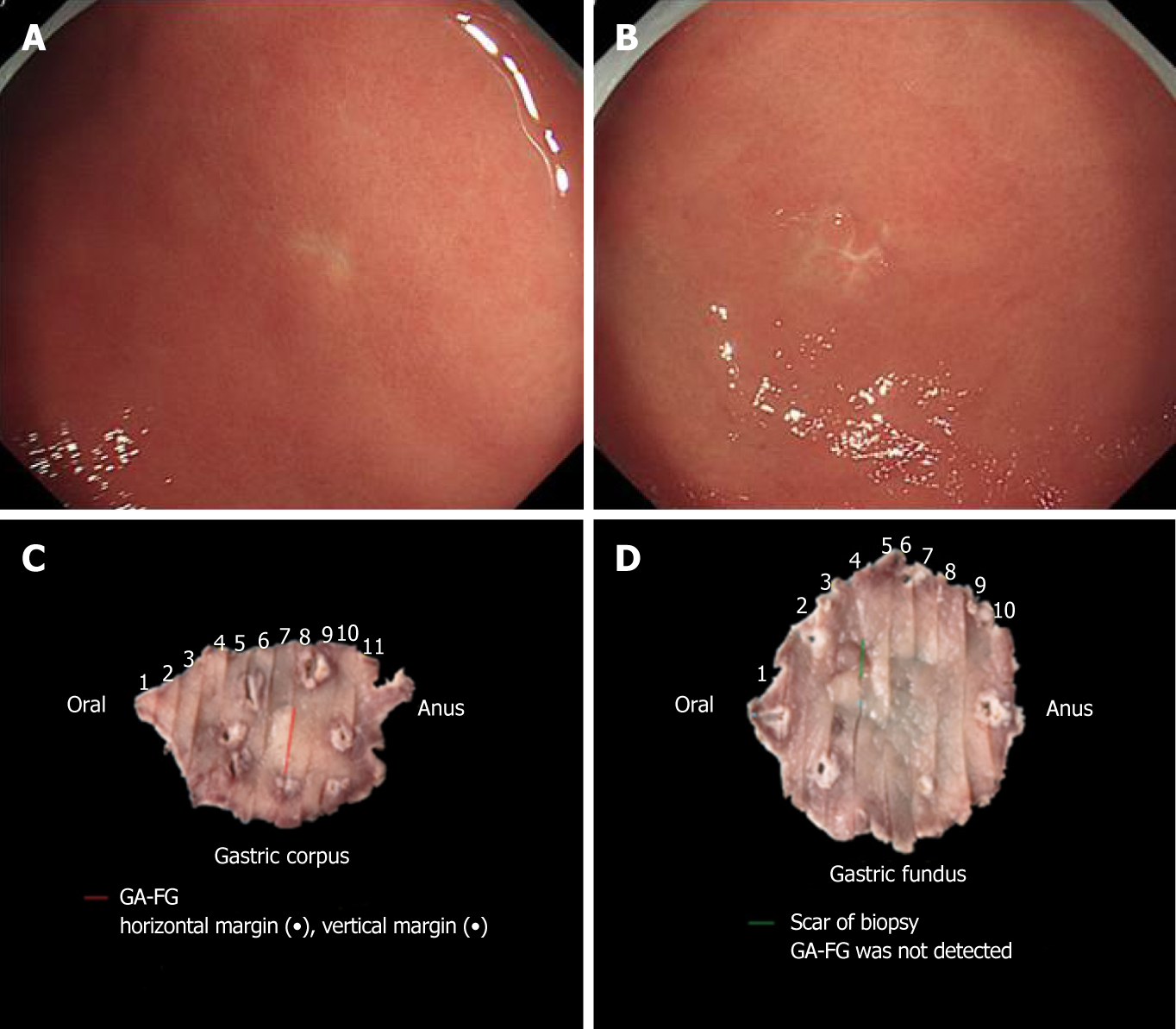
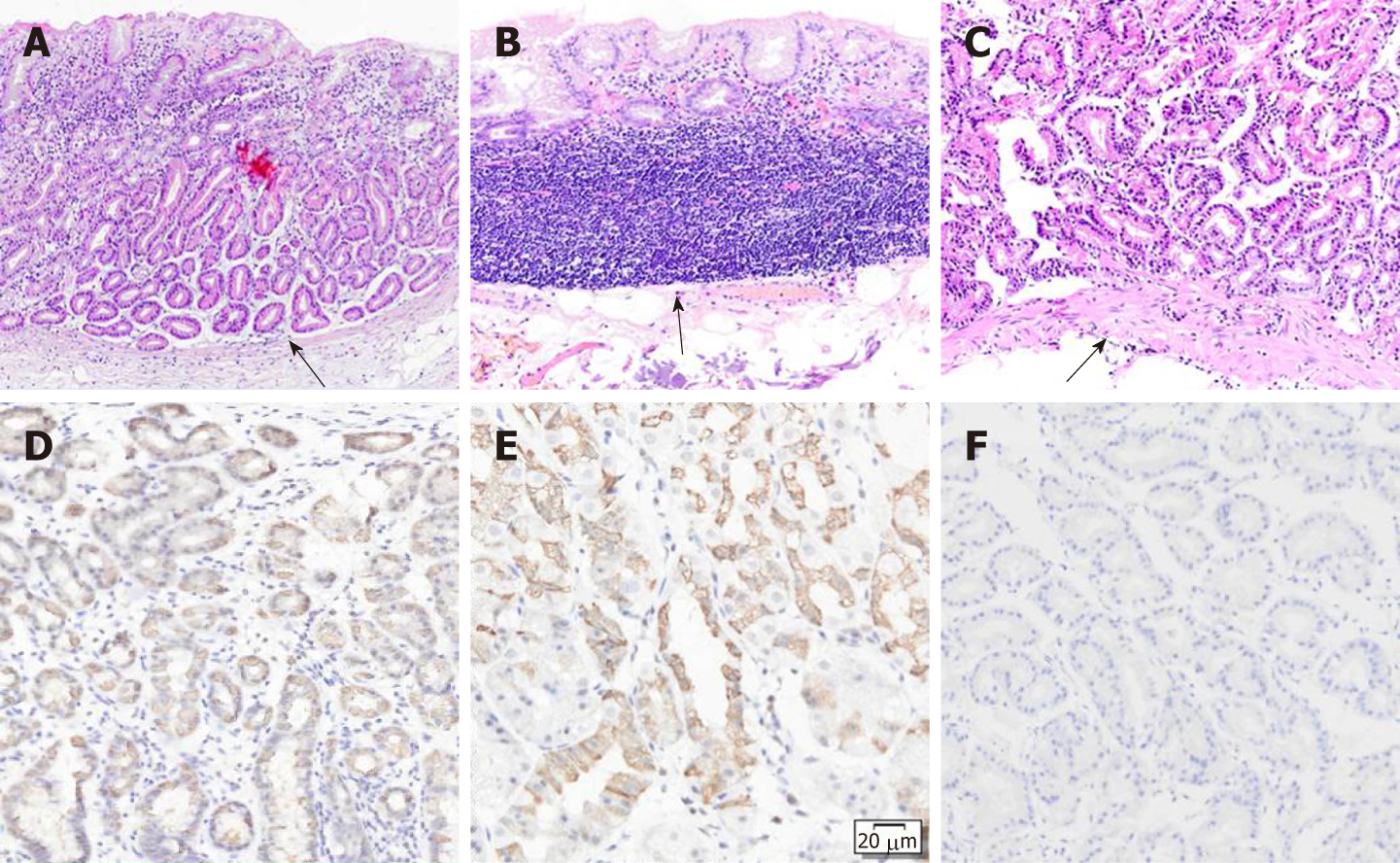
We performed endoscopic submucosal dissection (ESD) of the two lesions (Figure 3C and D). The scar from the biopsy could be easily found before the ESD (Figure 3A and B). The ESD-resected specimens were subjected to conventional HE staining and immunohistochemical staining on 2-millimeter thick tissue sections. Upon magnification, the main portion of the lesion on the gastric corpus was located in the deep layer of the lamina propria. The entire lesion was confined within the mucosa and composed of fundic chief cell-like basophilic columnar cells (Figure 4A). Immunohistochemical staining showed that the lesion was positive for pepsinogen I (Figure 4D) and MUC6 (Figure 4E) and partially positive for H+/K+- ATPase (Figure 4F). Interestingly, the ESD-resected specimen of the gastric fundus did not identify the lesion. Despite sectioning the entire specimen, the lesion could not be identified. The mucularis mucosa of the ESD-resected specimen was intact, and the scar from the biopsy was identified (Figure 4B). The mucularis mucosa of the biopsy specimen was intact (Figure 4C); therefore, we suspect that the lesion was biopsied and the lesion was located in the mucosal layer.
Based on the above data, the two lesions were diagnosed as GA-FG-CCP without invasion of the submucosal layer. The lesion on the gastric corpus was Type 0-IIa, 6 × 4 mm, PT1a, ly0, v0, PHM0, and PVM0. The lesion on the gastric fundus was Type 0-IIb, 4 × 4 mm, PT1a, ly0, v0, PHM0, and PVM0. Both lesions were determined to have undergone curative resection.
Three months after ESD, the patient underwent EGD and computed tomography. There was no evidence of recurrence or metastasis.
GA-FG is a rare type of well differentiated gastric adenocarcinoma towards chief cell-predominant differentiation. Staining for pepsinogen I is positive. The endoscopic features of GA-FG-CCP include: (A) Submucosal tumor shape, protruding, flat or depressed; (B) Faded or whitish, reddish color tone with a soft appearance; (C) Dilated vessels with branching architecture; and (D) No atrophic change, intestinal metaplasia, or chronic gastritis[2,3]. The macroscopic appearance of multiple lesions may be elevated, depressed, or flat. The color tone is yellowish, whitish, or faded, and the blood vessels are dilated, similar to the findings in a single lesion (Table 1).
| Author (yr) | Kino et al[13] | Watanabe et al [14] | Our case | ||||||||||||
| Patient number | 1 | 1 | 1 | ||||||||||||
| Age (yr) | 78 | 71 | 46 | ||||||||||||
| Sex | Male | Male | Female | ||||||||||||
| Number of lesions | 2 | 3 | 3 | ||||||||||||
| Location (U/ M/L) | U | U | M | M | M | U | M | ||||||||
| H. pylori infection | Eradication | Uninfective | Eradication | ||||||||||||
| Atrophic gastritis | Improved | Non-atrophy | Atrophy (C2) | ||||||||||||
| Size (mm, average) | 4 | 7 | 6 | 6 | 5 | 4 | 6 | ||||||||
| Macroscopic shape | Protruding Protruding | Depressed Depressed Depressed | Elevated | Flat | |||||||||||
| Color tone | Yellowish Yellowish | Faded Faded Faded | Whitish | Whitish | |||||||||||
| Dilated vessels | (+) (+) | (+) (+) ND | (+) | (+) | |||||||||||
| Depth (μm) | SM (120 μm) M | SM (200 μm) SM (11 μm) SM (38 μm) | M | M | |||||||||||
| Lymphatic invasion | (-) | (-) | (-) | (-) | (-) | (-) | (-) | ||||||||
| Venous invasion | (-) | (-) | (+) | (-) | (-) | (-) | (-) | ||||||||
| Pepsinogen I | (+) | (+) | (+) | (+) | (+) | (+) | (+) | ||||||||
| MUC6 | (+) | (+) | (+) | (+) | (+) | (+) | (+) | ||||||||
| H+/K+-ATPase | (+) | (+) | ND | ND | ND | (+) partial | |||||||||
| (+) partial | |||||||||||||||
The prevalence of GA-FG-CCP is 0.98% to 1.6% of gastric cancer cases[6,8,9]. In 2010 and 2014, Ueyama et al[10] reported ten cases of GA-FG and summarized the endoscopic and clinicopathological features. In 2012, Park et al[11] reported three cases in Korea and Singhi et al[12] reported about ten other cases. In 2015, Miyazawa et al[8] reported five cases, and in 2016, Chiba et al[6] reported 20 cases. The number of reported cases has increased rapidly. More and more doctors have become aware and understand this disease. Even more cases are anticipated in the future.
Most reports about GA-FG-CCP involve single lesions; multiple lesions are rare. Herein, we summarize the endoscopic and clinicopathologic characteristics of a case of multiple lesions (Table 1). Multiple lesions are usually located in the upper or middle stomach and can only develop in the fundus or gastric corpus or both. Two or three lesions may be present in one patient. The sizes of the lesions are small, with the largest being 7 mm, which accounts for the difficulty in finding them.
When finding GA-FG like lesions, we should carefully screen for the presence of similar tumors in the other portion in order to avoid a missed diagnosis. If uncertain of the lesion, biopsy may be a good approach to establish a diagnosis. However, because the lesions are typically small, sometimes the lesion may be biopsied or followed to consider the need for biopsy.
Our patient had received Helicobacter pylori eradication therapy. Her gastric mucosa showed grade C-2 atrophic gastritis, but no atrophy was seen around the lesions. In multiple cases, Kino et al[13] reported that the patients with two lesions had received Helicobacter pylori eradication therapy and that the atrophy of the gastric mucosa had improved. Watanabe et al[14] reported that a patient with three lesions was not infected with Helicobacter pylori and that the gastric mucosa was not atrophic. Chiba et al[6] reported that among 20 patients, 15 (75%) cases were of the closed type or open type atrophic gastritis. However, the lesions in 17 (85%) of 20 cases were present in the non-atrophic area, with three lesions located in moderate atrophic surrounding mucosa. The report also revealed that three-quarters of patients were thought to be “infected” with Helicobacter pylori; one-quarter was considered Helicobacter pylori-negative. Therefore, GA-FG-CCP can develop in a stomach infected or noninfected with Helicobacter pylori or after Helicobacter pylori eradication therapy with atrophic or non-atrophic mucosa. It is more likely to occur in non-atrophic areas. The state of Helicobacter pylori infection and atrophic mucosa may not be critical to the development of GA-FG-CCP. More cases and research are needed to evaluate the characteristics of GA-FG-CCP.
Regarding cases with multiple lesions, the depth of infiltration of our two lesions were limited to the mucosal layer. Watanabe et al[14] reported that the three lesions had infiltrated into the submucosa; the infiltration depths were 200 μm, 11 μm, and 38 μm; the resection they performed was also thought to be curative.
One lesion had venous invasion, but the patient did not have recurrence or metastasis during a follow-up period of one year and eight months. Kino et al[13] reported that one lesion was confined within the mucosal layer and the other infiltrated into the submucosa with a minimal invasion depth of 120 μm. One year later, EGD and computed tomography showed no evidence of recurrence or metastasis. These observations show that multiple GA-FG-CCP can still easily invade the submucosa and may likely show venous invasion but that the entity of multiple GA-FG-CCP still has low metastatic potential and low-grade malignancy.
Although the etiology and pathogenesis of GA-FG remain unclear, Lee et al[15] reported that PPP2R1A mutations may occur in gastric fundic gland-associated neoplasms. β-catenin expression and the mutation spectrum of PPP2R1A and Wnt pathway genes may be associated with the pathogenesis[16,17]. Kino et al[13] showed that multiple GA-FG occurred after conventional gastric cancer, causing them to hypothesize that they may have the same risk factors. Using immunohistochemistry, Takaoka et al[18] studied the mismatch repair (MMR) genes from 31 patients who developed 84 tumors (15 synchronous and 16 metachronous). Multiple early gastric cancers may occur at the same time (synchronously) or several years apart (metachronous) in the same patient, but their clonal origins were absolutely different. Multiple early-stage gastric cancers may be associated with “switching” or “mixing” of deficient mismatch repair (dMMR) genes and proficient-mismatch repair (pMMR) genes, Epstein-Barr virus, or chromosomal instability. It is still unclear whether multiple GA-FG has the same pathogenic mechanism, and more data are required to understand the pathogenesis of multiple GA-FG.
Multiple GA-FG-CCP is very rare in clinical practice. Most of its clinicopathologic characteristics are similar to those of single lesions. Our case provides diagnostic and therapeutic information about multiple GA-FG-CCP, but more data are needed to advance our understanding.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ueyama H, Lobo M S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Tsukamoto T, Yokoi T, Maruta S, Kitamura M, Yamamoto T, Ban H, Tatematsu M. Gastric adenocarcinoma with chief cell differentiation. Pathol Int. 2007;57:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 2. | Ueyama H, Yao T, Nakashima Y, Hirakawa K, Oshiro Y, Hirahashi M, Iwashita A, Watanabe S. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol. 2010;34:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Ueyama H, Matsumoto K, Nagahara A, Hayashi T, Yao T, Watanabe S. Gastric adenocarcinoma of the fundic gland type (chief cell predominant type). Endoscopy. 2014;46:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Yao T, Ueyama H, Kushima R. New Type of Gastric Carcinoma-Adenocarcinoma of the Fundic Gland Type: Its Clinicopathological Features and Tumor Development. Stom Intest. 2010;45:1192–1201. |
| 5. | Kushima R, Sekine S, Matsubara A, Taniguchi H, Ikegami M, Tsuda H. Gastric adenocarcinoma of the fundic gland type shares common genetic and phenotypic features with pyloric gland adenoma. Pathol Int. 2013;63:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Chiba T, Kato K, Masuda T, Ohara S, Iwama N, Shimada T, Shibuya D. Clinicopathological features of gastric adenocarcinoma of the fundic gland (chief cell predominant type) by retrospective and prospective analyses of endoscopic findings. Dig Endosc. 2016;28:722-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 740] [Article Influence: 43.5] [Reference Citation Analysis (3)] |
| 8. | Miyazawa M, Matsuda M, Yano M, Hara Y, Arihara F, Horita Y, Matsuda K, Sakai A, Noda Y. Gastric adenocarcinoma of fundic gland type: Five cases treated with endoscopic resection. World J Gastroenterol. 2015;21:8208-8214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Tohda G, Osawa T, Asada Y, Dochin M, Terahata S. Gastric adenocarcinoma of fundic gland type: Endoscopic and clinicopathological features. World J Gastrointest Endosc. 2016;8:244-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Ueyama H, Yao T, Matsumoto K. Establishment of Endoscopic Diagnosis for Gastric Adenocarcinoma of Fundic Gland Type (Chief Cell Predominant Type) Using Magnifying Endoscopy with Narrow-Band Imaging. Stom Intest. 2015;50:1533–1547. |
| 11. | Park ES, Kim YE, Park CK, Yao T, Kushima R, Kim KM. Gastric adenocarcinoma of fundic gland type: report of three cases. Korean J Pathol. 2012;46:287-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Singhi AD, Lazenby AJ, Montgomery EA. Gastric adenocarcinoma with chief cell differentiation: a proposal for reclassification as oxyntic gland polyp/adenoma. Am J Surg Pathol. 2012;36:1030-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Kino H, Nakano M, Kanamori A, Suzuki T, Kaneko Y, Tsuchida C, Tsuchida K, Tominaga K, Sasai T, Yamagishi H, Imai Y, Hiraishi H. Gastric Adenocarcinoma of the Fundic Gland Type after Endoscopic Therapy for Metachronous Gastric Cancer. Intern Med. 2018;57:795-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Watanabe R, Yada T, Ikegami Y, Ito K, Itakura Y, Koizuka H, Uemura N. A case of multiple gastric adenocarcinomas of fundic gland type. Progress of Digestive Endoscopy. 2018;92:104-5. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Lee SY, Saito T, Mitomi H, Hidaka Y, Murakami T, Nomura R, Watanabe S, Yao T. Mutation spectrum in the Wnt/β-catenin signaling pathway in gastric fundic gland-associated neoplasms/polyps. Virchows Arch. 2015;467:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Hidaka Y, Mitomi H, Saito T, Takahashi M, Lee S, Matsumoto K, Yao T, Watanabe S. Alteration in the Wnt/b-catenin signaling pathway in gastric neoplasias of fundic gland (chief cellpr9edominant) type. Hum Pathol. 2013;44:2438–48. [RCA] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Matsubara A, Sekine S, Kushima R, Ogawa R, Taniguchi H, Tsuda H, Kanai Y. Frequent GNAS and KRAS mutations in pyloric gland adenoma of the stomach and duodenum. J Pathol. 2013;229:579-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Takaoka S, Hirotsu Y, Ohyama H, Mochizuki H, Amemiya K, Oyama T, Ashizawa H, Yoshimura D, Nakagomi K, Hosoda K, Suzuki Y, Kojima Y, Omata M. Molecular subtype switching in early-stage gastric cancers with multiple occurrences. J Gastroenterol. 2019;54:674-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |