Published online Sep 26, 2019. doi: 10.12998/wjcc.v7.i18.2857
Peer-review started: June 19, 2019
First decision: August 2, 2019
Revised: August 6, 2019
Accepted: August 27, 2019
Article in press: August 26, 2019
Published online: September 26, 2019
Processing time: 103 Days and 2.1 Hours
Anaplastic large cell lymphoma (ALCL) is a type of non-Hodgkin’s lymphoma (NHL). ALCL is rare as a bone lesion and in pregnancy.
We present the first case of anaplastic lymphoma kinase (ALK)+ ALCL of the thoracic spine during pregnancy. A 25-year-old pregnant woman was presented to us at 24 wk’ gestation with severe back pain and weakness in the left lower limb. Imaging examination showed lesions at T10 and T11. She underwent posterior pedicle screw fixation and vertebroplasty. Pathological examination showed ALK+ ALCL. The patient chose to have therapeutic abortion after surgery and received chemotherapy in the hematology department. She now remains disease free with no neurological deficit after 30 mo’ follow-up.
ALK+ ALCL with the thoracic spine involvement is uncommon, especially in pregnancy. Many symptoms can be misunderstood during pregnancy; therefore, when a pregnant patient has persistent back pain or lower limb neurological symptoms, imaging examinations should be performed.
Core tip: We present the first case of anaplastic lymphoma kinase anaplastic large cell lymphoma of the thoracic spine during pregnancy. The patient underwent posterior spinal cord decompression and pedicle screw fixation surgery and chemotherapy. There were major improvements in the patient's status. We concluded that it is important to have a detailed examination of pregnant women with back pain and neurological deficits.
- Citation: Yang S, Jiang WM, Yang HL. ALK-positive anaplastic large cell lymphoma of the thoracic spine occurring in pregnancy: A case report. World J Clin Cases 2019; 7(18): 2857-2863
- URL: https://www.wjgnet.com/2307-8960/full/v7/i18/2857.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i18.2857
Anaplastic large cell lymphoma (ALCL) is a specific T-cell non-Hodgkin’s lymphoma (NHL) proposed by Stein et al[1] in 1985 that accounts for 2%-3% of all NHL[2]. Morris et al[3] found that the most common chromosomal abnormality in ALCL is t(2;5) (p23; q35); fusion of the anaplastic lymphoma kinase (ALK) gene on chromosome 2 and the nuclear phosphoprotein gene on chromosome 5. Depending on the expression of ALK, ALCL can be divided into ALK+ and ALK− forms, which have distinct clinical and pathological features. ALK+ ALCL usually manifests as superficial and abdominal lymphadenopathy[4] and is accompanied by fever and extranodal invasion in bones. Bone marrow, subcutaneous tissue, spleen, and central nervous system invasion is rare[5]. Given that ALK+ ALCL is rarely described during pregnancy, there is a lack of standardized treatment.
Here, we report a pregnant patient with ALK+ ALCL of the thoracic spine and hope to improve the understanding of the clinical manifestations and treatment of this disease.
A 25-year-old woman at 24 wk’ gestation presented to us with severe back pain and weakness in her left lower limb.
Two months ago, she began to notice weakness of the left lower limb and it gradually worsened over time. Four days ago, she felt severe pain in the back when she got out of bed and the pain did not show signs of improvement after then.
She had no past history of surgery and was not taking any medication. There was no abnormality in previous prenatal examinations.
She denied any family history of personal or family history of other diseases.
Physical examination revealed localized tenderness on the thoracic region of the spine and muscle strength was decreased to level 3 in the left lower limb. No sensory loss of the lower extremities was noted, and the pathological signs were negative. The patient had no fever or peripheral lymphadenopathy.
The patient had mild anemia with hemoglobin of 78 g/L. Hemostasis was within the normal limits, as was the biochemical test. Tumor marker levels were not increased.
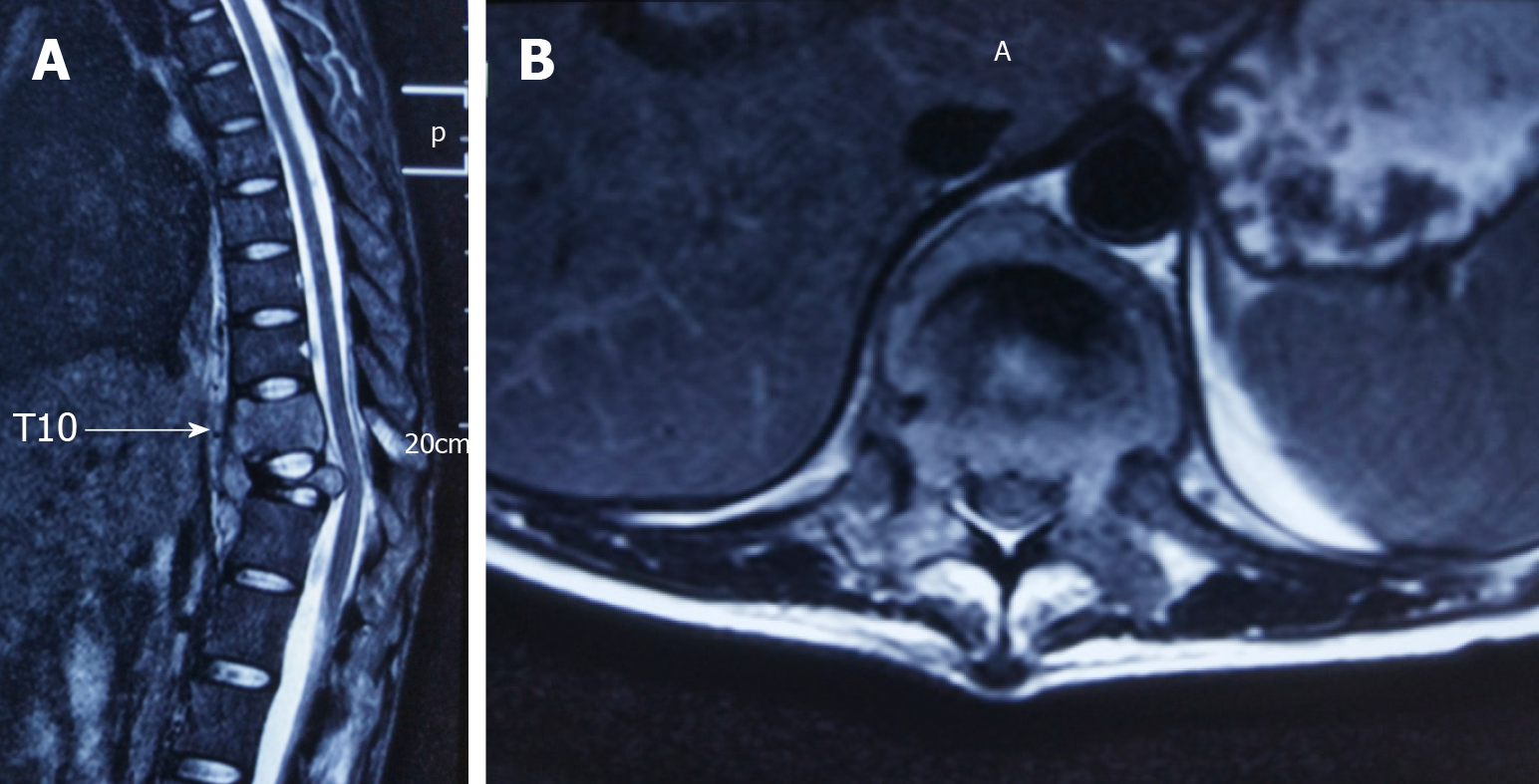
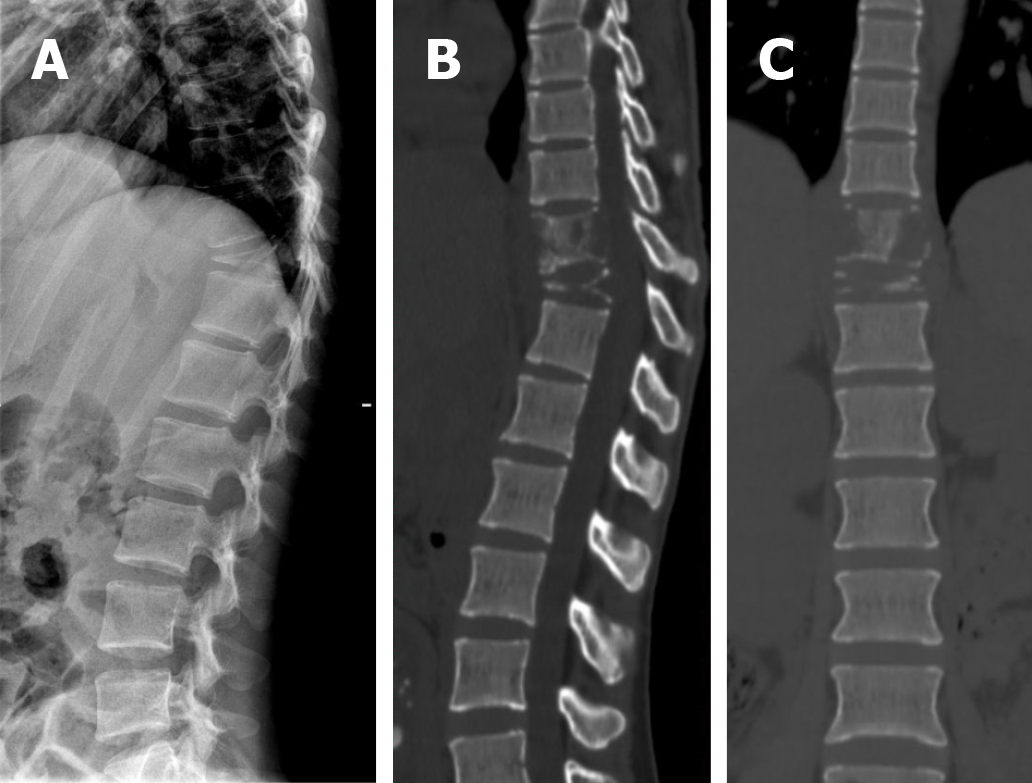
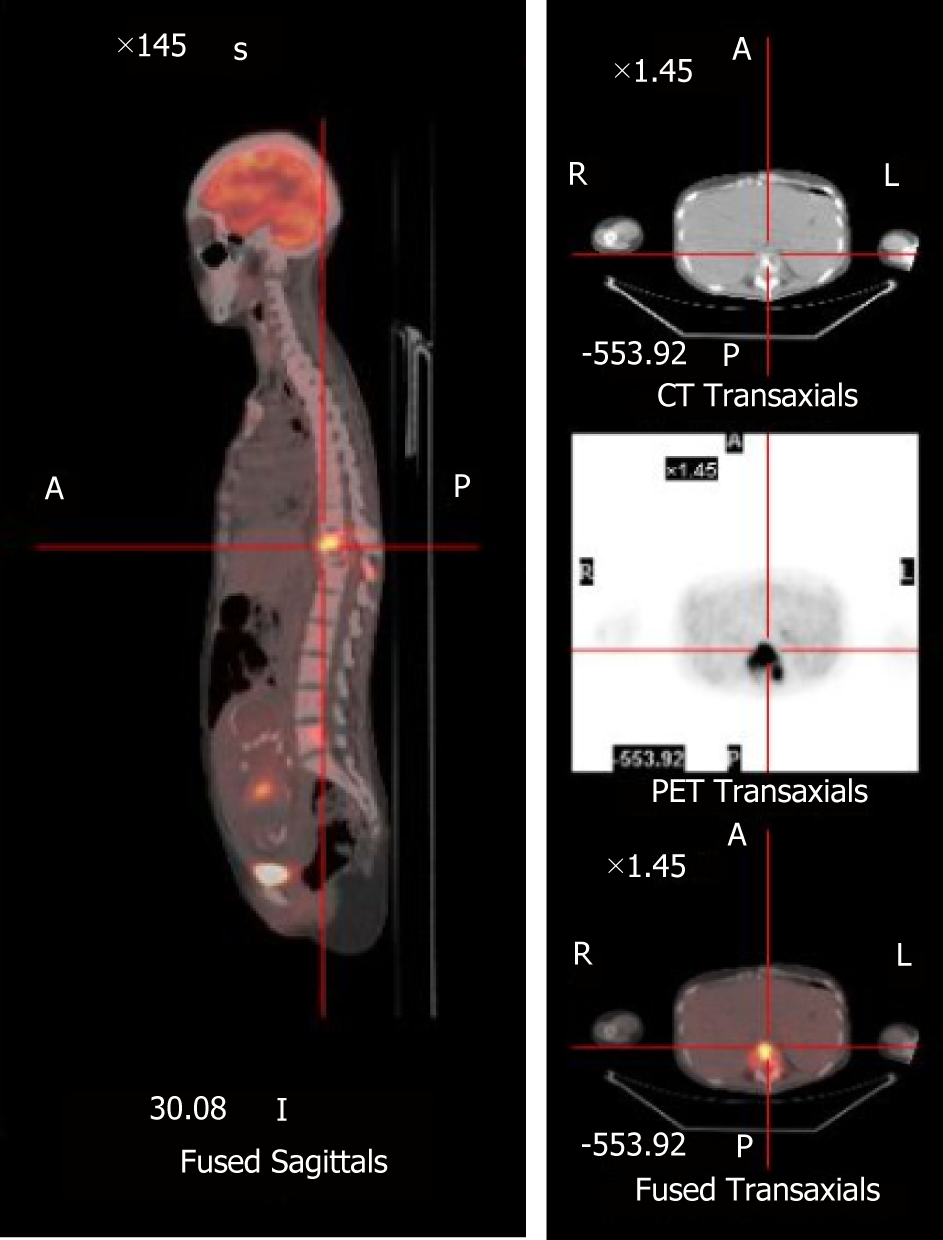
Magnetic resonance imaging (MRI) revealed an aggressive, abnormal signal involving the T10 and T11 vertebra with intraspinal extension. The T11 vertebral body was seriously collapsed and compressed the spinal cord backwards (Figure 1). X-ray and computed tomography (CT) reconstruction reported multiple bone destruction in the T10, T11 vertebrae (Figure 2). Fluoro-2-deoxy-D-glucose (FDG) -positron emission tomography-CT (PET-CT) was also performed and showed FDG-avid lesions in T10, T11 vertebrae (Figure 3).
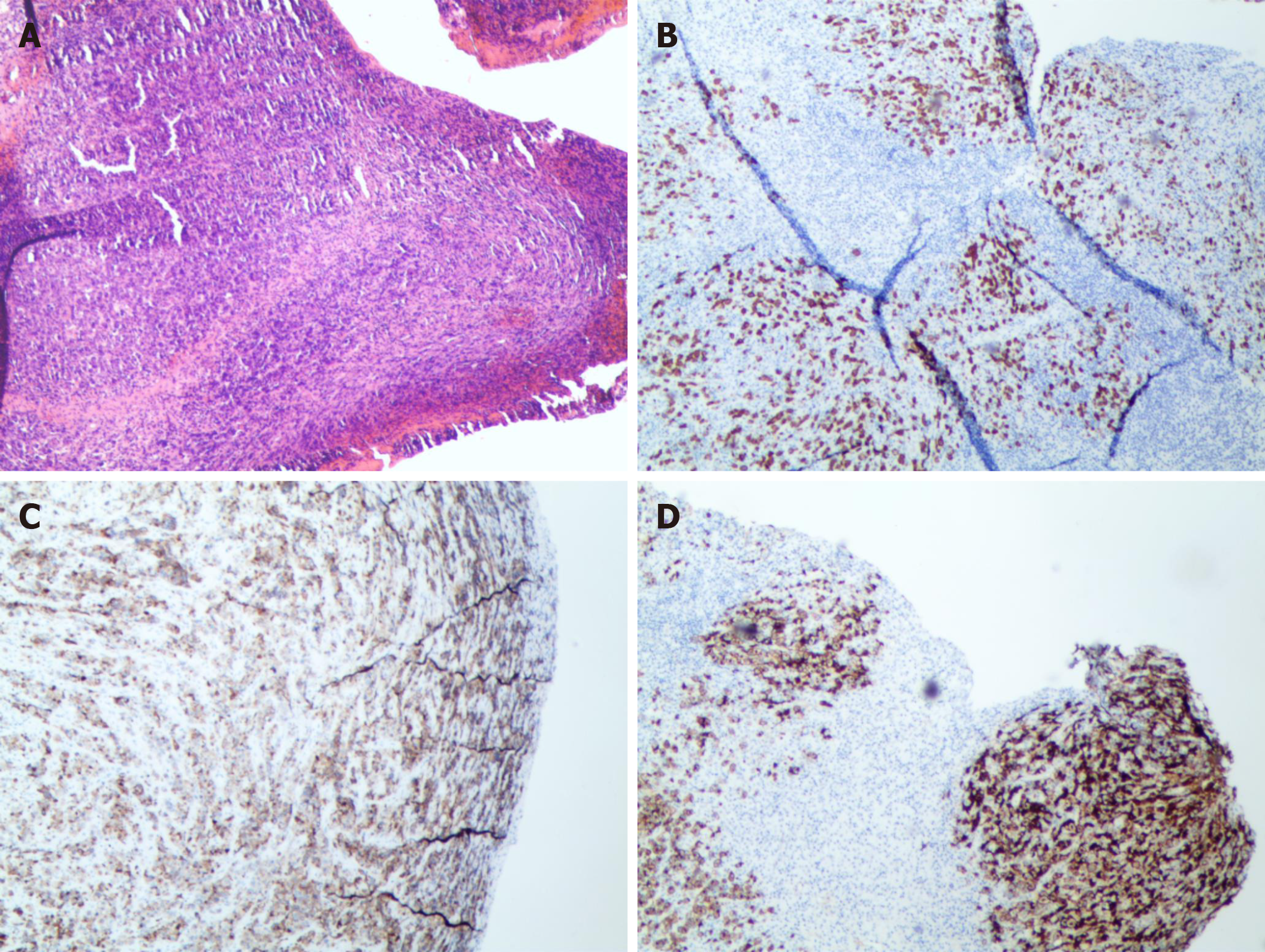
According to clinical symptoms and imaging findings, it is considered as a primary malignant tumor of the thoracic spine. CT-guided biopsy confirmed the diagnosis of ALK+ ALCL. Immunohistochemical stains showed positive staining for CD3, CD30 and CD246 (ALK protein) (Figure 4).
Taking into account the patient’s severe back pain and deterioration of neurological symptoms, we chose surgical treatment after full discussion with the patient. She underwent posterior spinal cord decompression and pedicle screw fixation from T8 to L1 to reconstruct the stability of the spine. Since CT reconstruction showed more destruction to the left of the T10 vertebral body, we chose unilateral vertebroplasty to avoid bone cement leakage. The patient was admitted to the obstetrics and hematology department for further evaluation. The patient eventually chose therapeutic abortion and received CHOPE (cyclophosphamide, doxorubicin, vincristine, prednisone and etoposide) chemotherapy.
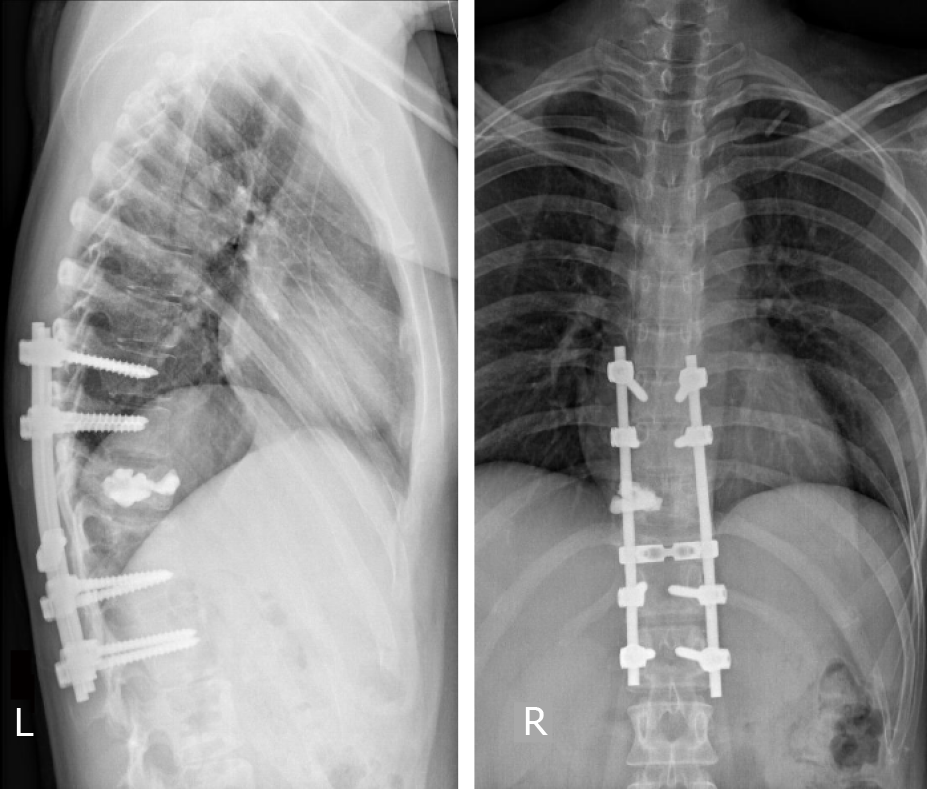
The muscle strength of her left lower limb gradually returned to normal and her back pain was significantly relieved. X-ray showed good stability (Figure 5) and she remained disease free with no neurological deficit 30 mo after therapy.
The most common form of NHL seen in pregnancy is diffuse large B-cell lymphoma, whereas ALCL rarely occurs[6-12]. Most NHL that occurs during pregnancy is invasive, disseminated, and often involve organs stimulated by progestogens, such as the breast, ovaries and uterus[13]. Our patient had no typical clinical manifestations of fever and lymphadenopathy, suggesting that the clinical manifestations of primary spinal lymphoma can be concealed and complex.
Bone involvement is only found in 9% of ALCL[14]. X-rays show osteolytic lesions and CT can show periosteal reactions, and “onion-skin” appearance often suggests more aggressive behavior and worse prognosis[15]. On MRI, the lesion is a T1-weighted low signal and a T2-weighted high signal unless there is a change related to fibrosis. Extranodal NHL, including bone lymphoma, exhibits high levels of FDG uptake[16,17]. According to our knowledge, this is the first report of ALK+ ALCL of the spine occurring in pregnancy.
For patients presenting with incapacitating pain and progressive neurological deficit, immediate treatment is advocated even during pregnancy[18]. Postponing treatment to childbirth may have a greater detrimental effect on the patient’s prognosis. Han et al[19] proposed guidelines for the management of spinal surgery in pregnant women. For patients after 34 wk’ gestation, if they have progressive neurological deficit, induction of labor or cesarean section should be carried out before the spinal surgery. Prepartum surgery can be taken into consideration for patients at < 34 wk’ gestation. Meng et al[20] also suggested that surgical treatment should be performed as soon as possible when there is rapidly progressing motor defect in pregnant patients with spinal tumor. It has been reported that exposure to radiation or teratogenic drugs in the first or second trimester is likely to cause congenital anomalies, and pregnancy may also lead to deterioration of spinal tumors through the effects of hormones and immunosuppression[21]. Aggressive NHL itself and the potential adverse reaction to treatment can endanger both mother and fetus[22].
Klezl et al[23] reported a similar case in 2002 with the diagnosis of NHL with immunoblastic and plasmacytoid infiltration. The patient’s back pain persisted and gradually worsened after pregnancy, but both physicians and physical therapists believed that the pain was related to pregnancy, and no further examination was performed. Immediately after delivery, she became paraparetic, and it was not until this time that the T12 lesion was discovered by MRI examination. The patient underwent posterior spinal decompression and fusion surgery and chemotherapy. Considering the situation of these two patients, we believe that timely diagnosis and intervention should be the first priority in patients with back pain and neurological deficits during pregnancy. This may avoid serious consequences from spinal compression. As for our patient, therapeutic abortion is a personal decision due to concerns about radiation doses and side effects of chemotherapy drugs. For an individual case report, there are still limitations about the choice of treatment and final outcome. We hope that the results of this study will improve the current understanding of the diagnosis and treatment of spinal lymphoma occurring during pregnancy. In the face of patients with different stages of pregnancy, the timing of surgery and chemotherapy, as well as the impact on the mother and fetus, remains to be further studied.
Sustained back pain and neurological deficits during pregnancy may be mistaken by the patient or clinician, so it is important to have a detailed examination of pregnant women with the above symptoms. Surgical treatment and chemotherapy should be considered after definitive diagnosis if the symptoms progress rapidly.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anand A S-Editor: Gong ZM L-Editor: Filipodia E-Editor: Qi LL
| 1. | Stein H, Mason DY, Gerdes J, O'Connor N, Wainscoat J, Pallesen G, Gatter K, Falini B, Delsol G, Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Morris SW, Kirstein MN, Valentine MB, Dittmer K, Shapiro DN, Look AT, Saltman DL. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1995;267:316-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 137] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Agnelli L, Mereu E, Pellegrino E, Limongi T, Kwee I, Bergaggio E, Ponzoni M, Zamò A, Iqbal J, Piccaluga PP, Neri A, Chan WC, Pileri S, Bertoni F, Inghirami G, Piva R; European T-Cell Lymphoma Study Group. Identification of a 3-gene model as a powerful diagnostic tool for the recognition of ALK-negative anaplastic large-cell lymphoma. Blood. 2012;120:1274-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Ferreri AJ, Govi S, Pileri SA, Savage KJ. Anaplastic large cell lymphoma, ALK-positive. Crit Rev Oncol Hematol. 2012;83:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Kisacik B, Akdogan A, Maras Y, Kalyoncu U, Karadag O, Kilickap S, Calguneri M. Anaplastic large cell lymphoma presenting with symmetric polyarthritis in pregnancy. Rheumatol Int. 2008;28:909-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Iyengar P, Reid-Nicholson M, Moreira AL. Pregnancy-associated anaplastic large-cell lymphoma of the breast: a rare mimic of ductal carcinoma. Diagn Cytopathol. 2006;34:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Kato M, Ichimura K, Hayami Y, Iida S, Wakita A, Ueda R, Nakamura S. Pregnancy-associated cytotoxic lymphoma: a report of 4 cases. Int J Hematol. 2001;74:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Lesesve JF, Buisine J, Grégoire MJ, Raby P, Lederlin P, Béné MC, Froment N, Labouyrie E. Leukaemic small cell variant anaplastic large cell lymphoma during pregnancy. Clin Lab Haematol. 2000;22:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Meguerian-Bedoyan Z, Lamant L, Hopfner C, Pulford K, Chittal S, Delsol G. Anaplastic large cell lymphoma of maternal origin involving the placenta: case report and literature survey. Am J Surg Pathol. 1997;21:1236-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Kwee TC, Kwee RM, Nievelstein RA. Imaging in staging of malignant lymphoma: a systematic review. Blood. 2008;111:504-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 12. | Alalade AO, Odejinmi FO. A rare and potentially fatal cause of pelvic pain in pregnancy: anaplastic large cell lymphoma. J Obstet Gynaecol. 2006;26:691-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Falkson HC, Simson IW, Falkson G. Non-Hodgkin's lymphoma in pregnancy. Cancer. 1980;45:1679-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Gianelli U, Patriarca C, Moro A, Ponzoni M, Giardini R, Massimino M, Alfano RM, Armiraglio E, Nuciforo P, Bosari S, Coggi G, Parafioriti A. Lymphomas of the bone: a pathological and clinical study of 54 cases. Int J Surg Pathol. 2002;10:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Krishnan A, Shirkhoda A, Tehranzadeh J, Armin AR, Irwin R, Les K. Primary bone lymphoma: radiographic-MR imaging correlation. Radiographics. 2003;23:1371-83; discussion 1384-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Paes FM, Kalkanis DG, Sideras PA, Serafini AN. FDG PET/CT of extranodal involvement in non-Hodgkin lymphoma and Hodgkin disease. Radiographics. 2010;30:269-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 211] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 17. | O'Neill J, Finlay K, Jurriaans E, Friedman L. Radiological manifestations of skeletal lymphoma. Curr Probl Diagn Radiol. 2009;38:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Ortega J. Multiple agent chemotherapy including bleomycin of non-Hodgkin's lymphoma during pregnancy. Cancer. 1977;40:2829-2835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Han IH, Kuh SU, Kim JH, Chin DK, Kim KS, Yoon YS, Jin BH, Cho YE. Clinical approach and surgical strategy for spinal diseases in pregnant women: a report of ten cases. Spine (Phila Pa 1976). 2008;33:E614-E619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Meng T, Yin H, Li Z, Li B, Zhou W, Wang J, Zhou L, Song D, Xiao J. Therapeutic strategy and outcome of spine tumors in pregnancy: a report of 21 cases and literature review. Spine (Phila Pa 1976). 2015;40:E146-E153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Divers WA, Hoxsey RJ, Dunnihoo DR. A spinal cord neurolemmoma in pregnancy. Obstet Gynecol. 1978;52:47S-50S. [PubMed] |
| 22. | Pavlidis NA. Coexistence of pregnancy and malignancy. Oncologist. 2002;7:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 140] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Klezl Z, Krbec M, Gregora E, Stritesky J. Rare presentation of non-Hodgkin lymphoma of the thoracolumbar spine in pregnancy with 7 years' survival. Arch Orthop Trauma Surg. 2002;122:308-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |