Published online Sep 26, 2019. doi: 10.12998/wjcc.v7.i18.2802
Peer-review started: March 4, 2019
First decision: July 30, 2019
Revised: August 4, 2019
Accepted: August 20, 2019
Article in press: August 20, 2019
Published online: September 26, 2019
Processing time: 207 Days and 0.3 Hours
Calcifying fibrous tumor (CFT) is a rare, benign soft tissue tumor usually occurring in children or young adults. Gastrohepatic ligament CFT with adhesion to the stomach is very rare. We present a case here.
A 25-year-old woman visited our hospital with abdominal pain. Computed tomography and endoscopy were performed, and a gastric submucosal tumor (SMT) with a size of 6.7 cm × 2.7 cm was detected, so endoscopic ultrasonography-guided fine needle biopsy was performed. The tumor was not diagnosed histologically, so surgical resection was planned and performed. The histopathologically confirmed mass size was 6.5 cm × 4.0 cm × 1.0 cm, and a calcified fibrous tumor that originated at the gastrohepatic ligament and adhered to the lesser curvature of the gastric antrum was identified.
Gastrohepatic ligament CFT is a very rare benign tumor. Since this disease may be confused with gastric SMT, the possibility of CFT should be kept in mind during clinical assessment of this disease.
Core tip: Gastrohepatic ligament calcifying fibrous tumor (CFT) is a very rare benign tumor. In addition, the tumor could not be distinguished from the submucosal tumor of the stomach because the tumor adhered to the lesser curvature of the gastric antrum. Since this disease may be confused with gastric submucosal tumor, the possibility of CFT should be kept in mind during clinical assessment of this disease.
- Citation: Kwan BS, Cho DH. Calcifying fibrous tumor originating from the gastrohepatic ligament that mimicked a gastric submucosal tumor: A case report. World J Clin Cases 2019; 7(18): 2802-2807
- URL: https://www.wjgnet.com/2307-8960/full/v7/i18/2802.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i18.2802
Calcifying fibrous tumor (CFT) is a rare, benign soft tissue tumor that typically occurs in children or young adults. The first CFT was reported in 1988 by Rosenthal and Abdul-Karim[1], and was called childhood fibrous tumor with psammoma bodies. Subsequently, Fetsch et al[2] reported 10 cases and named the entity a calcifying fibrous pseudotumor because it is similar to an inflammatory reaction. Nascimento et al[3] reported that true tumors can recur locally, and they are referred to as CFTs according to the 2002 World Health Organization (WHO) guidelines. It has now been confirmed that CFT can occur at various ages and various sites[4].
CFT is characterized by some fibrous tissue and focally infiltrative growth in fat tissue. Histologically, paucicellular hyalinized collagen and scattered calcification have been observed. Inflammatory cells, consisting of lymphocytes and plasma cells, infiltrate, and bland spindle cells are embedded[4,5].
Gastrohepatic ligament CFT with adhesion to the stomach is very rare, and the case presented here mimicked a gastric submucosal tumor (SMT). We present the details of this case together with a review of the literature and differential diagnosis.
A 25-year-old woman visited our hospital with recurrent abdominal pain.
There was intermittent epigastric pain that started 1 day before her visit to our hospital.
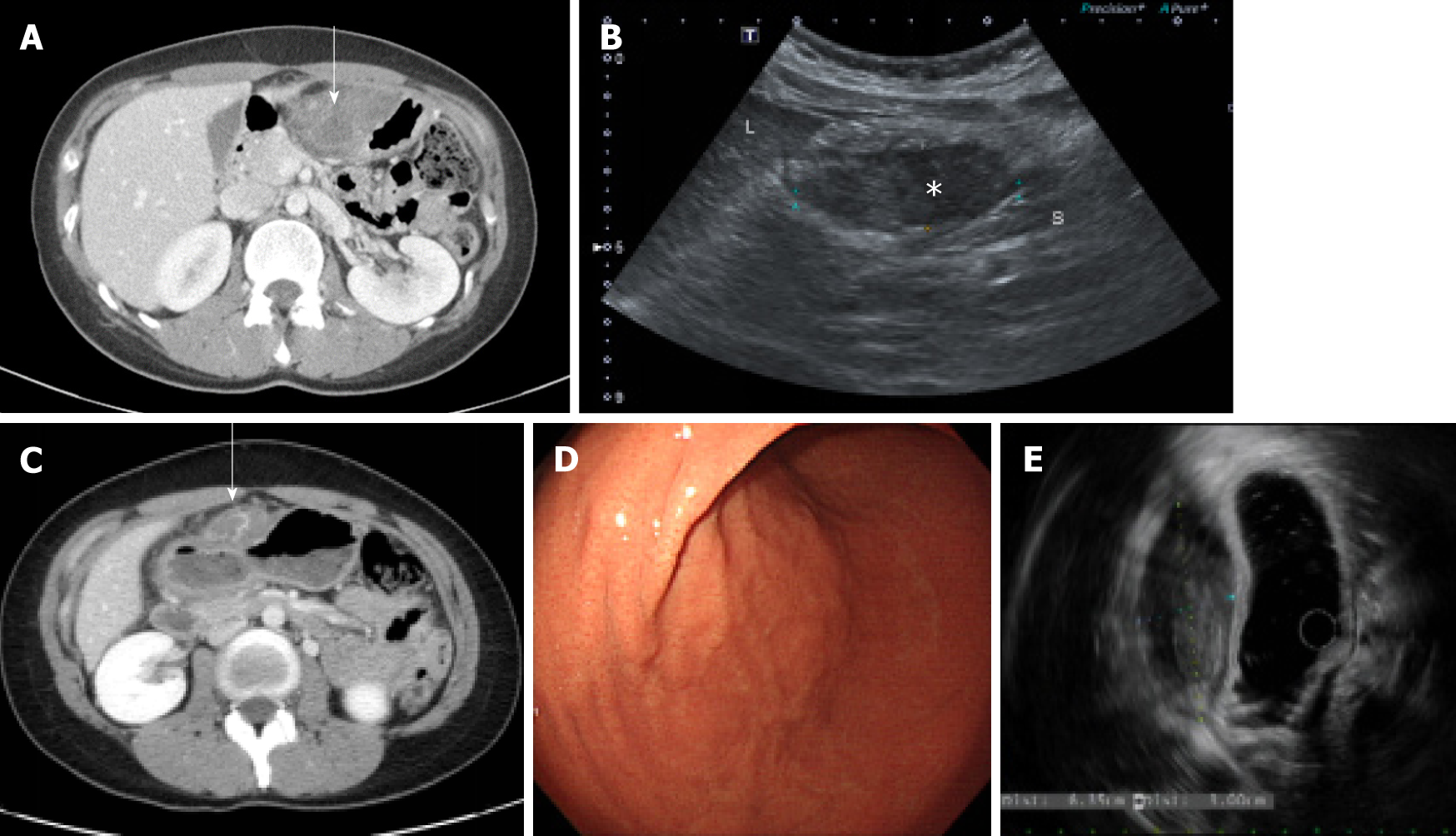
The patient had no specific history of past illness such as trauma or medication. Due to abdominal pain 1 year prior, she underwent computed tomography (CT) of the abdomen and pelvis at another hospital and was transferred to our hospital. A contrast-enhanced CT scan revealed a poorly enhanced heterogenous mass adjacent to the anterior wall of the gastric lower body (Figure 1A). A perigastric abscess or hematoma or a gastric SMT was suspected at that time because the patient had mild fever, abdominal pain, and a poorly enhanced heterogenous mass, and was hospitalized. The patient underwent sonography for percutaneous drainage insertion after admission and a focal hypoechoic solid-like lesion with an internal echogenic component on the anterior wall of the gastric antrum was found, but there was no evidence of an internal cystic lesion, suggesting that the hematoma was suspicious rather than an abscess because a small amount of blood component had drained (Figure 1B). She was treated with antibiotics, tranexamic acid, a proton pump inhibitor, and conservative therapy. Because her symptoms improved, the patient was discharged without any further examination (such as endoscopic ultrasound) and underwent regular follow-up in the outpatient clinic.
The patient had no personal or family history.
The patient exhibited tenderness over the entire abdominal, with epigastric pain dominant, and a fever of 38.4°C.
Blood analysis showed a mild leukocytosis (white blood cell count, 11770/µL), with dominant neutrophils (78.7%) and a normal hemoglobin level and platelet count. The serum C-reactive protein level was elevated, at 31.2 mg/L (normal range, 0-5 mg/L). The other laboratory findings were unremarkable.
Abdominal CT showed a well-circumscribed, highly attenuated, and slightly homogenous round mass in the peri-gastric region (Figure 1C). A SMT, peri-gastric hematoma, and gastric endometriosis were identified by differential diagnosis on CT scan. Thus, upper gastrointestinal endoscopy was performed, and a 6.7 cm × 2.7 cm subcutaneous lesion on the endoscopic anterior wall of the great curvature of the gastric antrum was identified (Figure 1D). Endoscopic ultrasonography (EUS) was performed next, and the findings were a heterogeneous hypoechoic mass of approximately 6.35 cm × 3.00 cm that appeared similar to a gastric SMT of fourthlayer origin (Figure 1E). EUS-guided fine needle biopsy (EUS-FNAB) was performed due to suspicion of a gastric SMT based on the EUS findings, but because the tumor was not diagnosed histologically, surgical resection was planned.
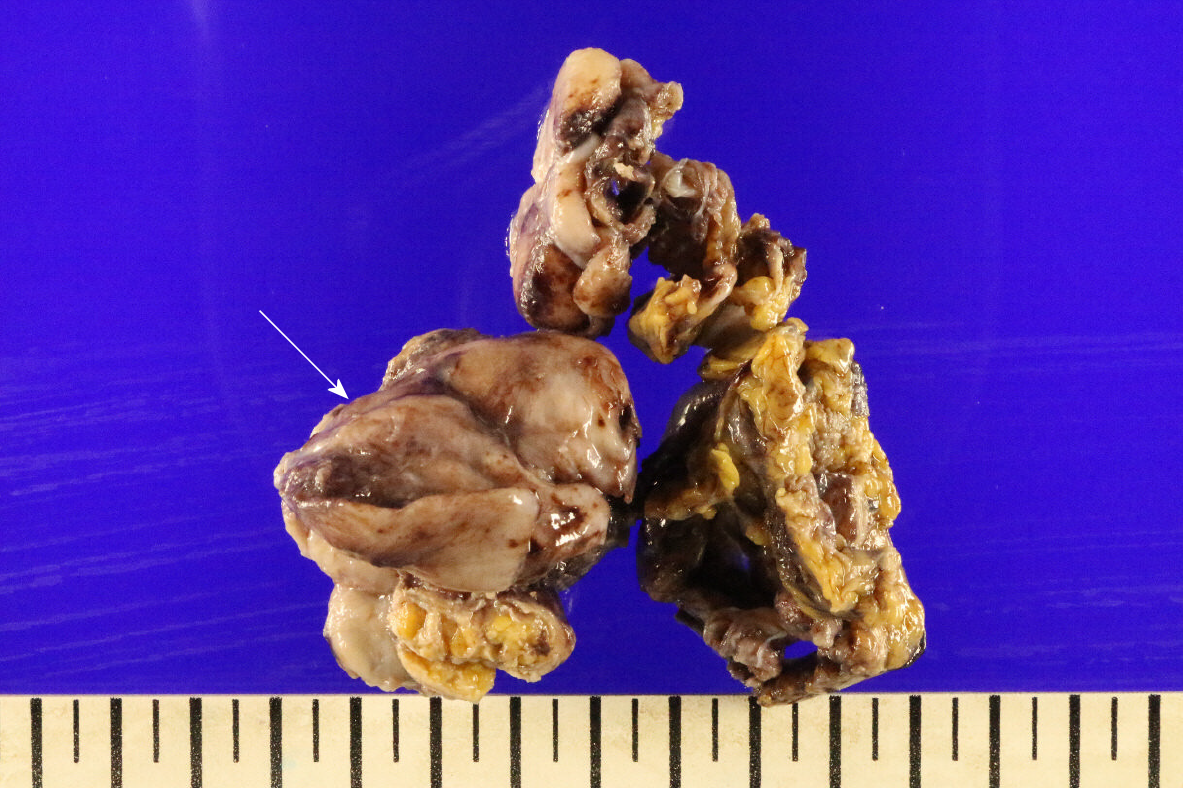
The mass was mostly well circumscribed with a white-tan cut surface, showed focally infiltrative growth in fat tissue. The grossly confirmed mass was 6.5 cm × 4.0 cm × 1.0 cm, and a calcified fibrous tumor that originated from the gastrohepatic ligament and adhered to the lesser curvature of the gastric antrum was identified (Figure 2).
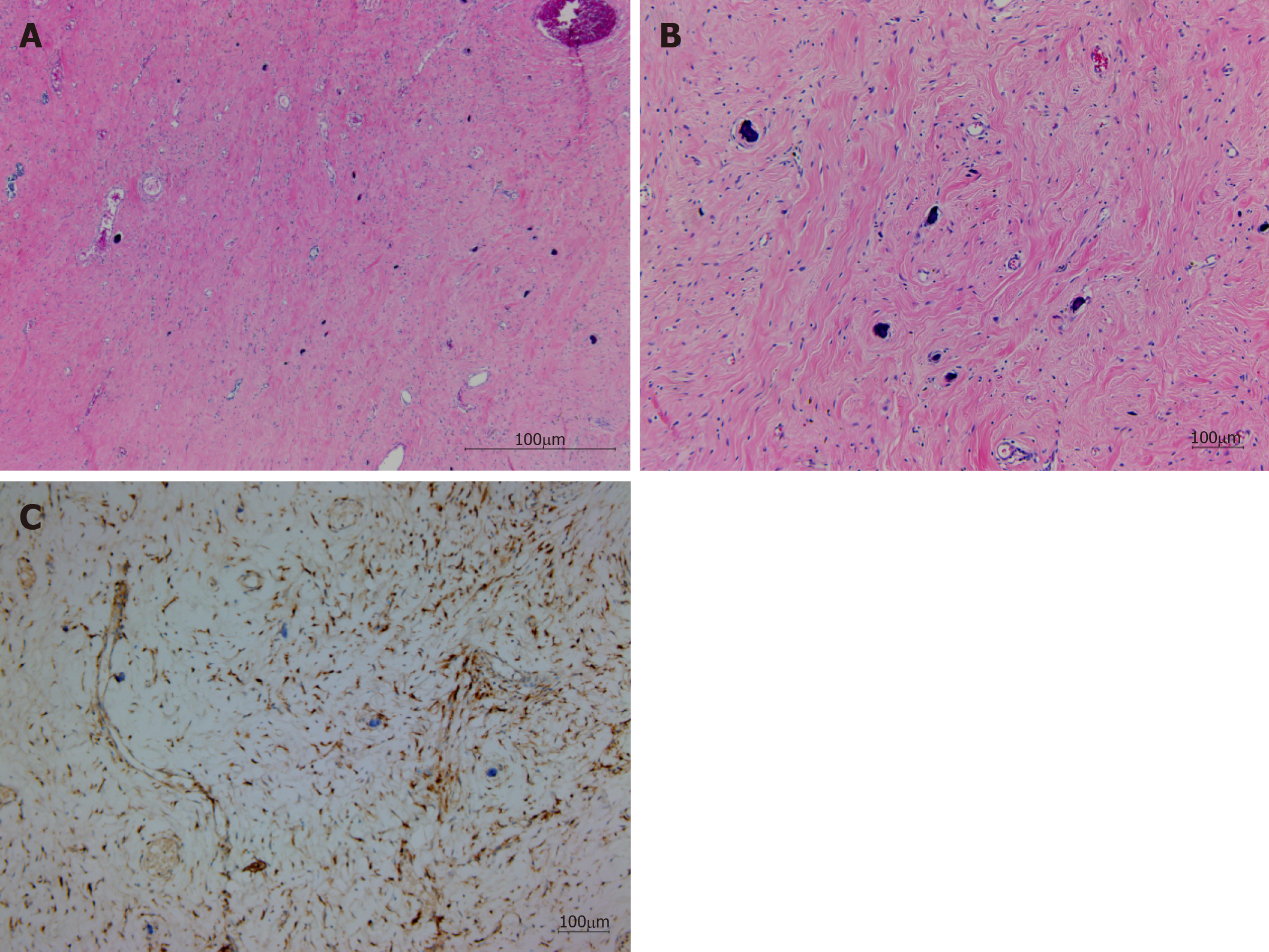
The tumor consisted of a sparse population of fibrous cells, hyalinized collagen, and a haphazard collagenous matrix; a scattered dystrophic calcification was observed. Inflammatory cells, consisting of lymphocytes and plasma cells, were the dominant infiltrating cells, and bland spindle cells were embedded (Figure 3A, 3B). No germinal center was observed. A fibrinoid degenerative lesion due to a previous histologic examination by EUS-FNAB was present. Immunohistochemical staining results indicated that the tumor was CD34 negative, SMA negative, CD117 negative, Bcatenin negative, and Factor XIIIa positive for spindle cells (Figure 3C).
A CFT was diagnosed.
Laparoscopic surgery was conducted for diagnostic and therapeutic purposes, and complete resection of the tumor was performed. After insertion of the laparoscope, the operator identified adhesion of the stomach, falciform ligament, and peritoneum. Although the mass strongly adhered to the wall of the stomach, the tumor was able to be completely separated from the stomach by harmonic scalpel dissection, and the stomach was completely preserved without abstinence.
No recurrence was observed during the 6-mo follow-up period.
CFT is a rare, benign tumor characterized by a grossly well-demarcated, unencapsulated, lobulated mass with a white cut surface. Microscopically, CFT was identified by the tumor’s paucicellular trait and its containing hyalinized collagen with interspersed dystrophic or psammomatous calcification, and infiltrating inflammatory cells composed of lymphocytes and plasma cells.
Cases in which gastric CFT mimicked gastric SMT have been reported in Japan[6]. CFT of gastric-wall origin has also been reported[7-10]. Although this case was not a CFT of gastric origin, it appeared to be a mass of gastric origin due to its strong adhesion to the gastrohepatic ligament CFT. Therefore, this case is rare. In fact, CFT can occur at various sites. According to a meta-analysis by Chorti et al[4], the most common site of occurrence is the stomach (18%), followed by the small intestine, (8.7%), pleura (9.9%), neck (6.2%), mesentery (5%), mediastinum (5%), and peritoneum (6.8%). A meta-analysis suggested that this case is very rare in terms of the site of occurrence. Furthermore, no report of CFT of gastrohepatic ligament origin was found in the literature; therefore, this may be the first case report.
In a study by Chorti et al[4], females were found to be 1.27-fold more likely to have CFT than males; indeed, the case reported here was in a female. In addition, the age of onset was within one of the high-incidence age groups according to Chorti et al[4]. A tumor of less than 5 cm is found in most cases, but the tumor in this case was also a relatively common size, 6.5 cm. The symptoms of CFT are heterogeneous, and only 30% of cases are symptomatic. This case belonged to the group with symptoms of abdominal pain and fever. Thus, this case is rare in terms of the site of occurrence and in being clinically similar to gastric SMT, but its epidemiology was relatively typical.
For diagnosis, CFT must be differentiated from various other types of tumors. The most important differential diagnoses in intra-abdominal CFT are GIST, leiomyoma, inflammatory myofibroblastic tumor (IMT), solitary fibrous tumor, and IgG4-related disease. Due to their heterogenous symptoms, these tumors are diagnosed based on imaging and pathologic findings, including immunohistochemical staining. Pathologically, calcification, inflammatory cells, and dense hyalinized collagen are characteristic of CFT, as is immunohistochemical staining for vimentin and Factor XIIIa staining[4,11,12]. This case required discrimination from gastric SMT, which was facilitated by immunochemical staining. CD117 and CD34 are helpful for distinguishing CFT and GIST[6,13-16].
Desmin and SMA are useful for distinguishing leiomyoma[7]. IMT, which is difficult to distinguish from CFT, is positive for SMA and ALK-1[3,12-14,16-18]. Solitary fibrous tumors have a hypercellular component, a heterogenous pattern of spindle cells, and are positive for CD34, CD99, and bcl-2. In some cases, CD34, CD68, IgG, and IgG4 may be partially expressed[4]. Larson et al[19] suggested that CFT may be an IgG4-related disease, because it shows a histopathologic pattern of dense lymphoplasmacytic infiltration, fibrosis, and obliterative phlebitis[4,19]. However, this idea requires further investigation because Chorti et al[4] reported that fewer than 10 cases exhibited positive IgG4 staining. Unfortunately, in the present case, IgG4 was not assayed.
The prognosis of CFT is good, but Nascimento et al[3]. reported that local recurrence can occur. Indeed, six cases experienced recurrence in the study by Chorti et al[4]. Therefore, follow-up is needed. However, because this is a recent case, no information on follow-up is yet available.
Gastrohepatic ligament CFT is a very rare benign tumor. Because this disease may be confused with gastric SMT, the possibility of CFT should be kept in mind during clinical assessment of patients with this disease.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lim SC, Kapoor S, Fujino Y S-Editor: Dou Y L-Editor: A E-Editor: Qi LL
| 1. | Rosenthal NS, Abdul-Karim FW. Childhood fibrous tumor with psammoma bodies. Clinicopathologic features in two cases. Arch Pathol Lab Med. 1988;112:798-800. [PubMed] |
| 2. | Fetsch JF, Montgomery EA, Meis JM. Calcifying fibrous pseudotumor. Am J Surg Pathol. 1993;17:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 204] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Nascimento AF, Ruiz R, Hornick JL, Fletcher CD. Calcifying fibrous 'pseudotumor': clinicopathologic study of 15 cases and analysis of its relationship to inflammatory myofibroblastic tumor. Int J Surg Pathol. 2002;10:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Chorti A, Papavramidis TS, Michalopoulos A. Calcifying Fibrous Tumor: Review of 157 Patients Reported in International Literature. Medicine (Baltimore). 2016;95:e3690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Larson BK, Dhall D. Calcifying Fibrous Tumor of the Gastrointestinal Tract. Arch Pathol Lab Med. 2015;139:943-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Sato S, Ooike N, Yamamoto T, Wada M, Miyamoto A, Matsukawa M, Morohoshi T. Rare gastric calcifying fibrous pseudotumor removed by endoscopic submucosal dissection. Digest Endosc. 2008;20: 84-86. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Elpek GO, Küpesiz GY, Ogüs M. Incidental calcifying fibrous tumor of the stomach presenting as a polyp. Pathol Int. 2006;56:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Vasilakaki T, Skafida E, Tsavari A, Arkoumani E, Koulia K, Myoteri D, Grammatoglou X, Moustou E, Firfiris N, Zisis D. Gastric calcifying fibrous tumor: a very rare case report. Case Rep Oncol. 2012;5:455-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Delbecque K, Legrand M, Boniver J, Lauwers GY, de Leval L. Calcifying fibrous tumour of the gastric wall. Histopathology. 2004;44:399-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Jang KY, Park HS, Moon WS, Lee H, Kim CY. Calcifying fibrous tumor of the stomach: a case report. J Korean Surg Soc. 2012;83:56-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Hill KA, Gonzalez-Crussi F, Chou PM. Calcifying fibrous pseudotumor versus inflammatory myofibroblastic tumor: a histological and immunohistochemical comparison. Mod Pathol. 2001;14:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Hill KA, Gonzalez-Crussi F, Omeroglu A, Chou PM. Calcifying fibrous pseudotumor involving the neck of a five-week-old infant. Presence of factor XIIIa in the lesional cells. Pathol Res Pract. 2000;196:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Im S, Jung JH, Yoo C, Choi HJ, Yoo J, Kang CS. Calcifying fibrous tumor presenting as rectal submucosal tumor: first case reported in rectum. World J Surg Oncol. 2014;12:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Wesecki M, Radziuk D, Niemiec S, Waniczek D, Lorenc Z. Calcifying fibrous tumor of the small bowel mesentery in a 27-year old male patient - case report. Pol Przegl Chir. 2014;86:436-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | George SA, Abdeen S. Gastric Calcifying Fibrous Tumor Resembling Gastrointestinal Stromal Tumor: A Case Report. Iran J Pathol. 2015;10:306-309. [PubMed] |
| 16. | Voltaggio L, Montgomery E. Gastric mesenchymal lesions other than gastrointestinal stromal tumor. Diagnostic Histopath. 2014;20: 228-238. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Ogasawara N, Izawa S, Mizuno M, Tanabe A, Ozeki T, Noda H, Takahashi E, Sasaki M, Yokoi T, Kasugai K. Gastric calcifying fibrous tumor removed by endoscopic submucosal dissection. World J Gastrointest Endosc. 2013;5:457-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Shi Q, Xu MD, Zhong YS, Zhou PH, Wu HF, Yao LQ. The laparoscopic-endoscopic cooperative surgery for the colonic calcifying fibrous tumor: one case report. J Laparoendosc Adv Surg Tech A. 2012;22:996-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Larson BK, Balzer B, Goldwasser J, Dhall D. Calcifying fibrous tumor: an unrecognized IgG4--related disease? APMIS. 2015;123:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |