Published online Sep 26, 2019. doi: 10.12998/wjcc.v7.i18.2776
Peer-review started: March 20, 2019
First decision: May 31, 2019
Revised: August 19, 2019
Accepted: September 9, 2019
Article in press: September 9, 2019
Published online: September 26, 2019
Processing time: 191 Days and 9.6 Hours
An epidemic of Mycobacterium chimaera (M. chimaera) infections following cardiac surgery is ongoing worldwide. The outbreak was first discovered in 2011, and it has been traced to a point source contamination of the LivaNova 3T heater-cooler unit, which is used also in Italy. International data are advocated to clarify the spectrum of clinical features of the disease as well as treatment options and outcome. We report a series of M. chimaera infections diagnosed in Treviso Hospital, including the first cases notified in Italy in 2016.
Since June 2016, we diagnosed a M. chimaera infection in nine patient who had undergone cardiac valve surgery between February 2011 and November 2016. The time between cardiac surgery and developing symptoms ranged from 6 to 97 mo. Unexplained fever, psychophysical decay, weight loss, and neurological symptoms were common complaints. The median duration of symptoms was 32 wk, and the longest was almost two years. A new cardiac murmur, splenomegaly, choroidoretinitis, anaemia or lymphopenia, abnormal liver function tests and hyponatremia were common findings. All the patients presented a prosthetic valve endocarditis, frequently associated to an ascending aortic pseudoneurysm or spondylodiscitis. M. chimaera was cultured from blood, bioprosthetic tissue, pericardial abscess, vertebral tissue, and bone marrow. Mortality is high in our series, reflecting the poor outcome observed in other reports. Three patients have undergone repeat cardiac surgery. Five patients are being treated with a targeted multidrug antimycobacterial regimen.
Patients who have undergone cardiac surgery in Italy and presenting with signs and symptoms of endocarditis must be tested for M. chimaera.
Core tip: A prolonged epidemic of Mycobacterium chimaera (M. chimaera) infections following cardiac surgery is ongoing worldwide. The outbreak was first discovered in Switzerland in 2011, and it has been traced to a point source contamination of the LivaNova (formerly Sorin) 3T heater-cooler unit, which is the most used device in Italy. International data are advocated in order to clarify the spectrum of clinical, laboratory, echocardiographic, and radiological features of the disease as well as treatment options and outcome. Here we report the clinical features of a case series of M. chimaera infections diagnosed in our Hospital, including the first cases notified in Italy in 2016.
- Citation: Inojosa WO, Giobbia M, Muffato G, Minniti G, Baldasso F, Carniato A, Farina F, Forner G, Rossi MC, Formentini S, Rigoli R, Scotton PG. Mycobacterium chimaera infections following cardiac surgery in Treviso Hospital, Italy, from 2016 to 2019: Cases report. World J Clin Cases 2019; 7(18): 2776-2786
- URL: https://www.wjgnet.com/2307-8960/full/v7/i18/2776.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i18.2776
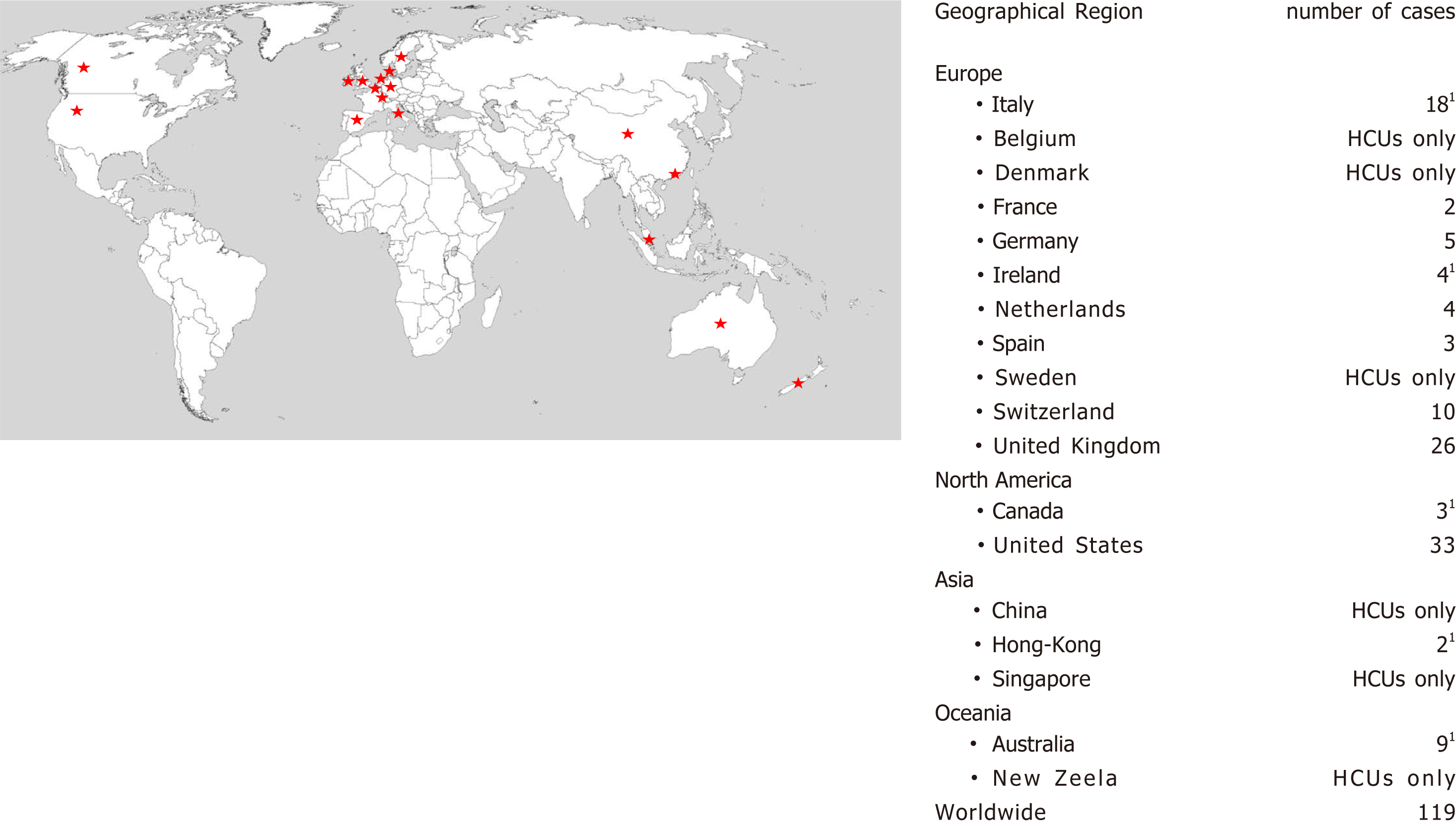
A prolonged epidemic of severe Mycobacterium chimaera (M. chimaera) infections following cardiac surgery is ongoing worldwide (Figure 1)[1]. After the first clinical reports from Switzerland in 2011, and then from Germany, the Netherlands and the United States, investigators identified an epidemiological link between M. chimaera infections and exposure during cardiopulmonary bypass to the LivaNova (formerly Sorin) 3T heater-cooler unit (HCU), which is also the most used device in Italy[2-6]. Extensive molecular epidemiological investigations in Europe, the United Kingdom, the United States, and Australia have traced the epidemic to a point source contamination of the LivaNova 3T HCU at the site of production in Germany[7-9].
A series of experimental studies have clarified the transmission pathway: Mycobacterium chimaera is a slow-growing non-tuberculous mycobacteria (NTM), that has been identified in household water[10,11]. It has been also isolated from the drinking water dispensers in hospital and from the water circuit of the HCU[3,12]. When the HCU was experimentally operating, M. chimaera was detected in the exhaust air of the HCU and in the area around the operating table[12]. Water turbulences induced by stirring devices inside the tank of the HCU produce air bubbles known to aerosolize NTM like M. chimaera at high concentration[13]. It is believed that when these bubbles burst, the ejected water droplets may escape through openings in the water circuit[13]. Smoke dispersal experiments and aerobiological investigations show that infectious aerosol, with a particle size < 1 μm, is released into the operating room and disseminated via the rear cooling fans of an HCU[14,15]. Infectious aerosol dissemination may result in contamination of the operative field and the bioprosthesis exposed prior to implantation, as M. chimaera can be identified on sedimentation plates at 5 m distance from the contaminated HCU[14]. Finally, M. chimaera may form biofilm on the contaminated intravascular device, leading to endocarditis and disseminated infections[13].
International data are advocated in order to clarify the spectrum of clinical, laboratory, echocardiographic, and radiological features of the disease as well as treatment options and outcome[16]. Here we report the clinical and laboratory features of a case series of M. chimaera infections diagnosed in our Unit of Infectious Diseases (UID), including the first cases notified in Italy in 2016 (Table 1).
| Case | sex/age | Cardiac surgery | Date surgery | Symptoms beginning | Date hospitali-zation | Micro-biological diagnosis (date) and disease history | Clinical syndromes | Medical therapy (mo) | Repeat surgery and disease history | Outcome |
| 1 | M 37 | CARR | 12/2012 TH | After 35 mo | 05/2016 | 09/2016, after 10 mo | PVE, AAP, DI | Anti-MAC (1) | After 8 mo | Deceased |
| 2 | M 60 | AVR + MVA | 01/2013 oH | After 40 mo | 05/2016 | 10/2016, after 11 mo | PVE, AAP, DI | No | No (see text) | Deceased |
| 3 | M 42 | AVR + ARR | 05/2015 TH | After 6 mo | 10/2016 | 11/2016, after 12 mo | PVE, AAP, DI | No | No | Deceased |
| 4 | M 75 | AVR | 02/2011 TH | After 64 mo | 07/2016 | 01/2017, after 7 mo | PVE, DI, SD | Anti-MAC (13) | After 15 mo | Deceased |
| 5 | M 80 | MVR + AW | 03/2016 TH | After 12 mo | 08/2018 | 09/2018, after 18 mo | PVE, SD | Targeted (14) | No | On therapy |
| 6 | M 56 | AVR | 06/2016 TH | After 27 mo | 11/2018 | 10/2018, after 1 mo | PVE, DI, SD | Targeted (12) | After 8 mo | On therapy |
| ARR | 05/2015 TH | |||||||||
| 7 | M 75 | AVR | 01/2014 oH | After 21 mo | 02/2019 | 04/2019, after 8 months | PVE | Targeted (4) | No | On therapy |
| AVR | 11/2016 oH | |||||||||
| 8 | M 76 | AVR + CABG | 02/2011 TH | After 97 mo | 05/2019 | 05/2019, after 2 mo | PVE, DI, SD | Targeted (3) | No | On therapy |
| 9 | M 82 | AVR + CABG | 08/2016 oH | After 12 mo | 06/2019 | 06/2019, after 22 mo | PVE | Targeted (2) | No | On therapy |
Patient 1: In May 2016, a 37-year-old male was admitted to the UID with an 8-mo history of fever and psychophysical decay.
Patient 2: In May 2016, a 60-year-old man was referred to our UID for recurrent endocarditis of the prosthetic valve, progressive renal failure, chronic liver disease, pancreatitis and type II diabetes mellitus.
Patient 1: Eight months prior to the current admission, he had been hospitalized for pneumonia in Romania, his native country. One month later, he was hospitalized again for unspecified chronic active hepatitis and splenomegaly. A presumptive diagnosis of pulmonary tuberculosis was made, based on a reticular pattern on the chest X-ray and unspecific granulomatous inflammation in lung biopsy. The patient received a first line four-drug anti-tuberculosis therapy for two months, and then he was maintained on rifampicin and isoniazid treatment. However, he arrived at the follow-up cardiologic visit with fever, severe weight loss, psychic decay, anaemia and kidney failure. Therefore, he was referred to our tertiary care hospital.
Patient 2: Ten days before the current hospitalization, he was admitted to another hospital with a several month histories of fatigue, 8 kg weight loss, and biochemical evidence of anaemia and elevated liver enzymes.
Patient 1: In 2012, the patient had undergone composite BioBentall aortic root replacement.
Patient 2: His past medical history included hospitalization in November 2012 for Streptococcus gallolyticus aortic endocarditis complicated by L5-S1 spondyloscitis and parietal forehead ischemia, for which in January 2013 he underwent concomitant bioprosthetic aortic valve replacement and mitral annuloplasty.
Patient 1: The patient’s temperature was 39.4 °C, heart rate was 102 bpm, respiratory rate was 22 breaths per minute, blood pressure was 100/70 mmHg and oxygen saturation in room air was 94%. His clinical examination was remarkable for diminished vesicular murmur at the lung bases, and hepatosplenomegaly.
Patient 2: On admission, the patient’s temperature was 37.8 °C, heart rate was 82 bpm, respiratory rate was 16 breaths per minute, blood pressure was 110/70 mmHg and oxygen saturation in room air was 98%. His clinical examination was remarkable for a grade III/VI systolic ejection murmur at the upper right sternal base and splenomegaly.
Patient 1: Pertinent laboratory findings (reference ranges provided parenthetically) included haemoglobin 8.2 g/dL (14.0-18.0 g/dL), platelet count 101 × 109/L (140-454 × 109/L), serum creatinine 2.25 mg/dL (0.7-1.2 mg/dL), blood urea nitrogen 73 mg/dL (6-20 mg/dL), aspartate aminotransferase (AST) 61 U/L (4-40 U/L), alanine transferase 68 U/L (4-41 U/L); lactate dehydrogenase (LDH) 480 U/L (122-222 U/L), C-reactive protein (CRP) level 3.09 mg/dL (< 0.50 mg/dL). The lymphocyte and CD4 cell counts were 824 and 368 cells/mmc, respectively. HIV infection was excluded. All microbiological investigations were negative except for the cultures of sputum and bronchoalveolar lavage that turned out positive for Mycobacterium intracellulare.
Patient 2: Pertinent laboratory findings included haemoglobin 10.6 g/dL, serum creatinine 1.7 mg/dL, blood urea nitrogen 42 mg/dL, AST 59 U/L; LDH 808 U/L, gammaglutamiltranspeptidase 216 U/L (8-61 U/L), ferritin 1292 ng/mL (< 300 ng/mL); CRP 1.4 mg/d, amylase 232 U/L (< 46 U/L), lipase 262 U/L (< 63 U/L). Conventional blood cultures were negative.
Patient 1: In the initial workup, a transthoracic echocardiogram (TTE) showed no evidence of endocarditis, while a computerized tomography (CT) of the thorax and abdomen showed pulmonary infiltrates on the right superior lobe, mediastinal and retroperitoneal lymphadenopathy, hepatomegaly and splenomegaly with ischemia at the upper pole. A kidney biopsy revealed an interstitial nephritis. One month later, the patient was transferred to the Coronary Unit for heart failure. This time the transoesophageal echocardiogram (TOE) showed an abscessual pseudoaneurysm of the aortic root and endocarditis vegetations on the cusps of the bioprosthetic valve.
Patient 2: In the initial work up before the transfer to our hospital, TTE and TOE were negative for signs of endocarditis but a positron emission tomography/computed tomography (PET/CT) demonstrated an increased metabolic activity in the left ventricle at the aortic level. A repeat TOE showed evidence of a pseudo-aneurysm of the aortic root.
Prosthetic valve endocarditis (PVE), ascending aortic pseudoaneurysm and disseminated infection due to M. chimera (post-mortem diagnosis, see “outcome and follow-up” paragraph).
PVE, ascending aortic pseudoaneurysm and disseminated infection due to M. chimaera and methicillin susceptible Staphylococcus aureus (MSSA).
Our clinical consideration was a PVE due to unknown aetiology, therefore empiric treatment was started with ampicillin and oxacillin i.v. After multidisciplinary evaluation the patient underwent aortic bioprosthesis replacement and left ventricular outflow tract reconstruction. The histopathological examination showed a fibrous and calcified valve tissue with giant cells infiltrates but without acid-fast-positive bacteria. The molecular analysis upon the valve tissue turned out positive for M. intracellulare. The patient started anti-mycobacterial therapy with rifampin, ethambutol, azithromycin and linezolid.
Our clinical consideration was a PVE due to unknown aetiology, therefore empiric treatment was started with daptomycin 700 mg i.v. as the patient was allergic to beta-lactams. The patient underwent aortic breach repair with bovine pericardium patch, maintaining his prosthetic valve. Intraoperative cultures for conventional bacteria turned out negative and he completed 6 wk of antibiotic therapy with some clinical improvement.
Post-operative course was complicated by haemorrhage, cardiac tamponade and Candida mediastinitis and the patient underwent four cardiothoracic interventions. An ophthalmologic exam was positive for bilateral peripheral chorioretinitis. He died after a massive cerebral haemorrhage. Culture from a pericardial abscess drained in the last intervention turned out positive for M. intracellulare. A post-mortem molecular analysis carried out on defrosted mycobacterial cultures from sputum and the pericardial abscess allowed the identification of Mycobacterium chimaera.
In the post-operative period, the patient developed severe acute renal failure requiring dialysis treatment. After three months, the patient became febrile again and his conditions soon deteriorated requiring the hospitalization in the Intensive Care Unit of another hospital. Blood cultures turned-out positive for MSSA and treatment with daptomycin 700 mg i.v. was resumed accordingly. The ophthalmologic exam was positive for bilateral chorioretinitis. A multidisciplinary evaluation advised to send mycobacterial cultures to the referral Microbiology. His clinical conditions were considered too serious to support a new cardiac surgery. He died of progressive heart failure a few wk later. Mycobacterial blood cultures grew M. chimaera after one month.
Microbiological diagnosis was carried out at the referral Microbiology of Padua University (patient 1, 2, 3 and 8) and at Treviso Hospital (patient 1, 4, 5, 6, 7 and 9). For patients 4, 5, 6, 7 and 8 antimicrobial susceptibility testing by broth microdilution was carried out at Padua University, while patients 1, 2, and 3 were tested only by molecular characterization of drug susceptibility for nontuberculous mycobacteria.
Blood collected in BD BACTEC™ Myco/F Lytic Medium and tissue specimens were cultured for mycobacteria by standard methods on Middlebrook 7H10 agar plates, followed by MGIT liquid and Lowenstaine solid cultures for recovering. Sequences were analyzed using GenoType Mycobacterium CM ver. 2.0 (Hain Lifescience, Nehren, Germany) and GenoType NTM-DR ver. 1.0 (Hain Lifescience, Nehren, Germany). The assignment of Mycobacterium chimaera was based on 16S rRNA gene sequencing results with the GenoType NTM-DR assay[10,17].
Since June 2016, nine patients with M. chimaera infection following cardiac surgery have been diagnosed in Treviso Hospital (Table 1). Consistent with earlier reports, our case series show that identifying patients with M. chimaera infection is difficult due to a prolonged incubation period, the insidious onset, and unspecific symptoms[4,13,16]. The median time between cardiac surgery and developing symptoms was 27 mo, but ranged from 6 mo to more than 8 years (Table 1). Unlike what was observed in a large case series in the United Kingdom, where 80% of patients became ill within 2 years of surgery, 55% of our patients developed symptoms after 2 years following surgery, and in one case after 97 mo: therefore, clinical surveillance should be prolonged for at least 10 years before considering the patient reasonably out of danger[16]. All the patients had undergone cardiac valve surgery between February 2011 and November 2016, in two cases with concomitant coronary artery bypass graft (CABG). Until now, we have never diagnosed any M. chimaera infection following only CABG surgery. All the patients were male and their age ranged from 37 to 82 years. The median duration of symptoms was 32 wk, but the longest was 22 mo in a patient operated in another hospital where previously no other M. chimaera cases had ever been diagnosed. Unexplained fever (78%), malaise (78%), psychophysical decay with weight loss (66%) were frequently reported at admission (Table 2). Neurological symptoms (55%) such as amnesia, confusional state, or hemisyndrome were often associated to a severe disease. A new cardiac murmur (66%), splenomegaly (66%), choroidoretinitis (55%), and radiological signs of spondylodiscitis (55%) were common findings. Anemia (100%), lymphopenia, thrombocytopenia, increased LDH and alkaline phosphatase, abnormal liver function tests, and hyponatremia were common laboratory findings. Renal function impairment with reduced estimated glomerular filtration rate was observed in some more severely ill patients, after a prolonged illness. All the cases occurred in the absence of a known primary or secondary immunodeficiency; in three cases, we observed a low CD4 count with a low CD4/CD8 percentage. Bone marrow biopsy performed on patient 6 and 8 revealed granulomatous lymphohistiocytic infiltrates suggesting a secondary immune suppression caused by the disseminated M. chimaera infection.
| Variable | UK patients (n = 30) | Italian patients (n = 9) |
| Clinical finding, n (%) | ||
| Fever | 24 (80) | 7 (78) |
| Malaise (astenia) | 24 (80) | 7 (78) |
| Weight loss | 18 (60) | 6 (66) |
| Cough | 11 (37) | 3 (33) |
| Dyspnoea | 10 (33) | 2 (22) |
| Arthralgia | 6 (20) | 0 |
| Chest pain | 6 (20) | 0 |
| Abdominal pain | 3 (10) | 0 |
| Back pain | 2 (7) | 5 (55) |
| New cardiac murmur | 9 (30) | 6 (66) |
| Oedema | 6 (20) | 1 (11) |
| Crepitations | 6 (20) | 0 |
| Splenomegaly | 8 (27) | 6 (66) |
| Hepatomegaly | 6 (20) | 3 (33) |
| Lymphadenopathy | 1 (3) | 1 (11) |
| Sternal wound | 4 (13) | 0 |
| Skin lesion | 2 (7) | 0 |
| Choroiditis | 2 (7) | 5 (55) |
| Neurological symptoms | NA | 5 (55) |
| Laboratory findings, median (IQR) | ||
| Haemoglobin (g/L) | 110 (96-127) | 105 (95-114) |
| WBC (× 109/L) | 3.9 (2.2-5.4) | 3.9 (2.8-6.7) |
| Neutrophils (× 109/L) | 2.4 (1.3-3.3) | 2.6 (1.4-3.8) |
| Lymphocytes (× 109/L) | 0.9 (0.6-1.3) | 0.76 (0.56–1.05) |
| Platelets (× 109/L) | 175 (86-223) | 166 (76-257) |
| Albumin (g/L) | 30 (26-37) | 34 (27-39) |
| ALT (IU/L) | 43 (33-85) | 41 (15-70) |
| ALP (IU/L) | 256 (132-357) | 242 (157-254) |
| Sodium (µmol/L) | 134 (131-136) | 131 (128-136) |
| eGFR (mL/min) | 66 (53-80) | 52 (35-81) |
| CRP (mg/L) | 33 (17-46) | 19 (14-31) |
| ESR (mm/h) | NA | 65 (36-111) |
| LDH (IU/L) | NA | 463 (244-680) |
| gammaGT (IU/L) | NA | 104 (44-156) |
All the patients were affected by a PVE, in three cases associated to an ascending aortic pseudo-aneurysm as the prominent cardiovascular manifestation. Four patents died of progressive heart failure, in one case associated with a severe disseminated infection and ischemic stroke.
For 4 out of 9 patients (44%), the diagnosis of M. chimarea infection was preceded 1-6 mo earlier by a PVE from conventional bacteria: Staphylococcus aureus for patient 2, Staphylococcus epidermidis for patient 4, and Enterococcus faecalis for patient 7 and 9. Therefore, our limited case series indicates a particularly high risk of PVE by conventional bacteria in patients with M. chimaera infection, and mycobacterial blood cultures should always be performed after a diagnosis of bacterial PVE to identify a possible concomitant infection from M. chimaera[18].
As we consider TOE more sensitive than TTE in diagnosing bacterial PVE, all nine patients underwent TOE, with five positive results (55%). However, PET/CT resulted positive for PVE in all the eight patients for which it was performed (100%), appearing more sensitive than TOE for patients 3 and 5 (negative TOE), and patient 6 and 8 (doubtful TOE). We suggest that PET/CT should always be considered after a first negative TOE, as it proved to be quite sensitive in the diagnosis of M. chimaera PVE in our case series, as well as having proved useful in the diagnosis of PVE and infections of implantable cardiac devices caused by other bacteria[19]. PET/CT has also the potential to simultaneously diagnose systemic complications such as septic emboli and spondylodiscitis, which appear to be a common localization of M. chimaera infection in our series.
Culture of M. chimaera from peripheral blood was the most common method of microbiological diagnosis, having been conducted in eight patients with seven positive cases (Table 3). M. chimaera was also cultured from bioprosthetic tissue, pericardial abscess, vertebral tissue, and bone marrow. When multiple samples were collected on separate days, all cultures turned out positive. The median time between sample collection and microbiological diagnosis was 5 wk (range 2-12 wk). Unfortunately, we did not conduct 16S rRNA gene sequencing for M. chimaera of tissue specimens such as excised cardiac material, a rapid method that, in light of the average culture times, could anticipate the diagnosis of more than one month. However, a presumptive rapid diagnosis in these cardio surgery patients was obtained with direct microscopy after enrichment on the bioprosthetic tissue (patient 4 and 6) or bone marrow biopsy (patient 6), or with auramine rhodamine fluorescent staining of the vertebral specimen (patient 5). Moreover, the rapid molecular diagnosis of M. intracellulare on the excised bioprosthetic valve of patient 1 raised suspicion in light of the current M. chimaera epidemic.
| Case | Specimens | Time for cultural identification | Other positive tests | Antimicrobial susceptibility test |
| 11 | Sputum | 8 wk | Macrolid (S)2 | |
| Broncholavage | 10 wk | |||
| Bioprosthesis | np | PCR | ||
| Pericardial abscess | 8 wk | |||
| Blood | np | |||
| 2 | Blood | 4 wk | Macrolid (S)2 | |
| 3 | Blood | 4 wk | Macrolid (S)2 | |
| 4 | Blood (2) | 3 wk | Clarithromycin (S); Linezolid (R); Moxifloxacin (S); Aminoglycoside (S)2; Macrolid (S)2 | |
| Bioprosthesis | 6 wk | Microscopy after enrichment | ||
| Vertebral bone | 12 wk | |||
| 5 | Vertebral bone (3) | 3, 5, and 10 wk | Auramine rhodamine stain | Clarithromycin (S); Linezolid (I); Moxifloxacin (R) |
| Blood (2) | Negative | |||
| 6 | Blood (3) | 3, 4, and 5 wk | Clarithromycin (S); Linezolid (I); Moxifloxacin (R) | |
| Bone Marrow | 2 wk | Microscopy after enrichment | ||
| Bioprosthesis (5) | 3 wk | Microscopy after enrichment | ||
| 7 | Blood | 6 wk | Clarithromycin (S); Linezolid (I); Moxifloxacin (S) | |
| 8 | Blood (2) | 4 wk | Clarithromycin (S); Linezolid (S); Moxifloxacin (S) | |
| 9 | Blood | 4 wk | ip |
Until recently, M. chimaera would have been identified by most laboratories as M. intracellulare or M. avium complex by using GenoType Mycobacterium CM for sequences analysis, and indeed this was the case of our patient 1, whose M. chimaera infection was identified only retrospectively by using GenoType NTM-DR on the defrosted culture of mycobacteria isolated from the pericardial abscess[20]. Interestingly, in this case and in a number of other cases observed in Treviso and Padua, gene sequencing with GenoType Mycobacterium CM showed a weak positivity on the band 13, in addition to the full positivity on the pathognomonic band 9.
Mortality due to M. chimaera is remarkably high (44%) in our series, and reflects the poor outcome observed in other reports[3,16]. Patient 1 did not respond to 6 mo empirical anti-tuberculosis treatment and one-month anti-mycobacterial treatment prescribed only late and before the patient was diagnosed with M. chimaera infection. Patients 2 and 3 had a late diagnosis and did not undergo anti-mycobacterial treatment. For patient 4 we followed the guidelines for Mycobacterium avium complex (MAC) treatment, but response to treatment was poor (cultures from blood, bioprosthetic valve, and vertebral specimen still positive after respectively 7, 8 and 11 months of treatment) and with many side effects[21]. Patients 5 and 6, and more recently patient 7 and 8 have started a five drug AST-guided antimycobacterial therapy including a macrolid, amikacin, a ryfamycin, ethambutol, and a fluorquinolone or clofazimine according to recent recommendations[1,22]. After 8-mo treament, patient 5, who was diagnosed after a prolonged illness, shows negative mycobacterial blood cultures with stable cardiac and bioprosthesis functions to serial echocardiographic controls, but not clinical improvement of the spondylodiscitis. This patient, for whom it was decided to retain the infected prosthetic valve, may require a chronic lifelong suppression[1]. Although a non-significant trend of better survival among patients who underwent repeat surgery had been reported, this was not the case of patients 1 and 4 who had undergone repeat cardiac surgery only belatedly, after a prolonged illness[16]. Patient 6 shows negative mycobacterial blood cultures after 1 and 2 mo of targeted therapy, and underwent a new cardiac surgery for valve replacement after 8 mo of targeted antimycobacterial therapy. However, cultures on several fragments of the removed bioprosthesis were still positive for M. chimaera. He continues four drug antimycobacterial treatment, with negative mycobacterial blood cultures collected two wk after surgery.
More than 40000 patients undergo cardiac surgery every year in Italy, but a national survey endorsed by the Italian Society of Cardiac Surgery (ISCS) in 2017 found only three patients affected by M. chimaera infection in addition to those reported in our series, bringing the total number of published cases to 12[6]. According to ISCS, the estimated national risk of M. chimaera infections following cardiac surgery in Italy resulted to be 0.3 patients every 10000 operations. However, diagnosing M. chimaera infections may be a challenge not only for the clinician, but also for the health authorities who attempt to estimate the epidemiological risk[24]. Our study shows that a number of M. chimaera infections were diagnosed in our hospital, where after the first cases, our clinical surveillance and the laboratory skills gained significant experience. We estimate that the risk of M. chimaera infections following cardiac surgery in the period 2011-2016 has currently risen to 1.5 patients every 1000 operations in our hospital.
Strict environmental control measures were implemented since 2017 in Treviso Hospital, and since 2018 all the hospitals of the Veneto Region that had used the 3T HCU had removed it from the operating room (Table 4). Regional Health Authorities issued an alert in October 2018, adopting the control measures promoted by the Centre for Disease Control and Prevention in the United States and Europe[1,13,24,25]. Similarly to what has been implemented with appreciable results in the United States, to enhance case findings and early detection of the infection, all the patients who underwent cardiopulmonary bypass surgery during 2010-2017 were sent a letter explaining the potential exposure and instructing them to seek care if they experienced signs or symptoms consistent with M. chimaera infection, such as unexplained fever, weight loss, cough, dyspnoea, night sweats, or wound infection[26]. A call center has been set up to answer patients’ questions and refer to the local physician or to the outpatient clinic of the UID, as appropriate. Hospital clinicians were alerted to be aware of the disease and to notify all M. chimaera cases to the Regional Health Authorities[27]. During the first nine months of surveillance, only a few paucisymptomatic and apyretic patients have come to our attention by active case finding: in all cases the TOE was negative for signs of endocarditis and in three cases the mycobacterial blood cultures were negative after two months. The three patients diagnosed in 2019 were detected by passive case finding for the clinical awareness of their clinicians.
| Measures | |
| Environmental control measures | Mycrobiological surveillance of HCUs and operating room |
| Culture-negative HCUs: carry out maintenance and cleaning of the device according to the manufacturer's recommendations | |
| Culture-positive HCUs: remove the HCU from the operating room and/or send the device to the manufacturer for sterilization and cleaning | |
| Clinical control measures | Enhance active case finding |
| Alert clinicians for passive case findings | |
| Notify the confirmed or probable cases |
As stated by the European Centre for Disease Prevention and Control, it is crucial that the healthcare providers involved in caring for patients who have undergone open-heart surgery must be aware of risk of M. chimaera infections in patients with signs and symptoms of endocarditis or other cardiovascular and disseminated infections of unidentified origin, consider testing specifically for slow-growing NTM such as M. chimaera, submit suspected cases to TOE and PET/CT when appropriate, and notify the confirmed cases to the competent health authorities[28].
We thank Dr. Marta Peracchi, of the Department of Microbiology of Padua University Hospital, and Director Elvio Polesel, of the Cardiac Surgery Unit of Treviso Hospital, for their precious collaboration.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Raja SG S-Editor: Dou Y L-Editor: A E-Editor: Qi LL
| 1. | Kasperbauer SH, Daley CL. Mycobacterium chimaera Infections Related to the Heater-Cooler Unit Outbreak: A Guide to Diagnosis and Management. Clin Infect Dis. 2019;68:1244-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 2. | Achermann Y, Rössle M, Hoffmann M, Deggim V, Kuster S, Zimmermann DR, Bloemberg G, Hombach M, Hasse B. Prosthetic valve endocarditis and bloodstream infection due to Mycobacterium chimaera. J Clin Microbiol. 2013;51:1769-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Sax H, Bloemberg G, Hasse B, Sommerstein R, Kohler P, Achermann Y, Rössle M, Falk V, Kuster SP, Böttger EC, Weber R. Prolonged Outbreak of Mycobacterium chimaera Infection After Open-Chest Heart Surgery. Clin Infect Dis. 2015;61:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 239] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 4. | Kohler P, Kuster SP, Bloemberg G, Schulthess B, Frank M, Tanner FC, Rössle M, Böni C, Falk V, Wilhelm MJ, Sommerstein R, Achermann Y, Ten Oever J, Debast SB, Wolfhagen MJ, Brandon Bravo Bruinsma GJ, Vos MC, Bogers A, Serr A, Beyersdorf F, Sax H, Böttger EC, Weber R, van Ingen J, Wagner D, Hasse B. Healthcare-associated prosthetic heart valve, aortic vascular graft, and disseminated Mycobacterium chimaera infections subsequent to open heart surgery. Eur Heart J. 2015;36:2745-2753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 5. | Perkins KM, Lawsin A, Hasan NA, Strong M, Halpin AL, Rodger RR, Moulton-Meissner H, Crist MB, Schwartz S, Marders J, Daley CL, Salfinger M, Perz JF. Notes from the Field: Mycobacterium chimaera Contamination of Heater-Cooler Devices Used in Cardiac Surgery-United States. MMWR Morb Mortal Wkly Rep. 2016;65:1117-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Cappabianca G, Paparella D, D'Onofrio A, Caprili L, Minniti G, Lanzafame M, Parolari A, Musumeci F, Beghi C. Mycobacterium chimaera infections following cardiac surgery in Italy: results from a National Survey Endorsed by the Italian Society of Cardiac Surgery. J Cardiovasc Med (Hagerstown). 2018;19:748-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Haller S, Höller C, Jacobshagen A, Hamouda O, Abu Sin M, Monnet DL, Plachouras D, Eckmanns T. Contamination during production of heater-cooler units by Mycobacterium chimaera potential cause for invasive cardiovascular infections: results of an outbreak investigation in Germany, April 2015 to February 2016. Euro Surveill. 2016;21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 8. | Williamson D, Howden B, Stinear T. Mycobacterium chimaera Spread from Heating and Cooling Units in Heart Surgery. N Engl J Med. 2017;376:600-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | van Ingen J, Kohl TA, Kranzer K, Hasse B, Keller PM, Katarzyna Szafrańska A, Hillemann D, Chand M, Schreiber PW, Sommerstein R, Berger C, Genoni M, Rüegg C, Troillet N, Widmer AF, Becker SL, Herrmann M, Eckmanns T, Haller S, Höller C, Debast SB, Wolfhagen MJ, Hopman J, Kluytmans J, Langelaar M, Notermans DW, Ten Oever J, van den Barselaar P, Vonk ABA, Vos MC, Ahmed N, Brown T, Crook D, Lamagni T, Phin N, Smith EG, Zambon M, Serr A, Götting T, Ebner W, Thürmer A, Utpatel C, Spröer C, Bunk B, Nübel U, Bloemberg GV, Böttger EC, Niemann S, Wagner D, Sax H. Global outbreak of severe Mycobacterium chimaera disease after cardiac surgery: a molecular epidemiological study. Lancet Infect Dis. 2017;17:1033-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 10. | Tortoli E. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clin Microbiol Rev. 2014;27:727-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 11. | Wallace RJ, Iakhiaeva E, Williams MD, Brown-Elliott BA, Vasireddy S, Vasireddy R, Lande L, Peterson DD, Sawicki J, Kwait R, Tichenor WS, Turenne C, Falkinham JO. Absence of Mycobacterium intracellulare and presence of Mycobacterium chimaera in household water and biofilm samples of patients in the United States with Mycobacterium avium complex respiratory disease. J Clin Microbiol. 2013;51:1747-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Götting T, Klassen S, Jonas D, Benk Ch, Serr A, Wagner D, Ebner W. Heater-cooler units: contamination of crucial devices in cardiothoracic surgery. J Hosp Infect. 2016;93:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Marra AR, Diekema DJ, Edmond MB. Mycobacterium chimaera Infections Associated With Contaminated Heater-Cooler Devices for Cardiac Surgery: Outbreak Management. Clin Infect Dis. 2017;65:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Sommerstein R, Rüegg C, Kohler P, Bloemberg G, Kuster SP, Sax H. Transmission of Mycobacterium chimaera from Heater-Cooler Units during Cardiac Surgery despite an Ultraclean Air Ventilation System. Emerg Infect Dis. 2016;22:1008-1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 15. | Chand M, Lamagni T, Kranzer K, Hedge J, Moore G, Parks S, Collins S, Del Ojo Elias C, Ahmed N, Brown T, Smith EG, Hoffman P, Kirwan P, Mason B, Smith-Palmer A, Veal P, Lalor MK, Bennett A, Walker J, Yeap A, Isidro Carrion Martin A, Dolan G, Bhatt S, Skingsley A, Charlett A, Pearce D, Russell K, Kendall S, Klein AA, Robins S, Schelenz S, Newsholme W, Thomas S, Collyns T, Davies E, McMenamin J, Doherty L, Peto TE, Crook D, Zambon M, Phin N. Insidious Risk of Severe Mycobacterium chimaera Infection in Cardiac Surgery Patients. Clin Infect Dis. 2017;64:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | Scriven JE, Scobie A, Verlander NQ, Houston A, Collyns T, Cajic V, Kon OM, Mitchell T, Rahama O, Robinson A, Withama S, Wilson P, Maxwell D, Agranoff D, Davies E, Llewelyn M, Soo SS, Sahota A, Cooper MA, Hunter M, Tomlins J, Tiberi S, Kendall S, Dedicoat M, Alexander E, Fenech T, Zambon M, Lamagni T, Smith EG, Chand M. Mycobacterium chimaera infection following cardiac surgery in the United Kingdom: clinical features and outcome of the first 30 cases. Clin Microbiol Infect. 2018;24:1164-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Mok S, Rogers TR, Fitzgibbon M. Evaluation of GenoType NTM-DR Assay for Identification of Mycobacterium chimaera. J Clin Microbiol. 2017;55:1821-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Inojosa WO, Minniti G, Scotton PG. Is Mycobacterium chimaera infection after cardiac surgery a risk factor for bacterial prosthetic valve endocarditis? Clin Infect Dis. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Granados U, Fuster D, Pericas JM, Llopis JL, Ninot S, Quintana E, Almela M, Paré C, Tolosana JM, Falces C, Moreno A, Pons F, Lomeña F, Miro JM; Hospital Clinic Endocarditis Study Group. Diagnostic Accuracy of 18F-FDG PET/CT in Infective Endocarditis and Implantable Cardiac Electronic Device Infection: A Cross-Sectional Study. J Nucl Med. 2016;57:1726-1732. [PubMed] |
| 20. | Schweickert B, Goldenberg O, Richter E, Göbel UB, Petrich A, Buchholz P, Moter A. Occurrence and clinical relevance of Mycobacterium chimaera sp. nov., Germany. Emerg Infect Dis. 2008;14:1443-1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Winthrop K; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. [PubMed] |
| 22. | Maurer FP, Pohle P, Kernbach M, Sievert D, Hillemann D, Rupp J, Hombach M, Kranzer K. Differential drug susceptibility patterns of Mycobacterium chimaera and other members of the Mycobacterium avium-intracellulare complex. Clin Microbiol Infect. 2019;25:379.e1-379.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Sommerstein R, Hasse B, Marschall J, Sax H, Genoni M, Schlegel M, Widmer AF; Swiss Chimaera Taskforce. Global Health Estimate of Invasive Mycobacterium chimaera Infections Associated with Heater-Cooler Devices in Cardiac Surgery. Emerg Infect Dis. 2018;24:576-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Regione del Veneto. Documento tecnico di indirizzo per la prevenzione e la gestione delle infezioni da Mycobacterium chimaera associate ad interventi chirurgici con utilizzo dei dispositivi di riscaldamento/raffreddamento (heater cooler units). Decreto NO 125. 2018;. |
| 25. | European Centre for Disease Prevention and Control. EU protocol for case detection, laboratory diagnosis and environmental testing of Mycobacterium chimaera infections potentially associated with heater-cooler units: case definition and environmental testing methodology. Available from: https://ecdc.europa.eu/en/publications-data/eu-protocol-case-detection-laboratory-diagnosis-and-environmental-testing. |
| 26. | Jarashow MC, Terashita D, Balter S, Schwartz B. Notes from the Field: Mycobacteria chimaera Infections Associated with Heater-Cooler Unit Use During Cardiopulmonary Bypass Surgery-Los Angeles County, 2012-2016. MMWR Morb Mortal Wkly Rep. 2019;67:1428-1429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Regione del Veneto. Infezioni da Mycobacterium chimaera in pazienti sottoposti ad intervento cardiochirurgico. Protocollo NO 490036-C: 101 2018; . |
| 28. | European Centre for Disease Prevention and Control. Invasive cardiovascular infection by Mycobacterium chimaera associated with 3T heater-cooler system used during open-heart surgery – 18 November 2016. Available from: https://ecdc.europa.eu/.../Publications/RRA-mycobacterium-chimaera-November-2016.pdf. |