Published online Sep 26, 2019. doi: 10.12998/wjcc.v7.i18.2722
Peer-review started: May 21, 2019
First decision: July 30, 2019
Revised: August 5, 2019
Accepted: August 20, 2019
Article in press: August 20, 2019
Published online: September 26, 2019
Processing time: 130 Days and 2.1 Hours
Early prediction of transient ischemic attack (TIA) has important clinical value. To date, systematic studies on clinical, biochemical, and imaging indicators related to carotid atherosclerosis have been carried out to predict the occurrence of TIA. However, their prediction accuracy is limited.
To explore the role of combining wall shear stress (WSS) with conventional predictive indicators in improving the accuracy of TIA prediction.
A total of 250 patients with atherosclerosis who underwent carotid ultrasonography at Naval Military Medical University Affiliated Gongli Hospital were recruited. Plaque location, plaque properties, stenosis rate, peak systolic velocity, and end diastolic velocity were measured and recorded. The WSS distribution map of the proximal and distal ends of the plaque shoulder was drawn using the shear stress quantitative analysis software, and the average values of WSS were recorded. The laboratory indicators of the subjects were recorded. The patients were followed for 4 years. Patients with TIA were included in a TIA group and the remaining patients were included in a control group. The clinical data, laboratory indicators, and ultrasound characteristics of the two groups were analyzed. Survival curves were plotted by the Kaplan-Meier method. Receiver operating characteristic curves were established to evaluate the accuracy of potential indicators in predicting TIA. Logistic regression model was used to establish combined prediction, and the accuracy of combined predictive indicators for TIA was explored.
The intraclass correlation coefficients of the WSS between the proximal and distal ends of the plaque shoulder were 0.976 and 0.993, respectively, which indicated an excellent agreement. At the end of the follow-up, 30 patients suffered TIA (TIA group) and 204 patients did not (control group). Hypertension (P = 0.037), diabetes (P = 0.026), homocysteine (Hcy) (P = 0.022), fasting blood glucose (P = 0.034), plaque properties (P = 0.000), luminal stenosis rate (P = 0.000), and proximal end WSS (P = 0.000) were independent influencing factors for TIA during follow-up. The accuracy of each indicator for predicting TIA individually was not high (area under the curve [AUC] < 0.9). The accuracy of the combined indicator including WSS (AUC = 0.944) was significantly higher than that of the combined indicator without WSS (AUC = 0.856) in predicting TIA (z = 2.177, P = 0.030). The sensitivity and specificity of the combined indicator including WSS were 86.67% and 92.16%, respectively.
WSS at plaque surface combined with hypertension, diabetes, Hcy, blood glucose, plaque properties, and stenosis rate can significantly improve the accuracy of predicting TIA.
Core tip: Early prediction of transient ischemic attack (TIA) is of great importance. However, the accuracy of the predicted methods including clinical, biochemical, and imaging indicators related to carotid atherosclerosis is limited. The purpose of this study was to explore the role of combining wall shear stress (WSS) with conventional predictive indicators in improving the accuracy of TIA prediction. Our study indicated that the WSS at plaque surface combined with traditional indicators such as hypertension, diabetes, Hcy, blood glucose, plaque properties, and stenosis rate can significantly improve the accuracy of predicting TIA.
- Citation: Liu QY, Duan Q, Fu XH, Jiang M, Xia HW, Wan YL. Wall shear stress can improve prediction accuracy for transient ischemic attack. World J Clin Cases 2019; 7(18): 2722-2733
- URL: https://www.wjgnet.com/2307-8960/full/v7/i18/2722.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i18.2722
Acute ischemic stroke (AIS) is the primary cause of permanent disability in elderly people. The characteristics of AIS are high incidence, high disability rate, and high recurrence rate[1-3]. It is the second leading cause of death after cardiovascular disease[4,5]. It is of great clinical significance to predict AIS in an early stage in order to take action to prevent or minimize its harm to patients[6-8]. Transient ischemic attack (TIA) is a high-risk factor for AIS. It needs to be actively treated to stabilize the disease and prevent the occurrence of AIS. Carotid atherosclerosis is known to be the most important cause of TIA, and the degree of atherosclerosis and microthrombus caused by unstable plaque rupture are closely related to the occurrence of TIA[9-11]. To date, systematic studies have been carried out on clinical, biochemical, and ultrasonic indicators related to carotid atherosclerosis to predict the occurrence of TIA, even AIS[12-14]. However, due to individual factors and the influence of different environments, the occurrence of TIA is still not accurately predicted in clinical practice, and its prediction accuracy needs to be further improved. With advances in ultrasound technology, recent studies have reported that wall shear stress (WSS) in blood vessel walls is one of the important factors affecting atherosclerosis[15-17]. WSS is the tangential friction of blood flowing on the surface of blood vessel walls acting on arterial endothelial cells[18]. Studies have revealed that a decrease in WSS can increase the intima-medium thickness of the carotid artery and induces carotid atherosclerosis. An increase in WSS on the surface of plaque can cause plaque rupture and induce TIA[19,20]. Based on these studies, the present study performed a 4-year follow-up and recorded clinical, biochemical, and ultrasound indicators of the carotid atherosclerotic plaque and the WSS of plaque surface. The aim of this study was to investigate the improving effect of combining WSS with conventional predictive indicators in TIA prediction.
From December 2013 to December 2018, 250 patients with atherosclerosis who underwent carotid ultrasonography at Naval Military Medical University Affiliated Gongli Hospital were recruited. The inclusion criteria were as follows: (1) Plaque-induced area stenosis ≥ 30%; (2) Age > 18 years; and (3) No TIA history. Patients with a hemodynamically altered disease were excluded. The age, gender, family history, comorbidities (hypertension, diabetes, and hypercholesterolemia), smoking, and alcohol abuse in all patients were recorded. All subjects provided informed consent for this study. This study was approved by the Ethics Committee of Naval Military Medical University Affiliated Gongli Hospital and registered at the China Clinical Trial Registration Center (ChiCTR-OON-14005359).
Ultrasound examination: Patients were examined using a PHILIPS iu22 ultrasound system with an L9-3 line array probe. The patient was placed in a supine position to fully expose the neck during the examination. After the carotid plaque was detected, the plaque location (internal carotid artery, common carotid artery, or carotid sinus) was recorded. The plaque property (hard plaque, soft plaque, or mixed plaque) was determined according to the echo. The thickness of the plaque protruding into the lumen and the degree of carotid lumen stenosis were calculated. Doppler was turned on to observe the blood flow at the plaque surface, and peak systolic velocity (PSV) and end diastolic velocity (EDV) (cm/s) were recorded. The blood flow dynamic map was saved for post-processing analysis.
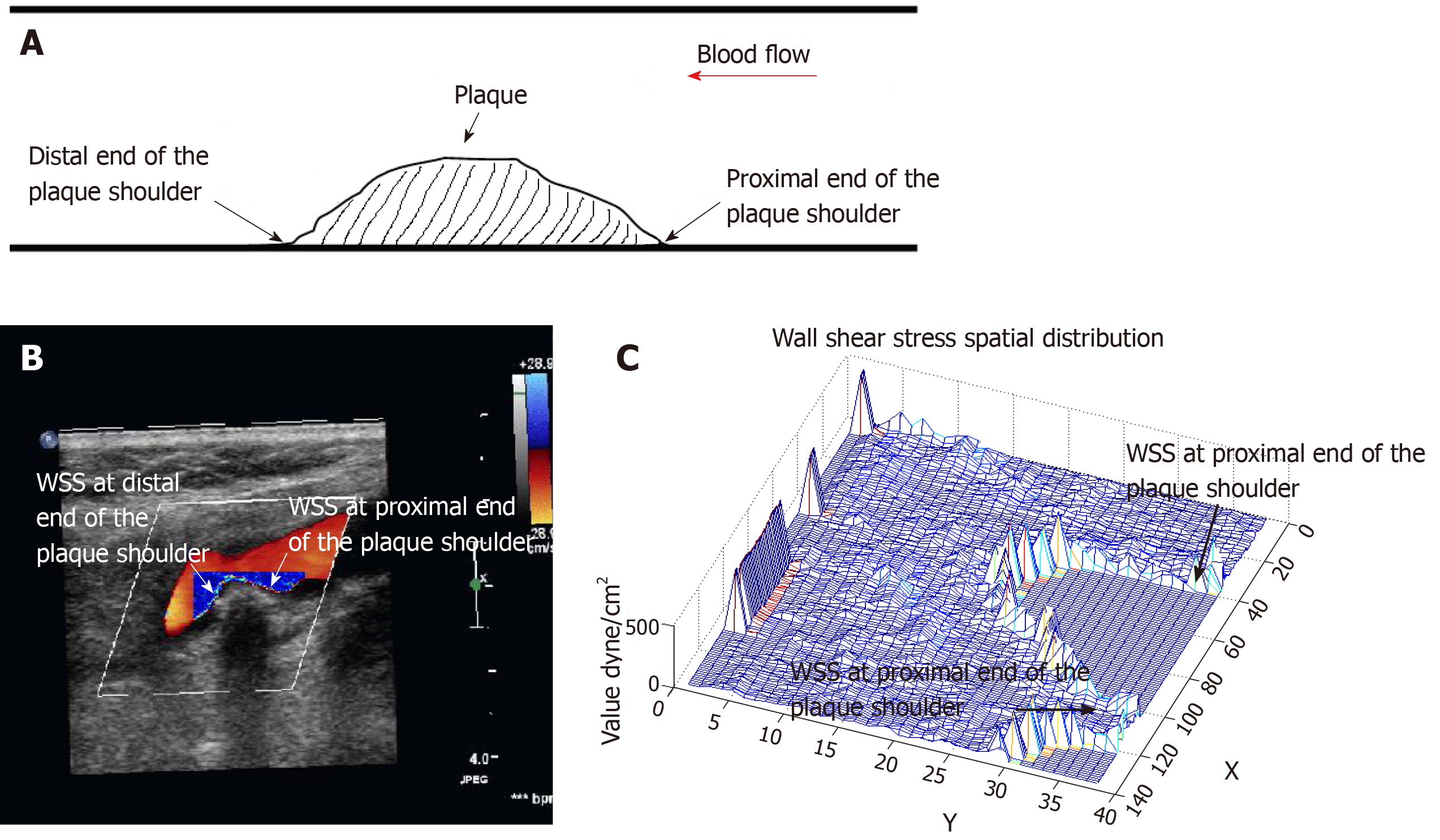
Analysis of the WSS distribution at carotid plaque: The Doppler blood flow dynamic maps were analyzed offline by two experienced sonographers using the shear stress quantitative analysis software (Software copyright registration: No. 2011SR089671). The WSS distribution of the proximal and distal ends of the plaque shoulder (the junction of the plaque and the normal carotid endothelium) was drawn, and the mean WSS values at this area were recorded. The shear stress quantitative analysis software can automatically generate the two-dimensional and three-dimensional WSS spatial distribution maps at the surface of the carotid plaque, and automatically calculate mean WSS value. The software represents the WSS value in color brightness. The brighter the color, the higher the WSS. The X and Y coordinates of the three-dimensional WSS spatial distribution map are in pixels, and the Z coordinate is in units of dyne/cm2. The X axis represents the length of the blood vessel, the Y axis represents the diameter of the blood vessel, and the Z axis represents the value of the WSS. The WSS is displayed in the form of amplitude on the three-dimensional WSS spatial distribution map (Figure 1)[21].
Laboratory examination: Fasting venous blood samples were taken 12 h after fasting, and direct bilirubin, total bilirubin, uric acid, fasting blood glucose (FBG), homocysteine (Hcy), blood lipids (including total cholesterol, triglyceride, high density lipoprotein cholesterol, and low density lipoprotein cholesterol) were determined using an automatic biochemical analyzer.
All patients enrolled in the study were followed for 4 years and an ultrasound examination was performed every 3 mo. TIA was defined as the endpoint event during patient follow-up. A patient’s refusal to examination or a midway withdrawal was defined as loss to follow-up. Based on the follow-up results, patients with endpoint events were included in a TIA group, and the remaining patients were included in a control group. The clinical data, laboratory indicators, and ultrasound characteristics of the two groups were analyzed.
Analyses were performed using Statistical Product and Service Solutions (SPSS 19.0) software. Measurement data are expressed as the mean ± standard deviation, and comparisons were performed using an independent sample t-test. Count data are expressed in cases (percentages), and the chi-square test and Fisher's exact probability method were used for comparison. Repeated tests were performed on WSS calculated by two sonographers using Bland-Altman analysis and intraclass correlation coefficient (ICC). An ICC > 0.75 indicates good repeatability, 0.4 < ICC ≤ 0.75 indicates repetitiveness, and ICC ≤ 0.4 indicates poor repeatability. Survival curves were plotted by the Kaplan-Meier method. The independent influencing factors that have a significant impact on the occurrence of AIS were analyzed using multivariate COX regression. Receiver operating characteristic (ROC) curves were established to evaluate the accuracy of potential indicators for predicting TIA. Logistic regression model was used to establish combined prediction, and the accuracy of combined predictive indicators for TIA was explored. The difference was considered statistically significant at P < 0.05.
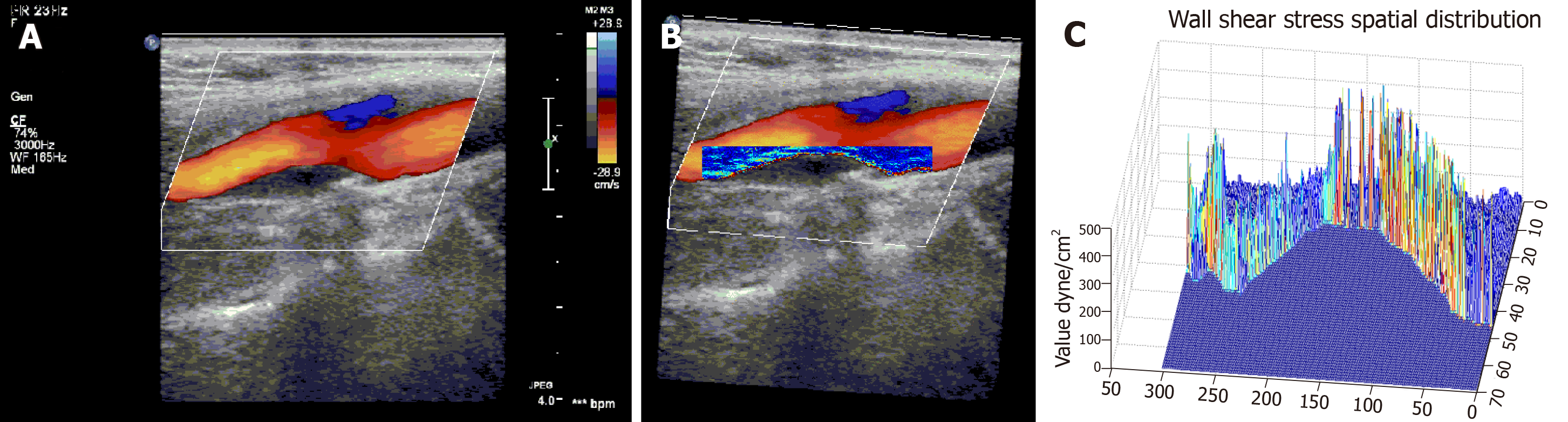
The shear stress quantitative analysis software successfully analyzed the WSS distribution at the proximal and distal ends of the carotid plaque shoulder in all patients with atherosclerosis. Typical images are shown in Figure 2. Figure 2A is a Doppler blood flow map of the internal carotid artery plaque, in which the blood flow near the carotid plaque can be visually observed. Figure 2B is a WSS distribution map at the carotid plaque, in which the WSS distribution at different locations near the carotid plaque can be visually observed. It could be found that the WSS color of the proximal end of the plaque shoulder was bright, and the color of the distal end was dim, suggesting that the WSS at the proximal end was higher than that of the distal end. Figure 2C is a three-dimensional WSS spatial distribution map. It can be found that the value of the Z-axis at the proximal end was significantly higher than that of the distal end, suggesting that the WSS at the proximal end of the shoulder was higher than that of the distal end.
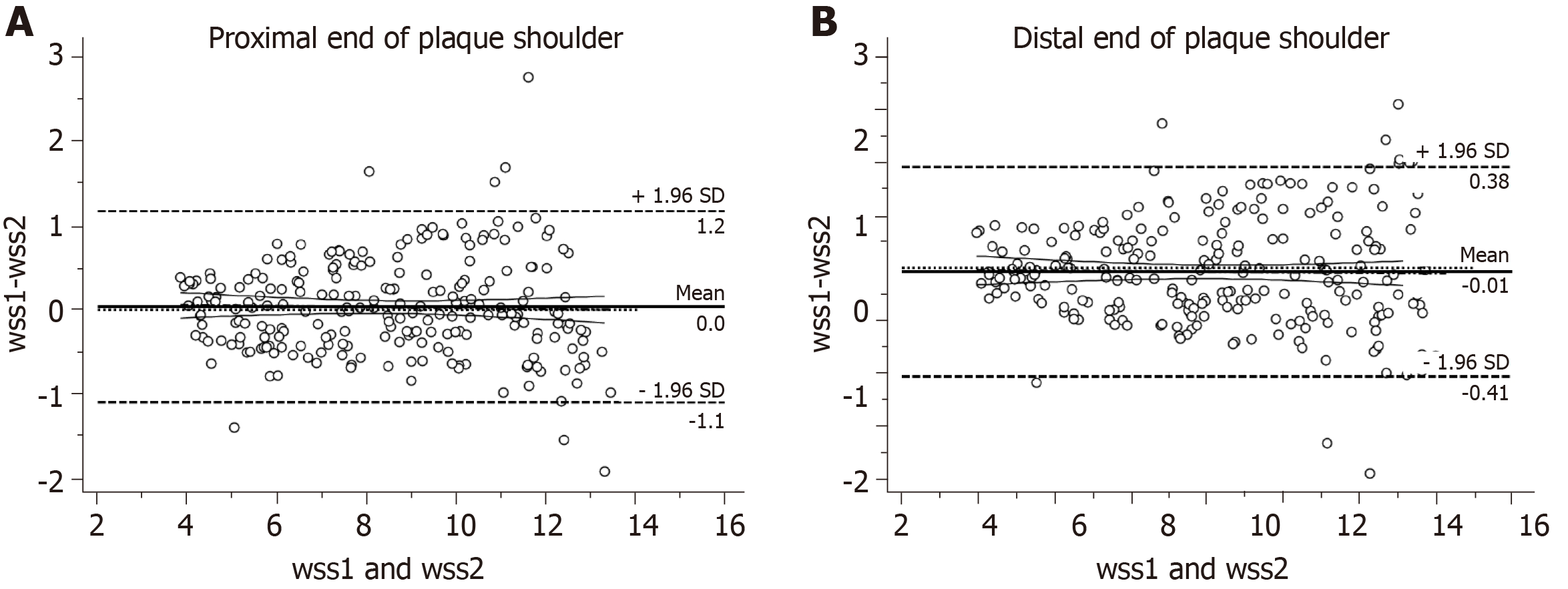
In order to evaluate the error of two sonographers in detecting WSS, this study utilized the Bland-Altman method to test the consistency of WSS at the proximal and distal ends of the plaque shoulder. The consistency analysis of WSS by two sonographers found that the mean difference between the WSS observers of the proximal end plaque shoulder was 0 dyne/cm2, and the 95% consistency range was between -1.1 and 1.2. The ICC result was 0.976, which indicated that the agreement was excellent (Figure 3A). The mean difference between the WSS observers of the distal end plaque shoulder was 0.01 dyne/cm2, and the 95% consistency range was between -0.41 and 0.38. The ICC result was 0.993, which indicated that the agreement was excellent (Figure 3B).
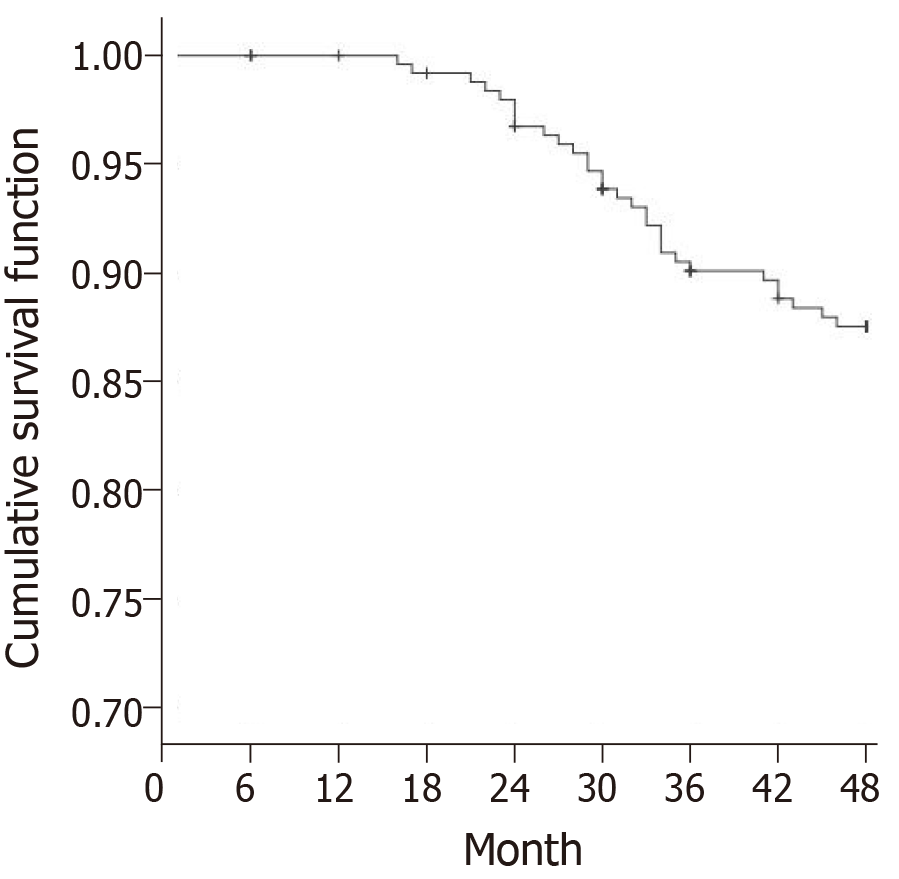
At the end of follow-up, 16 patients were lost to follow-up. Totally 204 patients did not develop TIA (control group) at the end of follow-up, and 30 patients developed TIA (TIA group). The Kaplan-Meier survival curve showed that the number of TIA cases increased over time, and the incidence of TIA was 15.2% (Figure 4).
The blood pressure, diabetes, Hcy, and FBG in the TIA group were significantly higher than those in the control group (P < 0.05). The remaining clinical data and laboratory indicators between the two groups were similar (P > 0.05) (Table 1).
| TIA Group (n = 30) | Control Group (n = 204) | t/X2 Value | P-value | |
| Age | 70.71 ± 10.92 | 65.93 ± 18.09 | 1.408 | 0.160 |
| Gender (Male/Female) | 18/12 | 126/78 | 0.034 | 0.853 |
| Family history | 4 (13.33) | 21(10.29%) | - | 0.5391 |
| Hypertension | 30 (100) | 178 (87.25) | 4.301 | 0.038 |
| Diabetes | 22 (73.33) | 104 (50.98) | 5.258 | 0.022 |
| Hypercholesterolemia | 10 (33.33) | 69 (33.82) | 0.003 | 0.958 |
| Smoking | 14 (46.67) | 104 (50.98) | 0.195 | 0.659 |
| Alcoholism | 4 (13.33) | 21 (10.29) | - | 0.5391 |
| DBIT (μmol/L) | 4.19 ± 1.08 | 3.98 ± 1.43 | 0.772 | 0.441 |
| TBIL (μmol/L) | 10.96 ± 3.96 | 9.77 ± 3.28 | 1.805 | 0.072 |
| UA (mmol/L) | 329.34 ± 84.39 | 337.09 ± 87.18 | 0.456 | 0.649 |
| TG (mmol/L) | 1.18 ± 0.43 | 1.15 ± 0.47 | 0.330 | 0.742 |
| TC (mmol/L) | 4.46 ± 1.20 | 4.29 ± 1.09 | 0.787 | 0.432 |
| HDL-C (mmol/L) | 1.17 ± 0.32 | 1.22 ± 0.34 | 0.757 | 0.459 |
| LDL-C (mmol/L) | 3.04 ± 0.95 | 2.89 ± 0.87 | 0.871 | 0.384 |
| Hcy (μmol/L) | 8.37 ± 2.76 | 7.29 ± 2.27 | 2.364 | 0.019 |
| FBG (mmol/L) | 6.97 ± 2.89 | 5.84 ± 2.58 | 2.205 | 0.028 |
The soft plaque ratio, stenosis rate, proximal end WSS, and distal end WSS were significantly higher in the observation group than in the control group (P < 0.05). The data of plaque location, PSV, and EDV were similar between the two groups (P > 0.05) (Table 2).
| TIA Group (n = 30) | Control Group (n = 204) | t/X2 Value | P-value | ||
| Plaque location | Common carotid artery | 8 | 51 | 0.046 | 0.977 |
| Carotid sinus | 18 | 124 | |||
| Internal carotid artery | 4 | 29 | |||
| Plaque property | Hard plaque | 9 | 104 | 6.062 | 0.048 |
| Soft plaque | 14 | 54 | |||
| Mixed plaque | 7 | 46 | |||
| Lumen stenosis rate (%) | 58.26 ± 13.28 | 44.29 ± 21.82 | 3.411 | 0.001 | |
| PSV (cm/s) | 90.34 ± 9.23 | 87.12 ± 9.42 | 1.753 | 0.081 | |
| EDV (cm/s) | 17.24 ± 5.12 | 15.93 ± 3.82 | 1.673 | 0.096 | |
| Proximal end WSS (dyne/cm2) | 9.15 ± 2.27 | 7.29 ± 3.86 | 6.720 | 0.000 | |
| Distal end WSS (dyne/cm2) | 7.42 ± 1.95 | 6.39 ± 1.74 | 2.980 | 0.003 | |
Multivariate COX regression analysis was further performed to compare the differences between the TIA group and the control group. The results showed that the effect of distal end WSS on TIA was not significant (P > 0.05). Hypertension (P = 0.037), diabetes (P = 0.026), Hcy (P = 0.022), FBG (P = 0.034), plaque properties (P = 0.000), lumen stenosis rate (P = 0.000), and proximal end WSS (P = 0.000) were independent factors affecting TIA during patient follow-up (Table 3).
| B | SE | Wald | P-value | RR | 95%CI | ||
| Lower limit | Upper limit | ||||||
| Hypertension | 1.365 | 0.293 | 2.813 | 0.037 | 3.914 | 2.204 | 6.951 |
| Diabetes | 1.462 | 0.428 | 3.792 | 0.026 | 4.316 | 1.865 | 9.986 |
| Hcy | 0.492 | 0.245 | 3.988 | 0.022 | 1.635 | 1.012 | 2.643 |
| FBG | 0.390 | 0.176 | 2.918 | 0.034 | 1.477 | 1.046 | 2.085 |
| Plaque property | 1.786 | 0.496 | 7.294 | 0.000 | 5.968 | 2.257 | 15.777 |
| Lumen stenosis rate (%) | 1.675 | 0.328 | 6.028 | 0.000 | 5.337 | 2.806 | 10.151 |
| Proximal end WSS (dyne/cm2) | 0.783 | 0.272 | 5.885 | 0.000 | 2.187 | 1.283 | 3.727 |
| Distal end WSS (dyne/cm2) | 0.459 | 0.482 | 1.125 | 0.192 | 1.582 | 0.615 | 4.069 |
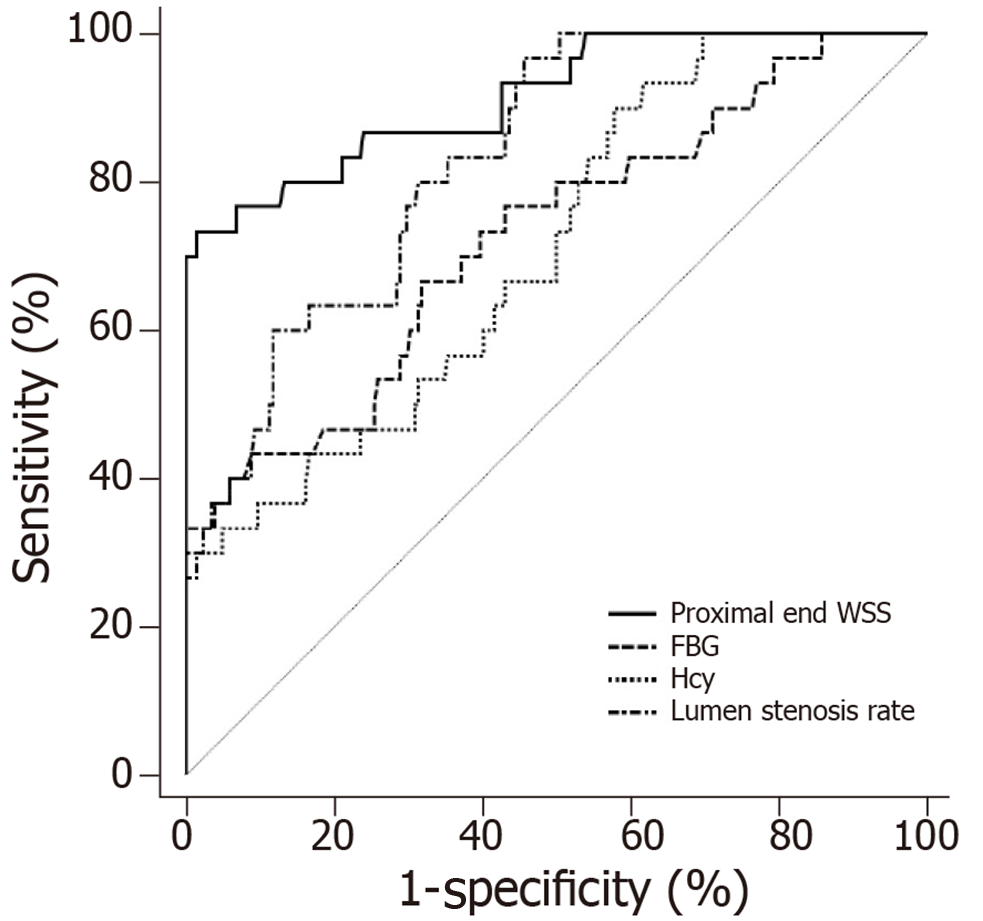
ROC curve analysis was performed to further assess the value of FBG, Hcy, lumen stenosis rate, and proximal end WSS in predicting TIA. The accuracy of each indicator for predicting TIA individually was not high (area under the curve [AUC] < 0.9). The prediction accuracy of proximal end WSS was the highest, with an AUC of 0.884 and a specificity of 95.51%, but the sensitivity was low (73.33%). The AUCs of lumen stenosis rate, Hcy, and FBG were significantly lower than that of proximal end WSS (P < 0.05) (Table 4 and Figure 5).
| AUC | 95%CI | Cutoff point | Sensitivity (%) | Specificity (%) | |
| FBG | 0.726 | 0.664-0.782 | 6.23 | 66.67 | 68.14 |
| Hcy | 0.711 | 0.648-0.768 | 7.65 | 90.00 | 42.16 |
| Lumen stenosis rate | 0.832 | 0.778-0.877 | 62.01 | 96.67 | 54.41 |
| proximal end WSS | 0.884 | 0.831-0.917 | 8.05 | 73.33 | 95.51 |
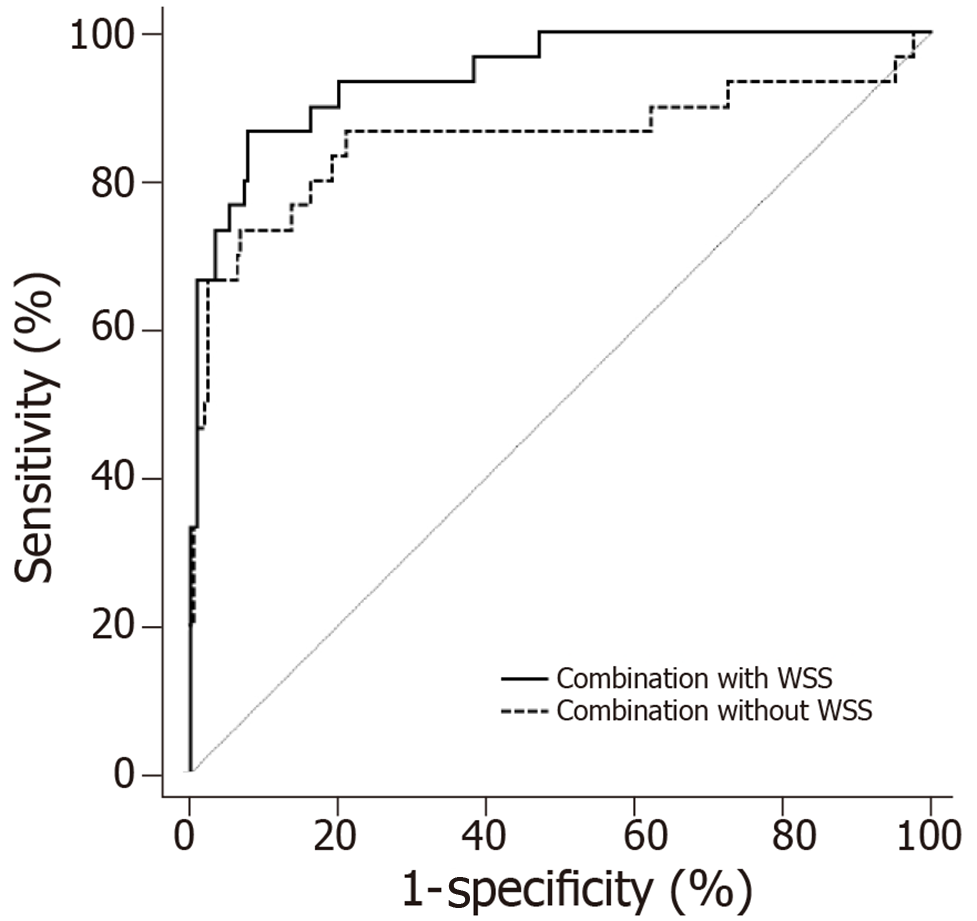
This study combined hypertension, diabetes, Hcy, FBG, plaque properties, lumen stenosis rate, and proximal end WSS based on a Logistic regression model, and compared the TIA prediction accuracy between the combined indicator with WSS and combined indicator without WSS. The results showed that the accuracy of the combined indicator with WSS (AUC = 0.944, 95%CI = 0.907-0.970) was higher than that of the combined indicator without WSS (AUC = 0.856, 95%CI = 0.805-0.899), and the difference was statistically significant (z = 2.177, P = 0.030). The sensitivity and specificity of the combined indicator with WSS were 86.67% and 92.16%, respectively (Figure 6).
AIS is an important cause of disability and death in humans. Although the prognosis of AIS has been greatly improved with the development of diagnosis and treatment techniques, the course of most patients cannot be reversed. Therefore, early prediction of high-risk people and early intervention is the best way to reduce the incidence of AIS. TIA is known to be one of the important risk factors for AIS. Studies have reported that the probability of stroke at 2, 7, 30, and 90 d after TIA was 3.5%, 5.2%, 8.0%, and 9.2%, respectively. If treated promptly, the risk of stroke at 90 d after TIA can be reduced to 1% to 3%[22-24]. Therefore, early diagnosis and individualized treatment of TIA patients are necessary to prevent the occurrence of AIS. TIA can be predicted clinically by biochemical indicators related to carotid atherosclerosis, such as blood pressure, FBG, Hcy, and ultrasound imaging indicators such as plaque properties and lumen stenosis rate. However, because different patients are affected by their individual factors and external environment, the prediction accuracy of these indicators is not satisfactory, hence the occurrence of TIA cannot be accurately predicted. There are still a large number of TIA patients in clinical practice. Therefore, in order to improve the accuracy of prediction, we need more targeted indicators to predict the development of TIA.
WSS is defined as the tangential friction of blood flowing on the surface of blood vessel walls acting on arterial endothelial cells. In the research of Corban et al[25], decreased WSS promoted the increase of carotid intima-media thickness and induced carotid atherosclerosis. Besides, increased WSS on the plaque surface was more prone to plaque rupture and could be a useful tool for the risk assessment of carotid plaque rupture, which was reported by Hung et al[26]. After plaque rupture, the ulcer is likely to form a microthrombus. Microthrombus can cause cerebral artery occlusion if it flows to the distal blood vessel, triggering TIA and even AIS. Therefore, the higher the WSS of the carotid plaque, the more likely it is to cause TIA. At present, researchers use ultrasound to detect blood vessel inner diameter and blood flow velocity, and calculate WSS according to Hagen-Poiseuille formula. But it is a simplified formula based on ideal state fluid, and is not suitable for the complex WSS calculation at carotid plaque.
The shear stress quantitative analysis software used in this study can quantitatively observe the distribution of WSS near the carotid plaque. The WSS distribution at the carotid plaque showed that the WSS color of the proximal end of the plaque shoulder was bright, and the color of the distal end of the plaque shoulder was dim, suggesting that the WSS of the proximal end of the plaque shoulder was higher than that of the distal end. From the three-dimensional WSS spatial distribution map, it could be observed that the value of the Z-axis of the proximal end of the plaque shoulder was significantly higher than that of the distal end, suggesting that the WSS of the proximal end of the plaque shoulder was higher than that of the distal end. Furthermore, according to the consistency analysis, the detection using our software was very consistent with different observers. Data acquisition was very stable, and could be widely used clinically.
To study the indicators that can improve the accuracy of predicting TIA, this study conducted a 4-year follow-up survey of 250 patients with carotid plaque but did not develop TIA. The results showed that the number of TIA cases increased gradually over time, and the incidence of TIA was 15.2%, which is in line with the research by Borné et al[27]. Although in this study, patients with carotid plaque received routine treatment interventions according to clinical guidelines, the data revealed that there was still a certain incidence of TIA, which confirmed the importance of early prediction and early intervention for TIA.
The incidence of TIA is affected by multiple factors, such as hypertension, diabetes, hyperlipidemia, and smoking. It is still uncertain how different risk factors affect the incidence of TIA[28]. By comparing the TIA group with the control group, a total of eight factors were significantly associated with TIA, including hypertension, diabetes, Hcy, FBG, soft plaque ratio, lumen stenosis rate, proximal end WSS, and distal end WSS. After COX multivariate analysis, hypertension, diabetes, Hcy, FBG, plaque property, lumen stenosis rate, and proximal end WSS were found to be independent factors influencing TIA during follow-up. Hypertension, diabetes, and Hcy are all clinically common risk factors. Hypertension easily stimulates plaque on the intima of the cerebral arteries to cause rupture[29]. The pathogenesis of TIA caused by diabetes is related to saccharification and peroxidation, vascular endothelial cell damage, platelet aggregation, and insulin resistance[30]. The mechanism of high Hcy-induced TIA involves three aspects including blood vessel wall, platelets, and coagulation factors[31]. However, the data of this study suggested that the effects of hypertension, diabetes, and Hcy on TIA were lower than those of other indicators. This may be related to the fact that they are indicators of systemic status, which are less targeted and were controlled with antihypertensive drugs and hypoglycemic agents.
Compared with hypertension, diabetes, and Hcy, indicators that directly describe plaque morphology and stability, such as plaque property, lumen stenosis rate, and proximal end WSS, had a greater impact on TIA. Because the larger the plaque volume, the higher the lumen stenosis, and the greater the likelihood that the vascular occlusion will cause TIA. The soft plaque is very unstable, which is easy to rupture so that platelets activate and adhere to form a thrombus. As the most important indicator of this study, the impact of WSS on TIA is very obvious, and the proximal end WSS of the plaque shoulder is more important than the distal end WSS. This is because the proximal end WSS of the plaque shoulder is significantly higher than the distal end WSS[32], while high WSS is more likely to cause plaque rupture. Hence, the proximal end of the plaque shoulder is more likely to rupture and trigger TIA.
In this study, ROC curves were used to further analyze the value of FBG, Hcy, lumen stenosis rate, and proximal end WSS for predicting TIA. The results showed that the accuracy of each indicator was not high (each AUC < 0.9). Of them, the prediction accuracy of proximal end WSS was the highest, and its AUC reached 0.884. This finding suggested that it is difficult to accurately predict TIA based on a single indicator. Therefore, the traditional risk factors such as hypertension and diabetes are not accurate, and they can only predict that TIA is more likely to occur. Subsequently, we performed a prediction by combining hypertension, diabetes, Hcy, blood glucose, plaque property, lumen stenosis rate, and proximal end WSS based on a logistic regression model, and then compared the accuracy between the combined prediction with proximal end WSS and the combined prediction without proximal end WSS. It revealed that when proximal end WSS was not included, the AUC of the combined indicator was higher compared with the individual indicators, but it still did not exceed 0.9. It indicated that the combination without proximal end WSS was still limited although the accuracy was higher than the individual indicators. However, the AUC of the combined prediction with proximal end WSS exceeded 0.9, and the sensitivity and specificity were satisfactory compared to the combined prediction without proximal end WSS. Therefore, we believe that WSS as an indicator of the blood flow environment near the plaque has an important role in predicting TIA, and the prediction accuracy of TIA will be significantly improved if including WSS.
There are certain deficiencies in this study. Patients with large plaques and high stenosis may be treated aggressively, and some patients with hypertension and hyperglycemia may take medicine for a long time. Therefore, the effects of various risk factors on patients are inevitably different. Further studies are suggested to perform on experimental animals. Animal experiments are more controllable, which can reduce unnecessary external environmental interference and improve the experimental accuracy.
In conclusion, WSS at the surface of carotid plaque can be used as an excellent indicator for predicting TIA. It is safe, non-invasive, and accurate. And the traditional indicators combined with WSS can significantly improve the prediction accuracy for TIA, which has great clinical significance for the early diagnosis of TIA.
Transient ischemic attack (TIA) is a common cause of acute ischemic stroke (AIS), and its early prediction is of great clinical significance for the prevention of AIS. Carotid atherosclerosis is known to be the most important cause of TIA. So far, some biochemical indexes related to carotid atherosclerosis, such as blood pressure, blood glucose, and Hcy and ultrasound imaging indicators including plaque property and lumen stenosis rate are the risk factors for TIA. However, the accuracy of their predictions of TIA is limited.
Wall shear stress (WSS) is defined as the tangential friction of blood flowing on the surface of blood vessel walls acting on arterial endothelial cells. Recent studies have found that increased WSS on the plaque surface is more prone to plaque rupture. After plaque rupture, the ulcer is likely to form a microthrombus, which may block distal blood vessels and cause TIA. Therefore, the higher the WSS of carotid plaques, the more likely it is to cause TIA. However, it is uncertain whether WSS can improve the accuracy of predicting the occurrence of TIA.
In this study, we analyzed the routine indicators and WSS data of patients with atherosclerosis. The aim of our study was to investigate the improving effect of combining WSS with conventional predictive indicators in TIA prediction.
A total of 250 patients with atherosclerosis were recruited. The laboratory indexes and imaging indexes of the patients were measured and recorded. The WSS distribution maps of the proximal and distal ends of the plaque shoulder were drawn using the shear stress quantitative analysis software, and the average values of WSS were recorded. The patients were followed for 4 years, and patients with TIA were included in a TIA group and the remaining patients were included in a control group. ROC curves were used to assess the accuracy of potential indicators in predicting TIA, and Logistic regression model was used to establish a combined prediction and explore its accuracy for predicting TIA.
The WSS between the proximal and distal ends of the plaque shoulder indicated an excellent agreement (ICC = 0.976 and 0.993, respectively). Besides, the WSS at the proximal end of the shoulder was significantly higher than that of the distal end (P < 0.05). According to the results of follow-up, patients with atherosclerosis were divided into a TIA group (n = 30) and a control group (n = 204). After COX multivariate analysis, hypertension, diabetes, homocysteine (Hcy), FBG, plaque property, lumen stenosis rate, and proximal end WSS were found to be independent factors influencing TIA during follow-up (P < 0.05). Among them, the proximal end WSS had the highest accuracy in predicting TIA, but its AUC was still less than 0.9. The Logistic regression results showed that the accuracy of the combination with WSS (AUC = 0.944) was significantly higher than that of the combination without WSS (AUC = 0.856) in predicting TIA (z = 2.177, P = 0.030). It is suggested that the traditional indexes combined with WSS can significantly improve the accuracy of TIA prediction.
WSS plays an important role in predicting TIA. WSS at plaque surface combined with hypertension, diabetes, Hcy, blood glucose, plaque properties, and stenosis rate can significantly improve the accuracy of TIA prediction.
In order to avoid the interference caused by the differences of individual and environmental factors among different patients, an animal experiment should be performed to explore if WSS can improve the accuracy of TIA prediction. Animal experiments are more controllable, which can reduce unnecessary external environmental interference and improve the accuracy. Therefore, the effect of the combination of the conventional prediction indexes and WSS in predicting TIA is expected to be further revealed by animal experiments.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Michael JH, Tomiyasu A S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Lim HS, Kim SM, Kang DW. Quantitative Predictive Models for the Degree of Disability After Acute Ischemic Stroke. J Clin Pharmacol. 2018;58:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Zheng L, Cheng W, Wang X, Yang Z, Zhou X, Pan C. Overexpression of MicroRNA-145 Ameliorates Astrocyte Injury by Targeting Aquaporin 4 in Cerebral Ischemic Stroke. Biomed Res Int. 2017;2017:9530951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Li L, Zhang LH, Xu WP, Hu JM. Risk assessment of ischemic stroke associated pneumonia. World J Emerg Med. 2014;5:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Kim J, Fann DY, Seet RC, Jo DG, Mattson MP, Arumugam TV. Phytochemicals in Ischemic Stroke. Neuromolecular Med. 2016;18:283-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Du EHY, Shankar JJS. Rapid Endovascular Treatment of Acute Ischemic Stroke: What a General Radiologist Should Know. Can Assoc Radiol J. 2017;68:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Zhang C, Wang Y, Zhao X, Liu L, Wang C, Pu Y, Zou X, Pan Y, Wong KS, Wang Y; Chinese IntraCranial AtheroSclerosis (CICAS) Study Group. Prediction of Recurrent Stroke or Transient Ischemic Attack After Noncardiogenic Posterior Circulation Ischemic Stroke. Stroke. 2017;48:1835-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Shahid F, Shantsila E, Lip GY. Recent advances in the understanding and management of atrial fibrillation: a focus on stroke prevention. F1000Res. 2016;5:2887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Shahid F, Lip GYH. Risk Stratification Models in Atrial Fibrillation. Semin Thromb Hemost. 2017;43:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Ghasemi M, Nolan DR, Lally C. An investigation into the role of different constituents in damage accumulation in arterial tissue and constitutive model development. Biomech Model Mechanobiol. 2018;17:1757-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Hoshino M, Shimizu T, Ogura H, Hagiwara Y, Takao N, Soga K, Usuki N, Moriya J, Nakamura H, Hasegawa Y. Intraplaque Microvascular Flow Signal in Superb Microvascular Imaging and Magnetic Resonance Imaging Carotid Plaque Imaging in Patients with Atheromatous Carotid Artery Stenosis. J Stroke Cerebrovasc Dis. 2018;27:3529-3534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Magenta A, Sileno S, D'Agostino M, Persiani F, Beji S, Paolini A, Camilli D, Platone A, Capogrossi MC, Furgiuele S. Atherosclerotic plaque instability in carotid arteries: miR-200c as a promising biomarker. Clin Sci (Lond). 2018;132:2423-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Kim Y, Faysel M, Balucani C, Yu D, Gilles N, Levine SR. Ischemic Stroke Predictors in Patients Presenting with Dizziness, Imbalance, and Vertigo. J Stroke Cerebrovasc Dis. 2018;27:3419-3424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Guzik A, Bushnell C. Stroke Epidemiology and Risk Factor Management. Continuum (Minneap Minn). 2017;23:15-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 206] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 14. | Nael K, Knitter JR, Jahan R, Gornbein J, Ajani Z, Feng L, Meyer BC, Schwamm LH, Yoo AJ, Marshall RS, Meyers PM, Yavagal DR, Wintermark M, Liebeskind DS, Guzy J, Starkman S, Saver JL, Kidwell CS. Multiparametric Magnetic Resonance Imaging for Prediction of Parenchymal Hemorrhage in Acute Ischemic Stroke After Reperfusion Therapy. Stroke. 2017;48:664-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Zhang B, Gu J, Qian M, Niu L, Zhou H, Ghista D. Correlation between quantitative analysis of wall shear stress and intima-media thickness in atherosclerosis development in carotid arteries. Biomed Eng Online. 2017;16:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Peiffer V, Sherwin SJ, Weinberg PD. Does low and oscillatory wall shear stress correlate spatially with early atherosclerosis? A systematic review. Cardiovasc Res. 2013;99:242-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 17. | Zhou H, Meng L, Zhou W, Xin L, Xia X, Li S, Zheng H, Niu L. Computational and experimental assessment of influences of hemodynamic shear stress on carotid plaque. Biomed Eng Online. 2017;16:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Buchanan CF, Verbridge SS, Vlachos PP, Rylander MN. Flow shear stress regulates endothelial barrier function and expression of angiogenic factors in a 3D microfluidic tumor vascular model. Cell Adh Migr. 2014;8:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 19. | De Wilde D, Trachet B, De Meyer GR, Segers P. Shear Stress Metrics and Their Relation to Atherosclerosis: An In Vivo Follow-up Study in Atherosclerotic Mice. Ann Biomed Eng. 2016;44:2327-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Kefayati S, Milner JS, Holdsworth DW, Poepping TL. In vitro shear stress measurements using particle image velocimetry in a family of carotid artery models: effect of stenosis severity, plaque eccentricity, and ulceration. PLoS One. 2014;9:e98209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Wang C, Chen M, Liu SL, Liu Y, Jin JM, Zhang YH. Spatial distribution of wall shear stress in common carotid artery by color Doppler flow imaging. J Digit Imaging. 2013;26:466-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Erdur H, Scheitz JF, Ebinger M, Rocco A, Grittner U, Meisel A, Rothwell PM, Endres M, Nolte CH. In-hospital stroke recurrence and stroke after transient ischemic attack: frequency and risk factors. Stroke. 2015;46:1031-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Sundararajan V, Thrift AG, Phan TG, Choi PM, Clissold B, Srikanth VK. Trends over time in the risk of stroke after an incident transient ischemic attack. Stroke. 2014;45:3214-3218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Grau AJ, Eicke M, Burmeister C, Hardt R, Schmitt E, Dienlin S. Risk of Ischemic Stroke and Transient Ischemic Attack Is Increased up to 90 Days after Non-Carotid and Non-Cardiac Surgery. Cerebrovasc Dis. 2017;43:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Corban MT, Eshtehardi P, Suo J, McDaniel MC, Timmins LH, Rassoul-Arzrumly E, Maynard C, Mekonnen G, King S, Quyyumi AA, Giddens DP, Samady H. Combination of plaque burden, wall shear stress, and plaque phenotype has incremental value for prediction of coronary atherosclerotic plaque progression and vulnerability. Atherosclerosis. 2014;232:271-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | Hung OY, Brown AJ, Ahn SG, Veneziani A, Giddens DP, Samady H. Association of Wall Shear Stress with Coronary Plaque Progression and Transformation. Interv Cardiol Clin. 2015;4:491-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Borné Y, Fagerberg B, Persson M, Östling G, Söderholm M, Hedblad B, Sallsten G, Barregard L, Engström G. Cadmium, Carotid Atherosclerosis, and Incidence of Ischemic Stroke. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Meschia JF, Brott T. Ischaemic stroke. Eur J Neurol. 2018;25:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 29. | Xiong H, Liu X, Tian X, Pu L, Zhang H, Lu M, Huang W, Zhang YT. A numerical study of the effect of varied blood pressure on the stability of carotid atherosclerotic plaque. Biomed Eng Online. 2014;13:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Shroff GR, Solid CA, Bloomgarden Z, Halperin JL, Herzog CA. Temporal trends in ischemic stroke and anticoagulation therapy for non-valvular atrial fibrillation: effect of diabetes. J Diabetes. 2017;9:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Spence JD. Homocysteine lowering for stroke prevention: Unravelling the complexity of the evidence. Int J Stroke. 2016;11:744-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Eshtehardi P, Brown AJ, Bhargava A, Costopoulos C, Hung OY, Corban MT, Hosseini H, Gogas BD, Giddens DP, Samady H. High wall shear stress and high-risk plaque: an emerging concept. Int J Cardiovasc Imaging. 2017;33:1089-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |