Published online Sep 6, 2019. doi: 10.12998/wjcc.v7.i17.2637
Peer-review started: April 22, 2019
First decision: June 12, 2019
Revised: June 27, 2019
Accepted: July 20, 2019
Article in press: July 20,2019
Published online: September 6, 2019
Processing time: 141 Days and 10.3 Hours
Calcifying fibrous tumor (CFT) is a rare benign mesenchymal tumor that often occurs in deep soft tissue of children and young adults. CFT rarely occurs in the mediastinum.
In this paper, we describe a 31-year-old male patient with CFT in the mediastinum. The patient did not have any symptoms, and the posterior mediastinal lesion was unintentionally found during routine re-examination of thyroid cancer. The tumor had no adhesion to the surrounding tissue and was successfully and completely removed. Pathology showed a large amount of collagen-rich fibrous connective tissue. There was scattered dystrophic calcification and gravel in the fibrous tissue and a small amount of lymphocyte and plasma cell infiltration and lymphoid follicle formation in the interstitial fluid. In addition, findings showed 20 IgG4+ plasma cells per high-powered field of the diseased tissue, an IgG4+/IgG ratio of about 20%, and normal serum IgG4 levels. The final diagnosis was CFT of the mediastinum (CFTM). No evidence of tumor recurrence was observed by computed tomography at 3 mo after surgery.
IgG4+ plasma cell enlargement may occur in CFTM, but clinical manifestations and serological tests suggest that it is not IgG4-related disease. We speculate that it may be an independent tumor subtype.
Core tip: Calcifying fibrous tumor of the mediastinum (CFTM) is a rare benign mesenchymal tumor that often occurs in children and young adults. Calcifying fibrous tumor rarely occurs in the mediastinum. Histological examination is the most important basis for diagnosis. Surgical resection is currently the primary means of treatment. There are no reports of recurrence of CFTM.
- Citation: Qi DJ, Zhang QF. Calcifying fibrous tumor of the mediastinum: A case report. World J Clin Cases 2019; 7(17): 2637-2643
- URL: https://www.wjgnet.com/2307-8960/full/v7/i17/2637.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i17.2637
Calcifying fibrous tumor (CFT) is formerly called calcifying fibrous pseudotumor (CFP) and childhood fibrous tumor with psammoma bodies[1,2]. CFT often occurs in children and adolescents[2], with an average age of incidence of about 34 years[3]. It is slightly more predominant in female patients, with a male to female ratio of 1:1.27[3]. To the best of our knowledge, no deaths from CFT have been reported in the international literature, thus CFT per se is a benign mesenchymal tumors; nonetheless, 10% of cases recur after resection[3]. The etiology of CFT is unclear and may be related to myofibroblastic tumors, genetic and embryologic factors, and trauma[3]. The occurrence of CFT is rare, with only slightly more than 100 cases reported in the international literature as of 2018[2,3]. The most common locations of CFT include the stomach, small intestine, pleura, peritoneum, and mesentery, but it may occasionally be found in other places such as the heart and maxilla[3-5]; in particular, there are just nine reports of CFT in the mediastinum (CFTM)[6-13].
A 31-year-old male patient underwent thyroidectomy for thyroid cancer six months previously. A postoperative computed tomography (CT) examination revealed an oval mass in the posterior mediastinum. The patient had no symptoms such as cough, pain, or difficulty breathing.
Six months previously, the patient was diagnosed with thyroid cancer and underwent thyroidectomy.
No meaningful positive results were noted in the physical examination, except for the thyroid incision in the neck.
Serum levels of tumor markers (α-fetoprotein, β-human chorionic gonadotrophin, and carcinoembryonic antigen) were normal. Serum IgG4 levels, erythrocyte sedimentation rate, rheumatoid factor, and anti-streptolysin and anti-nuclear antibodies were normal.
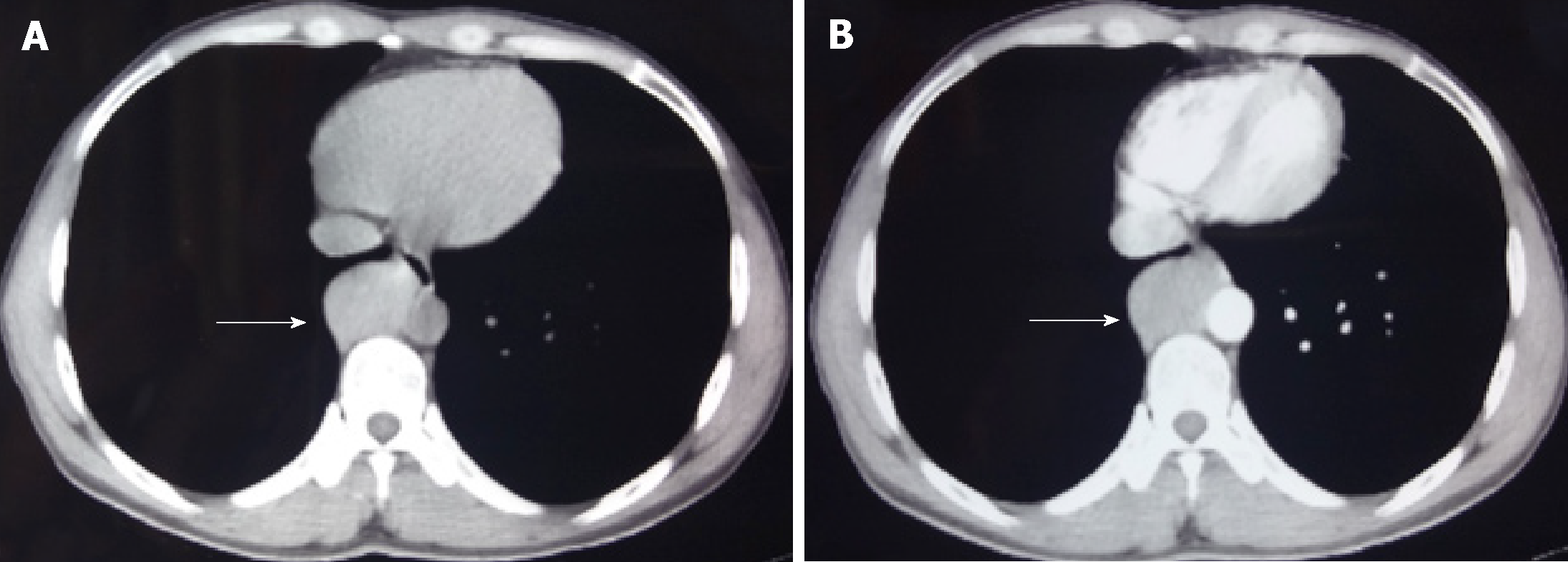
Chest CT examination revealed an oval tumor in the right posterior mediastinum, close to the spine; the tumor was of uniform density with a clear boundary and measured about 4.5 × 3.0 cm. The CT value was about 57 HU, and the enhanced CT value was about 113 HU (Figure 1). No tumor metastasis was detected on lung radiography, head CT, bone scan, or abdominal ultrasonography. The patient did not have a chest CT scan prior to the first surgery for thyroid cancer.
Intraoperative exploration revealed that the oval mass was located in the right lower posterior mediastinum, was adjacent to T7–T9 and the descending aorta, and had no adhesion to the surrounding tissue. After the vessel was processed, the tumor was completely removed. The resected tumor was 5.5 cm × 3.5 cm × 2.5 cm in size and firm; the cut surface was grayish white.
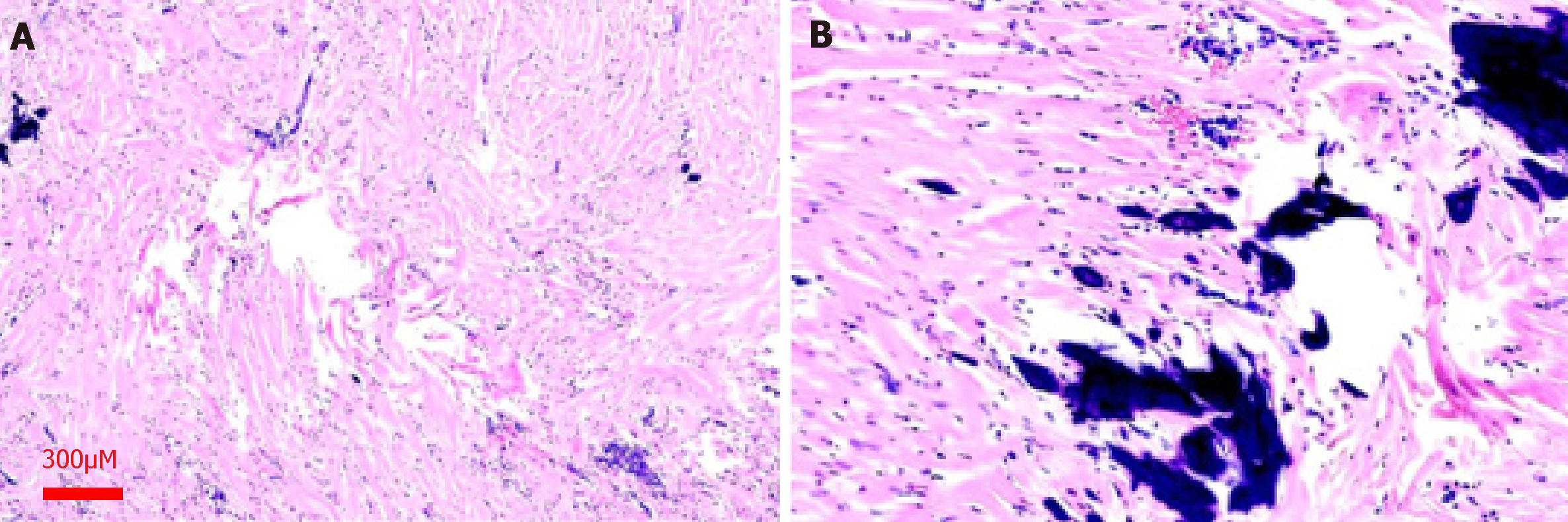
Histological manifestations are as follows. Hematoxylin and eosin staining showed a large amount of collagenous fibrous connective tissue. There was scattered dystrophic calcification and boulder formation in the fibrous tissue. A small amount of lymphocytes and plasma cells had infiltrated the interstitial space, and lymphoid follicle formation was observed locally at 40× magnification (Figure 2A and Figure 2B).
Immunohistochemical examination showed that the tumor cells expressed CD99 and CD38, thus revealing the presence of plasma cells. The remaining immunohistochemical findings were EMA (−), SOX-10 (−), S100 (−), CD34 (−), SMA (−), ALK l (−), FXIIIa (−), and beta-catenin (−), and the Ki-67 proliferation index was about 5%. There were 20 IgG4+ plasma cells per high-powered field (HPF) and an IgG4+/IgG ratio of about 20%. The patient recovered smoothly after surgery and was discharged three days later. He did not receive radiotherapy or chemotherapy.
The final diagnosis of the presented case was CFTM.
The patient was followed 4 mo after surgery. The incision healed well, and there was no discomfort. Chest CT findings showed no tumor recurrence.
Including the present case, there are ten reported cases of CFTM, with a male to female ratio of 1.5 (six females and four males); this is slightly lower than the ratio of male to female in CFT (1:1.27)[3]. The age range for CFTM is 8–54 years, with an average of 31.3 years; this is slightly lower than the average age of CFT (about 34 years old)[3]. Six patients had no symptoms, and masses were found during a regular radiological examination or a review of a chest radiographic test[7,9,11,13]. Regarding symptoms, three patients had persistent cough[6,10,12], one patient had sternal discomfort[6], and one patient had hemoptysis and weight loss[10]. Regarding location, seven tumors occurred in the anterior mediastinum, and three occurred in the posterior mediastinum[6-13]. Most CFTMs were single tumors, and only one case exhibited multiple tumors[10]. Most CFTMs had clear boundaries with the surrounding tissues, and only two cases invaded the surrounding tissues, including the vein and thoracic duct[11,13]. Tumors were generally oval, and the longest diameter of a single tumor ranged from 4–11 cm[6,11]. The longest diameter of multiple tumors was less than 2 cm[10].
The diagnosis of CFTM has the following characteristics. First, CT shows isolated or multiple solid soft tissue tumors with a clear boundary[10], and the CT signal of the tumor is slightly enhanced after contrast injection. Some tumors show calcification on CT[13], whereas others have no significant calcification. Second, gross examination shows an oval tumor, with the longest diameter of 3-11 cm, a clear boundary, and an incomplete capsule. The cut surface is grayish-white, solid, and hard and could be accompanied by gritty and scattered yellowish lesion. Third, microscopic histological features include aberrant hyalinized collagen, fibrotic proliferation, lymphoplasmacytic infiltration, and psammomatous or dystrophic calcification[2,6]. Immunohistochemical spindle cells are positive for vimentin, CD10, FXIIIa, and occasionally CD34; they are negative for actin, desmin, S-100, NF, CK, CD31, and ALK1. To date, there are no specific immunohistochemical or genetic ectopic abnormalities that have been discovered in CFTM.
CFTM needs to be differentiated from other pleural intrapulmonary lesions such as solitary fibrous tumor (SFT), inflammatory myofibroblastic tumor (IMT), fibromatosis, and sclerosing thymoma. First, for SFT, there is a morphological feature that contains collagen fibers of varying thickness and shape; these can be keloid-like and resemble CFT. However, SFT can alternate between cell-poor and cell-rich regions. The spindle-shaped nuclei are vacuolated, are diffusely positive for CD34 and STAT6, and do not contain widely distributed gravel or dystrophic calcification. Second, for IMT, there is a late histologically visible scar-like structure, which is rich in plate-shaped collagen and exhibits low cell density and a small amount of plasma cell infiltration; however, IMT occasionally shows gravel and coarse calcification as well as proliferating myofibroblasts with hyaline-denaturing, cell-free collagen. Immunohistochemical staining of the proliferating fibroblasts shows diffusely actin-positive (50%) and ALKI-positive (50%) spindle cells; in contrast, CFT is actin-negative and diffusely FVIII-positive[8]. Third, regarding fibromatosis, characteristics include an unclear tumor mass, invasive growth, no capsule, frequent invasion of adjacent tissues, and presence in the muscle, aponeurosis, and deep fascia. Microscopic examination shows bundles of fibroblasts in the tumor. Calcification is rare, and β-catenin is positive. Fourth, in sclerosing thymoma, the interstitium has rich and transparent degeneration-like collagen similar to CFT, but contains epithelial cells and lymphocytes and is positive for epithelial markers.
The pathogenesis of CFT remains unclear. It was previously thought that part of the CFT was late stage sclerosis of IMT, but many scholars objected to it on the grounds that ALK-1 expression in the two lesions was different[8,9]. So far, there has been no molecular evidence that CFT is clonal[13] (Table 1), and there is no evidence of genetic dislocation in CFT. The pathological changes of CFT and IgG4-related diseases (IgG4-RD) are similar, and in recent years, some CFT cases have been found to have IgG4+ plasma cells and observable transparent vascular cavity calcification in the CFT lesion site[14,15]; accordingly, it has been considered that vascular lumen calcification in CFT results from occlusive vasculitis[16]. Therefore, it is speculated that CFT is related to IgG4-RD and that CFT may be a stage of development for IgG4-RD or an undiscovered IgG4-RD[17]. A study[17] reported one case of CFT in the ileum in which the lesion site showed 122 IgG+ plasma cells/HPF, of which 69 were IgG4+ pulp cells (56.56% of IgG+ plasma cells). Prochaska et al[18] reported one case of adrenal CFT, with an average of 183 IgG+ and 11 IgG4+ plasma cells in the lesion at high magnification. Zhang et al[15] reported one case of gastric CFT, with 62 IgG4+ plasma cells in the lesion at high magnification; IgG4+ cells comprised 41% of IgG+ cells. Due to the current low number of cases and insufficient evidence, it is not clear whether the recurrence of cases is related to IgG4 levels and the effectiveness of the application of hormones or rituximab in treatment. As a result, the relationship between CFT and IgG4-RD needs further study. In addition, the report summarizes six other cases of IgG4-related CFT.
| Ref | Year | Age/sex | Symptoms duration | Localization | Gross examination | Treatment | Follow-up |
| Dumont et al[6] | 1997 | 23/F | Cough with retrosternal discomfort | Anterior mediastinum | Firm, gray-white, homogeneous, and well circumscribed, non-encapsulated, 11 × 8 × 5 cm, 235 g | OP | Alive, NED after 18 mo follow-up |
| Jeong et al[7] | 1997 | 54/F | - | Posterior mediastinum | 8.5 × 6 × 5 cm, 320 g, clear boundary, grayish white, scattered in light, yellow specks | OP | Alive, NED after 49 mo follow-up |
| Sigel et al[8] | 2001 | 37/F | NA | Anterior mediastinum | 7.0 cm | NA | NA |
| Nascimento et al[9] | 2002 | 27/M | - | Posterior mediastinum | 7 cm | OP | NA |
| Nascimento et al[9] | 2002 | 31/F | - | Anterior mediastinum | 5.5 cm | OP | NA |
| Sleigh et al[10] | 2010 | 22/F | Persistent cough, hemoptysis, weight loss | Anterior mediastinum and pleura | Circular mass, about 4.7 × 3.3 cm, clear boundary, uncoated film, gray-white; bilateral pleural nodules, 2–4.8 cm or smaller in diameter | OP (the anterior mediastinal and left pleural nodules were all removed, and the right side was not removed) | There was no recurrence in the left after 18 mo follow-up. No change in right lesion |
| Chang et al[11] | 2011 | 51/M | - | Anterior mediastinum, involving vein | 4.2 cm × 2.7 cm × 2.1 cm, clear boundary, uncoated film, gray to yellow | OP | NED after 11 mo follow-up |
| Chauhan et al[12] | 2014 | 8/M | Chronic cough | Anterior mediastinum | 7 cm × 5.5 cm × 3.6 cm, clear boundary, white, firm | OP | No recurrence (follow-up time not recorded) |
| Dissanayake et al[13] | 2016 | 29/F | - | Anterior mediastinum, involving the thoracic duct | 10 × 6 × 5.5 cm, 242 g, whitish tan, fleshy, homogeneous, surfaces with focal areas of yellowish tan, firm calcifications | OP (the thoracic duct was resected along with the mass) | NED after 3 mo follow-up |
| Present case | 2019 | 31/M | - | Posterior mediastinum | 5.5 cm × 3.5 cm × 2.5 cm, firm, grayish white | OP | Alive, NED after 4 mo follow-up |
The present case was also been found to have about 20 IgG4+ plasma cells per HPF in the lesion tissue, with IgG4+ comprising 20% of IgG+ cells; however, we do not believe that the case can be diagnosed as IgG4-RD, mainly based on the following reasons. First, the patient was relatively young, and the site of the disease was not an immune-related organ. Second, the patient's serum was IgG4-negative, and there were no abnormalities in erythrocyte deposition rate, rheumatoid factor, anti-Streptococcus hemolysin, or anti-nuclear antibody. Third, the ratio of the total number of IgG4+ plasma cells to IgG+ plasma cells under the microscope was less than 40%. Fourth, no other physiological system was affected. This is consistent with CFT, which appears to be a benign tumor with a main treatment method of complete surgical resection [11]. Moreover, in a few cases, CFT can recur, and the recurrent lesion shows the same pathological morphology as the primary lesion[14]. In the ten published cases of CFTM, none exhibited recurrence, although this may be related to the small sample size and short follow-up time[19].
This article reports a case of CFTM. In some cases, CFT may have an increase in IgG4+ plasma cells and a cross-sectional histomorphology. However, it lacks the systematic clinical manifestations and serological manifestations of IgG4-RD. Clinicians should be vigilant not to misdiagnose it as IgG4-RD based on a clinical diagnosis.
Author contributions: Qi DJ and Zhang QF conceived the study; Qi DJ searched the published articles, analyzed the data, and wrote the manuscript; Zhang QF reviewed and confirmed the final version of the manuscript and provided funding.
Manuscript source: Unsolicited Manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Movahed A S-Editor: Cui LJ L-Editor: Wang TQ E-Editor: Zhou BX
| 1. | Lee S, Jahng J, Han W. Gastric Calcifying Fibrous Tumor Manifesting as a Subepithelial Tumor. J Gastrointest Surg. 2018;22:1127-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Prucker J, Salaheddin-Nassr Y, Leidl S. Calcifying fibrous tumor of the terminal ileum mesentery: Case report. Medicine (Baltimore). 2018;97:e13351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Chorti A, Papavramidis TS, Michalopoulos A. Calcifying Fibrous Tumor: Review of 157 Patients Reported in International Literature. Medicine (Baltimore). 2016;95:e3690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Okamura K, Nawata K, Shimada S, Ono M. Complete resection of a giant calcifying fibrous tumor of myocardial origin. Gen Thorac Cardiovasc Surg. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Qureshi TA, Akhtar S, Abid M. Calcifying fibrous pseudotumour of maxilla: A rare entity mimicking malignancy: A case report. J Pak Med Assoc. 2018;68:1521-1524. [PubMed] |
| 6. | Dumont P, de Muret A, Skrobala D, Robin P, Toumieux B. Calcifying fibrous pseudotumor of the mediastinum. Ann Thorac Surg. 1997;63:543-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Jeong HS, Lee GK, Sung R, Ahn JH, Song HG. Calcifying fibrous pseudotumor of mediastinum--a case report. J Korean Med Sci. 1997;12:58-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Sigel JE, Smith TA, Reith JD, Goldblum JR. Immunohistochemical analysis of anaplastic lymphoma kinase expression in deep soft tissue calcifying fibrous pseudotumor: evidence of a late sclerosing stage of inflammatory myofibroblastic tumor? Ann Diagn Pathol. 2001;5:10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Nascimento AF, Ruiz R, Hornick JL, Fletcher CD. Calcifying fibrous 'pseudotumor': clinicopathologic study of 15 cases and analysis of its relationship to inflammatory myofibroblastic tumor. Int J Surg Pathol. 2002;10:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Sleigh KA, Lai W, Keen CE, Berrisford RG. Calcifying fibrous pseudotumours: an unusual case with multiple pleural and mediastinal lesions. Interact Cardiovasc Thorac Surg. 2010;10:652-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Chang JW, Kim JH, Maeng YH. Calcifying fibrous pseudotumor of the anterior mediastinum. Korean J Thorac Cardiovasc Surg. 2011;44:318-320. [PubMed] [DOI] [Full Text] |
| 12. | Chauhan KR, Shah HU, Trivedi PP, Shah MJ. Calcifying fibrous pseudotumor of the mediastinum: a rare case report. Indian J Pathol Microbiol. 2014;57:155-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Dissanayake SN, Hagen J, Fedenko A, Lee C. Calcifying Fibrous Pseudotumor of the Posterior Mediastinum With Encapsulation of the Thoracic Duct. Ann Thorac Surg. 2016;102:e39-e40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Vasilakaki T, Skafida E, Tsavari A, Arkoumani E, Koulia K, Myoteri D, Grammatoglou X, Moustou E, Firfiris N, Zisis D. Gastric calcifying fibrous tumor: a very rare case report. Case Rep Oncol. 2012;5:455-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Zhang H, Jin Z, Ding S. Gastric calcifying fibrous tumor: A case of suspected immunoglobulin G4-related gastric disease. Saudi J Gastroenterol. 2015;21:423-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Stone JH, Khosroshahi A, Deshpande V, Chan JK, Heathcote JG, Aalberse R, Azumi A, Bloch DB, Brugge WR, Carruthers MN, Cheuk W, Cornell L, Castillo CF, Ferry JA, Forcione D, Klöppel G, Hamilos DL, Kamisawa T, Kasashima S, Kawa S, Kawano M, Masaki Y, Notohara K, Okazaki K, Ryu JK, Saeki T, Sahani D, Sato Y, Smyrk T, Stone JR, Takahira M, Umehara H, Webster G, Yamamoto M, Yi E, Yoshino T, Zamboni G, Zen Y, Chari S. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012;64:3061-3067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 488] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 17. | Koizumi S, Kamisawa T, Kuruma S, Tabata T, Chiba K, Iwasaki S, Endo Y, Kuwata G, Koizumi K, Shimosegawa T, Okazaki K, Chiba T. Immunoglobulin G4-related gastrointestinal diseases, are they immunoglobulin G4-related diseases? World J Gastroenterol. 2013;19:5769-5774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Prochaska EC, Sciallis AP, Miller BS. Retroperitoneal calcifying fibrous tumor mimicking an adrenal tumor. J Surg Case Rep. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Pezhouh MK, Rezaei MK, Shabihkhani M, Ghosh A, Belchis D, Montgomery EA, Voltaggio L. Clinicopathologic study of calcifying fibrous tumor of the gastrointestinal tract: a case series. Hum Pathol. 2017;62:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |