Published online Sep 6, 2019. doi: 10.12998/wjcc.v7.i17.2630
Peer-review started: February 24, 2019
First decision: June 19, 2019
Revised: June 30, 2019
Accepted: July 20, 2019
Article in press: July 20, 2019
Published online: September 6, 2019
Processing time: 197 Days and 7.9 Hours
Wilson disease (WD) is a genetic disorder of hepatic copper excretion, leading to copper accumulation in various tissues. The manifestations are quite variable, and hemolytic anemia is the most common hematological presentation. WD associated with thrombocytopenia is very rare.
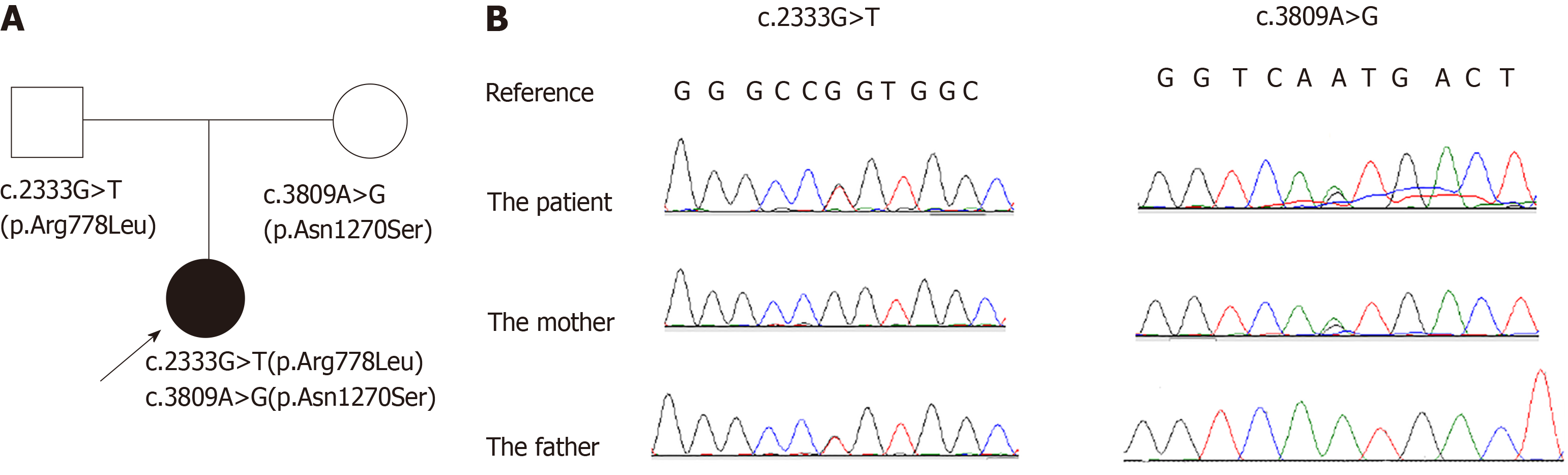
We report the case of an 11-year-old Chinese girl with WD that was associated with immune thrombocytopenia (ITP). Thrombocytopenia was the initial chief complaint for her to visit a hematologist, and ITP was diagnosed based on the results of a bone marrow biopsy and positive antiplatelet autoantibodies. About two weeks before the thrombocytopenia was found, the patient developed drooling. Tremors developed in her right hand about one week after being diagnosed with ITP, after which she was admitted to our hospital. Further evaluations were performed. Ceruloplasmin was decreased, with an increased level of copper in her 24-h urine excretion. Kayser Fleischer's ring (K-F ring) was positive. The ultrasound showed liver cirrhosis, and brain magnetic resonance imaging showed that the lenticular nucleus, caudate nucleus, and brainstem presented a low signal intensity in T1-weighted images and high signal intensity in T2-weighted images. WD was diagnosed and a genetic analysis was performed. A compound heterozygous mutation in ATP7B was detected; c.2333G>T (p.Arg778Leu) in exon 8 and c.3809A>G (p.Asn1270Ser) in exon 18. The former was inherited from her father and the latter from her mother. However, her parents showed normal liver function and negative K-F rings. Such a compound mutation in a case of WD associated with ITP in children has not been published previously.
WD can associate with thrombocytopenia but the mechanism is still unclear. We recommend that antiplatelet autoantibodies should be tested in WD patients with thrombocytopenia in future to verify the association.
Core tip: Our findings indicate that Wilson disease (WD) can associate with thrombocytopenia. Some recessive heterozygous mutations can induce WD in combination with other recessive heterozygous mutations in ATP7B. Thrombocytopenia patients with neurological signs or abnormal liver function should be screened for WD because early detection and treatment of WD lead to a better outcome. We recommend that antiplatelet autoantibodies should be tested in WD patients with thrombocytopenia in future to verify the association.
- Citation: Ma TJ, Sun GL, Yao F, Yang ZL. Wilson disease associated with immune thrombocytopenia: A case report and review of the literature. World J Clin Cases 2019; 7(17): 2630-2636
- URL: https://www.wjgnet.com/2307-8960/full/v7/i17/2630.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i17.2630
Wilson Disease (WD), first described in 1912, is a hereditary genetic disorder induced by the dysfunction of copper metabolism in the liver. WD is fatal if untreated, but the prognosis can be good with timely and lifelong management[1-2]. The initial signs for WD are quite varied, and some rare presentations may lead to delays in diagnosis and treatment[3-5]. It is caused by mutations in ATP7B, which encodes a membrane-bound P1B-type ATPase involved in copper excretion from hepatocytes. The accumulation of copper in the body can lead to multiple organ damage. ATP7B was first identified as the responsible gene in 1993, and now over 500 mutations have been detected and most patients with WD are compound heterozygotes[6-8]. Some mutations in ATP7B show relatively higher frequencies in special populations, such as the mutation resulting in p.Arg778Leu in the Far East[9-15]. The identification of a mutation supports the diagnosis of WD, while a compound heterozygous status confirms the diagnosis. Recently, Atox1 and COMMD1 were also concerned in WD patients, but there was no evidence to show their contribution[16].
Coombs-negative hemolytic anemia is the hematological presentation reported for WD and is rare. Immune thrombocytopenia (ITP) is an acquired hemorrhagic disease caused by the accelerated clearance of platelets induced by antiplatelet autoantibodies such as antiglycoprotein (GP) IIb/IIIa[17-19]. WD has been associated with ITP in an adult[20], but such association has not been reported in children.
Here, we report a case of genetically-confirmed WD caused by a compound ATP7B mutation that was inherited from the proband’s unaffected parents. The patient was diagnosed with ITP and revealed WD soon after the diagnosis of ITP, and we also discuss the association of WD with ITP.
The proband (Figure 1A) was an 11-year-old Chinese girl who was admitted to our hospital with chief complaints of thrombocytopenia for 15 d, and tremor in her right hand for 3 d.
About 15 d previously, she experienced coughing, drooling of saliva, and dysarthria without fever and was admitted to a local hospital with a diagnosis of epiglottitis. A routine blood test found a platelet count of 54 × 109/L, after which she visited a hematologist. A bone marrow examination and testing for antiplatelet antibodies were performed. HLA Class I antibody was negative, but antibodies against GP IIb/IIIa, GP Ib/Ix, and GP Ia/type IIa were positive. In bone marrow smears, granulocytic and erythrocytic series were normal, while megakaryocytes appeared immature and platelet-producing megakaryocytes were not found. According to these results, ITP was diagnosed. The child was not treated for ITP because her platelet count was greater than 50 × 109/L and she had no signs of hemorrhagic tendency. About 3 days previously, she exhibited an involuntary tremor, with numbness in her right hand.
She was healthy in the past.
Her medical and family histories were unremarkable, and she had not started menstruating. Her parents had no history of consanguinity.
On physical examination, she had dysarthria with normal orientation. She had no rashes, hemorrhagic signs, hepatomegaly, or splenomegaly. The pharyngeal reflex, abdominal reflexes, patellar tendon reflexes, finger-to-nose test, and Romberg’s sign were normal. Barbinski signs were negative. Alternating movements with hands were slow, and her right hand trembled. Sensitivity to heat or pain stimulation was normal. Kayser Fleischer's (K-F) rings were found under a slit lamp.
Laboratory investigations revealed serum platelet counts of 34-43 × 109/L (normal range, 100-300 × 109/L), and hemoglobin of 112-123 g/L (normal range, 120-140 g/L). Liver function tests revealed alanine aminotransferase (ALT) of 59 U/L (normal range, 7-40 U/L), aspartate aminotransferase (AST) of 65 U/L (normal range, 13-35 U/L), albumin (ALB) of 26 g/L (normal range, 40-55 g/L), total serum bilirubin of 45 µmol/L (normal range, 3.4-20.5 µmol/L), serum direct bilirubin (DBL) of 12.9 µmol/L (normal range, 0.3-8.5 µmol/L), and plasma ammonia of 35 µmol/L (normal range, 11-25 µmol/L). Her blood clotting profile showed a prothrombin time (PT) of 18.2 s (normal range, 11.0-14.3 s), prothrombin time activity (PTA) of 54% (normal range, 80-120 s), prothrombin time [international normalized ratio (INR)] of 1.53 (normal range, 08-1.15), activated partial thromboplastin time (APTT) of 55.5 s (normal range, 32.6-43.0 s), and fibrinogen of 1.27 g/L (normal range, 2.0-4.0 g/l). Plasma ceruloplasmin was 190 mmol/L (normal range, 220-330 mmol/L) and 24-h urine copper was 203 µg/24 h (normal value, <100 µg/24 h). Her lactic acid level and urinalysis were normal. Antibodies for viral hepatitis series (including hepatitis A, B, C, D, and E viruses) were negative. Tests of immunoglobin M for Coxsackie virus, herpes virus, cytomegalovirus, Toxoplasmosis gondii, rubella virus, adenovirus, respiratory syncytial virus, and Mycoplasma pneumoniae were negative. Antibodies for autoimmune hepatitis and connective tissue disease (including AMA-M2, LKM-1, LC-1, SLA/LP, Ro-52, PML, sp100, gp210, M2-3E, anti-nuclear antibodies, anti-ds-DNA, Sm, SS-A, SS-B, and ENA-Jo-1) were negative.
Liver biopsy was not recommended because of the patient’s thrombocytopenia and disturbances in blood clotting functions. With written consent from her parents, genetic analysis for WD was performed. DNA was extracted from the peripheral blood samples, which were collected from the proband and her parents using the QIAamp Blood DNA Mini Kit (Qiagen, Germany). PCR was performed to amplify each exon and its neighboring introns using an ABI9700 PCR amplifier (Life Technologies, United States). Direct sequencing was performed on the amplified DNA fragments using the ABI3500 sequencer (Life Technologies, USA) and the results were subjected to sequence analysis using Sequence Scanner v1.0 (Applied Biosystems, United States).
Genetic analysis showed that the proband had a compound heterozygous mutation in the ATP7B gene; c.2333G>T (p.Arg778Leu) in exon 8 and c.3809A>G (p.Asn1270Ser) in exon 18 (reference sequence: NM_000053.3). The former was inherited from her father and the latter was inherited from her mother (Figure 1B).
The detected mutations were interpreted according to the guidelines from the American College of Medical Genetics and Genomics and patient phenotype[21]. The PCR amplification and sequencing procedure were performed by Shenyang Kingmed for Clinical Laboratory (Shenyang, China), which provides third party inspection services. The hypothetical effects of the mutations on protein function were analyzed using the Polymorphism Phenotyping v2 (PolyPhen-2) prediction tool (http://genetics.bwh.harvard.edu/pph2/dbsearch.shtml), SIFT (http://sift.jcvi.org/www/SIFT_enst_submit.html), and MutationTaster (http://www.mutationtaster.org/index.html).
The c.2333G>T (p.Arg778Leu) mutation is known as a polymorphism (number rs28942074) and located in the transmembrane domain 4 (TM4). The p.Asn1270Ser mutation is located in the ATP hinge of ceruloplasmin. PolyPhen-2 and SIFT analyses suggested that the c.2333G>T mutation can negatively affect gene function, while MutationTaster suggested it is benign. PolyPhen-2, SIFT, and MutationTaster analyses suggested that the c.3809A>G mutation can be harmful (Table 1). A synonymous mutation, c.2310C>G (p.Leu770=) (rs398123136) in exon 8, was also detected in the proband and her father (data not shown).
| Base change | Exon number | Amino acid change | PolyPhen-2 analysis | SIFT analysis | MutationTaster analysis |
| c.2333G>T | 8 | p.Arg778Leu | Probably damaging | Damaging | Polymorphism |
| c.3809A>G | 18 | p.Asn1270Ser | Probably damaging | Damaging | Disease causing |
The electroencephalogram (EEG) was normal. Brain magnetic resonance imaging scans showed a low signal intensity in T1-weighted images, and high signal intensity in T2-weighted images from the lenticular nucleus, caudate nucleus, and brainstem. Ultrasonography showed that the liver decreased in volume with an unsmooth surface and blunt edge, and the internal echogenicity was enhanced with tortuous hepatic veins. Splenomegaly was also observed.
WD was diagnosed.
She was not treated for ITP because she had no hemorrhagic signs, but she was surveilled with the count of PLT. Liver protective therapy was begun when the patient’s liver function was found to be abnormal. After the diagnosis of WD, oral penicillamine (from 125 mg to 1000 mg per day in one week, administered in four doses finally) and zinc sulfate (300 mg per day, administered in three doses) were administered, and intramuscular injections of dimercapto propanol (100 mg per day for two weeks then weaned off over two weeks) were also given after evaluating the benefits and possible side effects.
The 24-h urine copper was reexamined and was 261 µg/24 h after one month. The oral therapies were continued, and dimercaptopropanol was administered for another two weeks, weaned off over two weeks, repeated over three months, and then injected once weekly. At the 6-mo follow-up, the drooling of saliva had disappeared and the tremors in the patient’s right hand and dysarthria had slightly improved, but the ultrasound results showed no marked changes. Platelet count increased and was sustained at about 60 × 109/L over the next month.
WD is an autosomal recessive disease. More than 500 mutations in ATP7B have been identified in patients with WD and most patients are compound heterozygotes[6]. In the proband’s family, c.2333G>T (p.Arg778Leu) and c.3809A>G (p.Asn1270Ser) were detected in the father and mother, respectively, and both mutations had been reported[13,15,22], in some cases as a compound heterozygote without thrombocytopenia[23,24]. The missense heterozygous mutations in the parents were autosomal recessives, even though the mutations induced abnormal protein function according to the bioinformatic analyses. It is possible that a normal allele produces sufficient protein to transport the copper in hepatocytes. But, when the patient inherited both mutations from her parents, the deleterious effects of the compound mutation could not be counteracted by a normal allele.
WD is characterized by the toxic accumulation of copper mainly in the liver and brain, but some other systems can be involved. Hemolysis has been reported as a presenting feature in 12% of 220 WD patients[25]. Thrombocytopenia with negative antiplatelet antibodies has been reported in children as a result of hypersplenism and/or a side effect of D-penicillamine therapy[26,27]. It is reported that the stability of biological membranes can be disturbed by an overload of copper which was accumulated on the membranes. If the membranes of erythrocytes are affected, hemolysis can be induced. We believe that platelets are more easily destroyed and cleared in patients with WD because the membranes of platelets can also be disturbed. It has been reported that the increased depolarization of the mitochondrial membranes can enhance the apoptosis of platelets in patients with chronic ITP[28]. The copper overload on membranes may also enhance apoptosis of platelets by disturbing mitochondrial membranes, but this will need to be investigated further. Serum copper is usually decreased in WD patients. Anemia and neutropenia were the most common hematologic abnormalities identified in copper deficiency patients[29,30]. It was considered that hypocupremia may be a reversible cause of bone marrow dysplasia that caused cytopenia[31]. Hypocupremia induced bone marrow dysplasia may be involved in thrombocytopenia.
In our case, autoimmune diseases and viral and Mycoplasma pneumoniae infections were excluded because of the absence of autoimmune, viral, and M. pneumoniae antibodies. Antibodies positive for GP IIb/IIIa, GP Ib/Ix, and GP Ia/type IIa and the bone marrow findings supported the diagnosis of ITP. Autoantibody-induced pathologies are quite complex[32]. In our case, platelets might initially have been destroyed because of the increased copper accumulation on the membranes. The GP was then released from platelet membranes and accumulated in blood. If the autoimmune response was triggered by GP, antibodies can be generated and the platelet count can decrease further. This is a reasonable explanation for the positive GP antibodies in our case, while these antibodies were negative in the other reported cases since the cases can be at different stages of the disease.
The ultimate therapy for WD is the clearance of copper, and the prognosis is better if therapy is started as early as possible. The first-line treatment for ITP is generally using corticosteroids, or intravenous immunoglobulin in severe cases[33,34]. But thrombocytopenia in WD cases has been reported to have a poor response to glucocorticoids[20,26,27]. After we evaluated the possible risks to the patient, glucocorticoids were not given. The observation that the patient’s platelet counts ceased to decrease after copper clearance therapy also indicated the association of clearance of platelets with copper burden.
WD can associate with thrombocytopenia but the mechanism is still unclear. We recommend that anti-platelet autoantibodies should be tested in WD patients with thrombocytopenia in future to verify the association.
Manuscript source: Unsolicited Manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Anis S, Pop TL, Samasca G S-Editor: Cui LJ L-Editor: Wang TQ E-Editor: Li X
| 1. | Zimbrean PC, Schilsky ML. Psychiatric aspects of Wilson disease: a review. Gen Hosp Psychiatry. 2014;36:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 2. | Dalvi A, Padmanaban M. Wilson's disease: etiology, diagnosis, and treatment. Dis Mon. 2014;60:450-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Millard H, Zimbrean P, Martin A. Delay in Diagnosis of Wilson Disease in Children With Insidious Psychiatric Symptoms: A Case Report and Review of the Literature. Psychosomatics. 2015;56:700-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Pfeiffer RF. Wilson Disease. Continuum (Minneap Minn). 2016;22:1246-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Członkowska A, Litwin T, Dusek P, Ferenci P, Lutsenko S, Medici V, Rybakowski JK, Weiss KH, Schilsky ML. Wilson disease. Nat Rev Dis Primers. 2018;4:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 548] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 6. | Ferenci P. Whom and how to screen for Wilson disease. Expert Rev Gastroenterol Hepatol. 2014;8:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Behari M, Pardasani V. Genetics of Wilsons disease. Parkinsonism Relat Disord. 2010;16:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Şimşek Papur Ö, Aşık Akman S, Terzioğlu O. Clinical and genetic analysis of pediatric patients with Wilson disease. Turk J Gastroenterol. 2015;26:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Kim EK, Yoo OJ, Song KY, Yoo HW, Choi SY, Cho SW, Hahn SH. Identification of three novel mutations and a high frequency of the Arg778Leu mutation in Korean patients with Wilson disease. Hum Mutat. 1998;11:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Shimizu N, Nakazono H, Takeshita Y, Ikeda C, Fujii H, Watanabe A, Yamaguchi Y, Hemmi H, Shimatake H, Aoki T. Molecular analysis and diagnosis in Japanese patients with Wilson's disease. Pediatr Int. 1999;41:409-413. [PubMed] |
| 11. | Okada T, Shiono Y, Hayashi H, Satoh H, Sawada T, Suzuki A, Takeda Y, Yano M, Michitaka K, Onji M, Mabuchi H. Mutational analysis of ATP7B and genotype-phenotype correlation in Japanese with Wilson's disease. Hum Mutat. 2000;15:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Chen C, Shen B, Xiao JJ, Wu R, Duff Canning SJ, Wang XP. Currently Clinical Views on Genetics of Wilson's Disease. Chin Med J (Engl). 2015;128:1826-1830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Wang LH, Huang YQ, Shang X, Su QX, Xiong F, Yu QY, Lin HP, Wei ZS, Hong MF, Xu XM. Mutation analysis of 73 southern Chinese Wilson's disease patients: identification of 10 novel mutations and its clinical correlation. J Hum Genet. 2011;56:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Gu YH, Kodama H, Du SL, Gu QJ, Sun HJ, Ushijima H. Mutation spectrum and polymorphisms in ATP7B identified on direct sequencing of all exons in Chinese Han and Hui ethnic patients with Wilson's disease. Clin Genet. 2003;64:479-484. [PubMed] |
| 15. | Wei Z, Huang Y, Liu A, Diao S, Yu Q, Peng Z, Hong M. Mutational characterization of ATP7B gene in 103 Wilson's disease patients from Southern China: identification of three novel mutations. Neuroreport. 2014;25:1075-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Bost M, Piguet-Lacroix G, Parant F, Wilson CM. Molecular analysis of Wilson patients: direct sequencing and MLPA analysis in the ATP7B gene and Atox1 and COMMD1 gene analysis. J Trace Elem Med Biol. 2012;26:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 959] [Cited by in RCA: 902] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 18. | Li R, Hoffmeister KM, Falet H. Glycans and the platelet life cycle. Platelets. 2016;27:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Grozovsky R, Giannini S, Falet H, Hoffmeister KM. Regulating billions of blood platelets: glycans and beyond. Blood. 2015;126:1877-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 20. | Donfrid M, Jankovic G, Strahinja R, Colovic R, Begic-Janeva A, Colovic M. Idiopathic thrombocytopenia associated with Wilson's disease. Hepatogastroenterology. 1998;45:1774-1776. [PubMed] |
| 21. | Richards CS, Bale S, Bellissimo DB, Das S, Grody WW, Hegde MR, Lyon E, Ward BE; Molecular Subcommittee of the ACMG Laboratory Quality Assurance Committee. ACMG recommendations for standards for interpretation and reporting of sequence variations: Revisions 2007. Genet Med. 2008;10:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 643] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 22. | Huster D, Kühne A, Bhattacharjee A, Raines L, Jantsch V, Noe J, Schirrmeister W, Sommerer I, Sabri O, Berr F, Mössner J, Stieger B, Caca K, Lutsenko S. Diverse functional properties of Wilson disease ATP7B variants. Gastroenterology. 2012;142:947-956.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Shimizu N, Aoki T. Wilson Disease. In: Oohashi T., Tsukahara H., Ramirez F., Barber C., Otsuka F. (eds) Human Pathobiochemistry. Springer. 2019;133-141. [DOI] [Full Text] |
| 24. | Rao R, Shu S, Han YZ, Chiu YJ, Han YS. A case report: Co-occurrence of Wilson disease and oculocutaneous albinism in a Chinese patient. Medicine (Baltimore). 2018;97:e13744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Ferenci P. Diagnosis of Wilson disease. Handb Clin Neurol. 2017;142:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Zhvania M, Gogberashvili K, Gagoshidze M, Uberi E. Wilson disease with thrombocytopenia (case report). Georgian Med News. 2014;61-64. [PubMed] |
| 27. | Erkan T, Aktuglu C, Gülcan EM, Kutlu T, Cullu F, Apak H, Tümay GT. Wilson disease manifested primarily as amenorrhea and accompanying thrombocytopenia. J Adolesc Health. 2002;31:378-380. [PubMed] |
| 28. | Deng G, Yu S, Li Q, He Y, Liang W, Yu L, Xu D, Sun T, Zhang R, Li Q. Investigation of platelet apoptosis in adult patients with chronic immune thrombocytopenia. Hematology. 2017;22:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Dzieżyc K, Litwin T, Sobańska A, Członkowska A. Symptomatic copper deficiency in three Wilson's disease patients treated with zinc sulphate. Neurol Neurochir Pol. 2014;48:214-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Halfdanarson TR, Kumar N, Li CY, Phyliky RL, Hogan WJ. Hematological manifestations of copper deficiency: a retrospective review. Eur J Haematol. 2008;80:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 31. | Rau AR, Usha M, Mallya P, Rau AT. Cytopenia and Bone Marrow Dysplasia in a Case of Wilson's Disease. Indian J Hematol Blood Transfus. 2014;30:433-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Ludwig RJ, Vanhoorelbeke K, Leypoldt F, Kaya Z, Bieber K, McLachlan SM, Komorowski L, Luo J, Cabral-Marques O, Hammers CM, Lindstrom JM, Lamprecht P, Fischer A, Riemekasten G, Tersteeg C, Sondermann P, Rapoport B, Wandinger KP, Probst C, El Beidaq A, Schmidt E, Verkman A, Manz RA, Nimmerjahn F. Mechanisms of Autoantibody-Induced Pathology. Front Immunol. 2017;8:603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 341] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 33. | Izak M, Bussel JB. Management of thrombocytopenia. F1000Prime Rep. 2014;6:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Neunert CE. Management of newly diagnosed immune thrombocytopenia: can we change outcomes? Blood Adv. 2017;1:2295-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |