Published online Sep 6, 2019. doi: 10.12998/wjcc.v7.i17.2487
Peer-review started: March 28, 2019
First decision: April 18, 2019
Revised: August 1, 2019
Accepted: August 20, 2019
Article in press: August 20, 2019
Published online: September 6, 2019
Processing time: 162 Days and 21.8 Hours
To date, there are no guidelines on the treatment of solid neoplasms in the transplanted kidney. Historically, allograft nephrectomy has been considered the only reasonable option. More recently, nephron-sparing surgery (NSS) and ablative therapy (AT) have been proposed as alternative procedures in selected cases.
To review outcomes of AT for the treatment of renal allograft tumours.
We conducted a systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 Checklist. PubMed was searched in March 2019 without time restrictions for all papers reporting on radiofrequency ablation (RFA), cryoablation (CA), microwave ablation (MWA), high-intensity focused ultrasound (HIFU), and irreversible electroporation (IRE) of solid tumours of the kidney allograft. Only original manuscripts describing actual cases and edited in English were considered. All relevant articles were accessed in full text. Additional searches included all pertinent references. Selected studies were also assessed for methodological quality using a tool based on a modification of the Newcastle Ottawa scale. Data on recipient characteristics, transplant characteristics, disease characteristics, treatment protocols, and treatment outcomes were extracted and analysed. Given the nature and the quality of the studies available (mostly retrospective case reports and small retrospective uncontrolled case series), a descriptive summary was provided.
Twenty-eight relevant studies were selected describing a total of 100 AT procedures in 92 patients. Recipient age at diagnosis ranged from 21 to 71 years whereas time from transplant to diagnosis ranged from 0.1 to 312 mo. Most of the neoplasms were asymptomatic and diagnosed incidentally during imaging carried out for screening purposes or for other clinical reasons. Preferred diagnostic modality was Doppler-ultrasound scan followed by computed tomography scan, and magnetic resonance imaging. Main tumour types were: papillary renal cell carcinoma (RCC) and clear cell RCC. Maximal tumour diameter ranged from 5 to 55 mm. The vast majority of neoplasms were T1a N0 M0 with only 2 lesions staged T1b N0 M0. Neoplasms were managed by RFA (n = 78), CA (n = 15), MWA (n = 3), HIFU (n = 3), and IRE (n = 1). Overall, 3 episodes of primary treatment failure were reported. A single case of recurrence was identified. Follow-up ranged from 1 to 81 mo. No cancer-related deaths were observed. Complication rate was extremely low (mostly < 10%). Graft function remained stable in the majority of recipients. Due to the limited sample size, no clear benefit of a single procedure over the other ones could be demonstrated.
AT for renal allograft neoplasms represents a promising alternative to radical nephrectomy and NSS in carefully selected patients. Properly designed clinical trials are needed to validate this therapeutic approach.
Core tip: Ablative therapy (AT) is a minimally invasive alternative to radical or partial nephrectomy for the treatment of renal allograft tumours. To date, limited data exist regarding long-term efficacy and safety. We performed a systematic review on radiofrequency ablation, cryoablation, microwave ablation, high-intensity focused ultrasound, and irreversible electroporation of neoplasms arising in the transplanted kidney and described treatment-specific and overall outcomes. In the considered cases, AT was successfully offered to all transplant recipients with benign tumours or with American Joint Committee on Cancer T1a N0 M0 renal cell carcinomas of the kidney allograft who were not suitable for more aggressive and demanding surgical treatments.
- Citation: Favi E, Raison N, Ambrogi F, Delbue S, Clementi MC, Lamperti L, Perego M, Bischeri M, Ferraresso M. Systematic review of ablative therapy for the treatment of renal allograft neoplasms. World J Clin Cases 2019; 7(17): 2487-2504
- URL: https://www.wjgnet.com/2307-8960/full/v7/i17/2487.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i17.2487
Kidney transplantation (KTx) is the best therapy for end-stage renal disease[1]. However, due to chronic exposure to immunosuppression, renal transplant recipients have higher incidences of malignancy than the general population[2-4]. Among cancer-related complications, neoplasms involving the allograft are particularly difficult to manage and deserve special consideration[5]. Ideally, optimal therapy should ensure tumour control while preserving as much transplant function as possible. In this complex group of patients, the benefit of complete allograft removal must be carefully weighed against the substantial risk of death arising from renal failure and return to chronic dialysis[5-7].
Like in the non-transplant population, for many years nephrectomy has been considered the gold standard treatment[8]. More recently, recognition of the advantages of nephron-sparing surgery (NSS) and ablative therapy (AT) in native kidneys has led to an increasing use of such alternative options in renal allografts[9-12]. Radiofrequency ablation (RFA), cryoablation (CA), microwave ablation (MWA), high-intensity focused ultrasound (HIFU), and irreversible electroporation (IRE) have all shown promising results in selected cases but solid evidence supporting their role in the management of kidney allograft neoplasms and long-term follow-up data are still missing.
To date, no clinical guidelines, comprehensive meta-analyses, or systematic reviews addressing this topic have been published. The aim of the present study was to systematically review characteristics and outcomes of AT for the treatment of solid masses of the transplanted kidney.
We conducted a systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 Checklist[13]. PubMed was searched in March 2019 for any papers reporting on AT of kidney allograft neoplasms. No time limits were applied. The following key word combinations were used: “thermal ablation”, “kidney ablation”, “renal ablation”, “allograft ablation”, “graft ablation”, “transplant ablation”, “allograft cancer”, “allograft neoplasm”, “allograft tumor”, “allograft mass”, “kidney transplant cancer”, “kidney transplant neoplasm”, “kidney transplant tumor”, “kidney transplant mass”, “renal transplant cancer”, “renal transplant neoplasm”, “renal transplant tumor”, and “renal transplant mass”. Only English manuscripts were considered.
Primary and secondary searches were performed by two independent groups of authors. Disagreements between the two groups were resolved by discussion with the lead author. Duplicate articles were removed. Remainder were screened out reading titles and abstracts. Manuscripts potentially describing cases of AT of kidney allograft tumours were assessed in full text. Only original studies actually reporting on AT of neoplasms in the transplanted kidney were included. Additional search of reference lists was performed. If available, the following data points were collected: recipient ethnicity, recipient gender, recipient age, donor type, donor gender, donor age, induction treatment, maintenance immunosuppression, time from transplant to tumour diagnosis, tumour type, tumour size, tumour histology, Fuhrman grade[14], diagnostic modality, staging modality, American Joint Committee on Cancer (AJCC) Tumour Nodes Metastasis (TNM) classification[15], treatment modality, primary treatment failure, secondary treatment failure, complications, allograft function before and after treatment, recurrence, cancer-specific survival, transplant survival, and length of follow-up. Extracted data were transferred to a dedicated database for analysis purpose.
Selected studies were assessed for methodological quality using a tool based on a modification of the Newcastle Ottawa scale as proposed by Murad et al[16]. As suggested by the authors, questions 4, 5, and 6 of the questionnaire were not considered since they were mostly relevant to cases of adverse drug events. Rather than using an aggregate score, we made an overall judgement considering the questions deemed most critical in the specific clinical scenario. Accordingly, quality of selected studies was classified as low, average or high.
Our review considers a large majority of single case reports and some small case series. No meta-analysis was performed as the small case series are composed of heterogeneous patients making any summary measures meaningless. In order to describe compactly the literature, we reported the number for the categorical variables and the range for the continuous ones. The tables must also be considered as a compact way of describing the results from the literature review. No inferences can be drawn from this study. The statistical methods were assessed by a senior biomedical statistician (Federico Ambrogi, Associate Professor from the Laboratory of Medical Statistics, Biometrics and Epidemiology of the Department of Clinical Sciences and Community Health of the University of Milan).
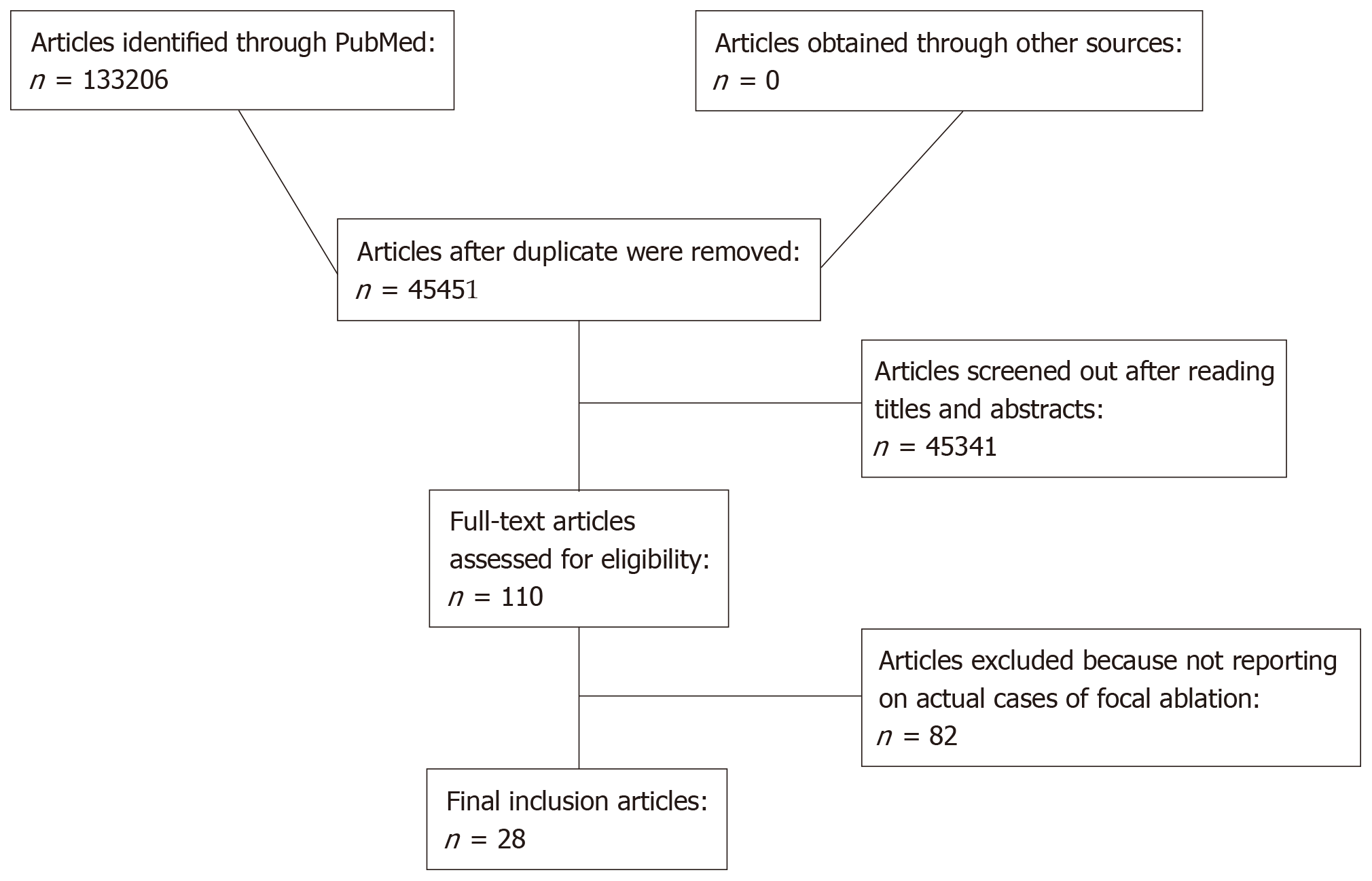
A flow diagram summarizing included articles and selection processes is depicted in Figure 1. The amount of reports preliminarily retrieved using each of the keyword combinations previously mentioned was 133206. More in details: thermal ablation, 5981; kidney ablation, 4130; renal ablation, 4374; allograft ablation, 172; graft ablation, 899; transplant ablation, 4205; allograft cancer, 6749; allograft neoplasm, 5502; allograft tumor, 7726; allograft mass, 1720; kidney transplant cancer, 13171; kidney transplant neoplasm, 11784; kidney transplant tumor, 14121; kidney transplant mass, 5495; renal transplant cancer, 14010; renal transplant neoplasm, 12406; renal transplant tumor, 14896; and renal transplant mass, 5865. After duplicates were removed (n = 87755), a pool of 45451 manuscripts remained for further evaluation. Following the inclusion criteria described above and after reviewing papers by title and abstract, 110 full text articles were identified. Articles not reporting original cases of AT of kidney allograft neoplasms were excluded (n = 82). No additional reports were found through searches of references. Eventually, 28 studies were selected[17-44]. No randomized clinical trials, prospective controlled studies or prospective uncontrolled studies were identified. At the end of the process, we included 12 retrospective case reports, 13 single-centre retrospective uncontrolled case series, 2 multi-centre retrospective uncontrolled case series, and 1 multi-centre retrospective controlled case series. Main characteristics and qualitative evaluations of the studies meeting the criteria for the systematic review are described in Table 1. In total, 100 KTx neoplasms in 92 recipients were treated by 100 primary AT procedures. This included 78 RFA, 15 CA, 3 MWA, 3 HIFU, and 1 IRE.
| Study | Design | Period | Total | Ablation | Ablation | Quality |
| sample size | sample size | technique | ||||
| (P/N) | (P/N) | |||||
| Charboneau et al[18] | R-CR | 2002 | 1/1 | 1/1 | RF | L |
| Shingleton et al[17] | R-CR | 2002 | 1/1 | 1/1 | CA | L |
| Baughman et al[19] | R-CR | 2004 | 1/1 | 1/1 | RF | L |
| Hruby et al[22] | R-CR | 2006 | 1/1 | 1/1 | CA | L |
| Goeman et al[21] | R-CR | 2006 | 1/1 | 1/1 | RF | L |
| Aron et al[23] | R-CR | 2007 | 1/1 | 1/1 | RF | L |
| Matevossian et al[24] | R-CR | 2008 | 1/1 | 1/1 | RF | L |
| Sanchez et al[26] | R-CR | 2009 | 1/1 | 1/1 | RF | L |
| Chakera A et al[27] | R-CR | 2010 | 1/1 | 1/1 | HIFU | L |
| Olivani et al[29] | R-CR | 2011 | 1/1 | 1/1 | RF | L |
| Silvestri et al[36] | R-CR | 2014 | 1/1 | 1/1 | CA | L |
| Christensen et al[38] | R-CR | 2015 | 1/1 | 1/1 | RF | L |
| Roy et al[20] | S-U-R-CS | 2005 | 2/2 | 1/1 | RF | L |
| Veltri et al[25] | S-U-R-CS | 2009 | 3/3 | 3/3 | RF | L |
| Elkentaoui et al[28] | S-U-R-CS | 2010 | 39/42 | 2/2 | RF | L |
| Leveridge et al[31] | S-U-R-CS | 2011 | 47/53 | 3/3 | RF | L |
| Ploussard et al[32] | S-U-R-CS | 2012 | 12/17 | 2/2 | CA | L |
| Swords et al[34] | S-U-R-CS | 2013 | 4/4 | 1/1 | RF | L |
| Vegso et al[35] | S-U-R-CS | 2013 | 9/9 | 5/5 | RF | L |
| Su et al[37] | S-U-R-CS | 2014 | 4/5 | 1/2 | RF | L |
| Hernàndez et al[39] | S-U-R-CS | 2015 | 4/4 | 1/1 | RF | L |
| Cool et al[41] | S-U-R-CS | 2017 | 10/12 | 10/12 | RF | A |
| Iezzi et al[42] | S-U-R-CS | 2018 | 3/3 | 3/3 | RF | L |
| Di Candio et al[43] | S-U-R-CS | 2019 | 3/4 | 3/4 | RF, HIFU | L |
| Gul et al[44] | S-U-R-CS | 2019 | 6/6 | 6/6 | CA, MW, IRE | L |
| Tillou et al[33] | M-U-R-CS | 2012 | 79/79 | 5/5 | RF | A |
| Cornelis et al[30] | M-U-R-CS | 2011 | 20/24 | 20/24 | RF, CA | A |
| Guleryuz et al[40] | M-C-R-CS | 2016 | 92/92 | 14/14 | RF, CA | H |
Only a few articles reported on the total number of KTx performed over the same period in which allograft neoplasms were diagnosed and treated. Consequently, no reliable estimate of cumulative incidence of these tumours could be calculated. Nevertheless, according to Tillou et al[33], incidence and prevalence of KTx neoplasms are 0.19% and 0.5%, respectively. Information regarding donor type, donor gender, donor age, recipient ethnicity, induction treatment, and maintenance immunosuppression were seldom given. Gender details were available for 69/92 (75%) recipients undergoing AT. Data on recipient age were reported in 64/92 (69.6%) patients. Recipient age at the time of tumour diagnosis ranged from 21 to 71 years. Time between transplantation and diagnosis was adequately reported in 48/100 (48%) cases. Time interval range was 0.1-312 mo.
Diagnosis was made by imaging in almost all the lesions. Albeit scarce (38/100, 38%), data showed that neoplasm distribution within the allograft was homogeneous. Tumour appearance was available in 48/100 (48%) cases. Most lesions were endophytic but both exophytic and mixed exo-endophytic masses were described. Size was reported in 73/100 (73%) neoplasms. Maximal tumour diameter ranged from 5 to 55 mm. The majority of the neoplasms showed a maximal diameter inferior to 20 mm. Only 2 cases exceeding 40 mm were reported. Biopsy was obtained for 93/100 (93%) masses. Final pathologist reports demonstrated: Papillary renal cell carcinoma (RCC), clear cell RCC, RCC not otherwise specified, chromophobe RCC, tubulo-papillary RCC, tubulo-cystic RCC, mixed clear cell and papillary RCC, and oncocytoma . Details on Fuhrman grading score were given for 38/100 (38%) neoplasms. Most lesions were grade I or grade II. No cases of locally advanced or metastatic disease were reported. Staging as per AJCC 2010 TNM classification was reported for 93/100 (93%) tumours. The vast majority were T1a N0 M0 with only 2 T1b N0 M0. Clinical presentation was described for 91/100 (91%) neoplasms. Most masses were asymptomatic and were diagnosed during routine ultrasound (US) follow-up or incidentally discovered during investigations carried out for other reasons. Symptoms not necessarily related to the tumour that prompted further assessment included haematuria, allograft dysfunction, abdominal pain, fever, flu-like syndrome, and asthenia. Characteristics of kidney allograft neoplasms treated by AT are summarized in Table 2.
| Variables | Range or number |
| Neoplasms | 100 |
| Imaging-based diagnosis | |
| RCC | 94 |
| Cystic mass | 4 |
| Oncocytoma | 1 |
| Not available | 1 |
| Localization | |
| Interpolar | 16 |
| Lower pole | 12 |
| Upper pole | 10 |
| Not available | 62 |
| Growth pattern | |
| Endophytic | 27 |
| Exophytic | 16 |
| Mixed exo-endophytic | 5 |
| Not available | 52 |
| Size | |
| Maximal diameter (mm) | 5-55 |
| Maximal diameter 0-20 mm | 37 |
| Maximal diameter 21-30 mm | 26 |
| Maximal diameter 31-40 mm | 9 |
| Maximal diameter > 40 mm | 1 |
| Not available | 27 |
| Histology-based diagnosis | |
| Papillary RCC | 48 |
| Clear cell RCC | 20 |
| Chromophobe RCC | 2 |
| Tubulo-papillary RCC | 2 |
| Tubulo-cystic RCC | 1 |
| Mixed clear cell and papillary RCC | 1 |
| RCC not otherwise specified | 17 |
| Oncocytoma | 1 |
| Indeterminate | 1 |
| Not available | 7 |
| Fuhrman grading score | |
| Grade I | 10 |
| Grade II | 24 |
| Grade I-II | 3 |
| Grade III | 1 |
| Not available | 62 |
| AJCC TNM classification | |
| T1a N0 M0 | 91 |
| T1b N0 M0 | 2 |
| Not available | 7 |
| Ablative treatment | |
| RFA | 78 |
| CA | 15 |
| MWA | 3 |
| HIFU | 3) |
| IRE | 1/100 (1) |
Detailed descriptions of diagnostic work up were available in 95/100 (95%) cases. Most lesions were initially detected by Doppler-US scan with or without contrast enhancement. The remaining masses were diagnosed by contrast-enhanced computed tomography (CT) scan, magnetic resonance imaging (MRI) with or without contrast dye, a combination of different imaging modalities or kidney allograft biopsy.
Information regarding staging modality could be retrieved for 49/100 (49%) tumours. Preferred imaging technique was contrast-enhanced CT scan followed by MRI with or without contrast dye and Doppler-US scan with or without contrast enhancement.
Technical details were obtained for 84/100 (84%) ablative treatments. A percutaneous approach was used in most of the procedures. Other access modalities were: Trans-osseous (n = 1), laparoscopic (n = 1), and open (n = 1).
Guidance modality was described for 71/100 (71%) procedures. US-guided and CT-guided procedures were the most frequently reported.
Follow-up protocol was mentioned for 69/100 (69%) treatments. In most cases, a combination of different imaging modalities was used. In one study an US-guided allograft biopsy was also obtained as a part of the routine surveillance program.
Twenty-eight studies describing 100 AT of KTx neoplasms in 92 patients were considered[17-44]. Patients and tumours characteristics as well as technical details of the procedures (access to the allograft and guidance modality) have been described above. Overall, 3 episodes of primary treatment failure (residual tumour present following treatment) were reported. In 2 cases, repeat AT was performed with successful ablation of the lesion. Follow-up range was 1-81 mo. One local recurrence which was successfully treated with further AT was described. One patient developed regional lymph node metastases without local recurrence 4 years after treatment. This recipient died from a cardiovascular event a few months later. No kidney allograft cancer-specific deaths were recorded. Eleven patients experienced peri-operative complications. Transplant function remained stable in the majority of the patients. In 5 recipients, there was a progressive deterioration of function eventually leading to allograft loss. Overall characteristics and outcome of AT are summarized in Table 3.
| Variables | Range or number |
| Procedures | 100 |
| Patients | 92 |
| Interventional access | |
| Percutaneous | 81 |
| Open | 1 |
| Laparoscopic | 1 |
| Transosseous | 1 |
| Not available | 16 |
| Guidance modality | |
| US | 31 |
| CT | 20 |
| MRI | 1 |
| US and CT | 19 |
| Not available | 29 |
| Primary treatment failure | 3 |
| Secondary treatment failure | 1 |
| Recurrence | 1 |
| Disease-specific mortality | 0 |
| Overall renal allograft loss | 5 |
| Peri-operative complication | 11 |
| Urinary leakage | 1 |
| Post-infarction syndrome | 1 |
| Hematoma | 2 |
| Infection of the ablation site | 2 |
| Leg pain due to nerve injury | 4 |
| Leg pain due to muscle injury | 1 |
| Follow-up (mo) | 1-81 |
RFA was by far the most widely used treatment modality with 22 studies reporting on 78 procedures in 70 patients[18-21,23-26,28-31,33-35,37-43]. Recipient age ranged from 21 to 77 years. Histological evaluation was obtained in 73/78 (93.6%) cases and it demonstrated papillary RCC (n = 41), clear cell RCC (n = 10), chromophobe RCC (n = 2), tubulo-papillary RCC (n = 2), tubulo-cystic RCC (n = 1), and RCC not otherwise specified (n = 17). Tumor size ranged from 5 to 40 mm. All lesions were T1a N0 M0. Almost all the procedures were executed percutaneously under US or CT guidance. Overall, 2 cases of primary treatment failures were identified. Both patients underwent repeat RFA obtaining successful tumour ablation. Post-operative follow-up range was 3-71 mo. Local recurrence was observed in one treatment. Recurrent disease was successfully managed by repeat RFA. No cancer-related deaths were recorded. Peri-operative complication rate was less than 15% in all the reports. Complications were: Transient leg pain due to thermal injury to the genitofemoral nerve (n = 3), urinary leakage (n = 1), cruralgia due to thermal injury to the ileopsoas muscle (n = 1), ablative site infection (n = 2), and post-infarction syndrome (n = 1). Renal function remained consistently stable in most cases. However, a few recipients developed irreversible allograft dysfunction. Characteristics and outcome of RFA are summarized in Table 4.
| Variables | Range or Number | |||||
| RFA | CA | HIFU | MWA | IRE | ||
| Procedures | 78 | 15 | 3 | 3 | 1 | |
| Patients | 70 | 15 | 3 | 3 | 1 | |
| Tumor size (mm) | 5-40 | 15-35 | 8-55 | 21-28 | 16 | |
| Tumor histology | ||||||
| Clear cell | 10 | 7 | 1 | 1 | 1 | |
| Papillary | 41 | 3 | 2 | 2 | 0 | |
| Mixed RCC | 0 | 1 | 0 | 0 | 0 | |
| Chromophobe | 2 | 0 | 0 | 0 | 0 | |
| Tubulo-papillary | 2 | 0 | 0 | 0 | 0 | |
| Tubulo-cystic | 1 | 0 | 0 | 0 | 0 | |
| RCC NOS | 17 | 0 | 0 | 0 | 0 | |
| Oncocytoma | 0 | 1 | 0 | 0 | 0 | |
| Indeterminate | 0 | 1 | 0 | 0 | 0 | |
| Not available | 5 | 2 | 0 | 0 | 0 | |
| Interventional access | ||||||
| Percutaneous | 67 | 8 | 3 | 2 | 1 | |
| Open | 0 | 1 | 0 | 0 | 0 | |
| Laparoscopic | 0 | 1 | 0 | 0 | 0 | |
| Transosseous | 0 | 0 | 0 | 1 | 0 | |
| Not available | 11 | 5 | 0 | 0 | 0 | |
| Guidance modality | ||||||
| US | 24 | 4 | 3 | 0 | 0 | |
| CT | 12 | 4 | 0 | 3 | 1 | |
| MRI | 0 | 1 | 0 | 0 | 0 | |
| US and CT | 18 | 1 | 0 | 0 | 0 | |
| Not available | 24 | 5 | 0 | 0 | 0 | |
| Primary treatment failure | 2 | 0 | 1 | 0 | 0 | |
| Re-treatment failure | 0 | - | 1 | - | - | |
| Recurrence | 1 | 0 | 0 | 0 | 0 | |
| Disease-specific mortality | 0 | 0 | 0 | 0 | 0 | |
| Renal allograft loss | 5 | 0 | 0 | 0 | 0 | |
| Complications | 8 | 2 | 0 | 1 | 0 | |
| Follow-up (mo) | 3-71 | 1-59 | 12-81 | 8-61 | 34 | |
CA of a renal allograft neoplasm has been described in 7 studies treating 15 patients[17,22,30,32,36,40,44]. Recipient age at the time of intervention ranged from 35 to 71 years. Histology was available in 13/15 (86.7%) cases. Treated tumours comprised clear cell RCC (n = 7), papillary RCC (n = 3), mixed clear cell and papillary RCC (n = 1), oncocytoma (n = 1), and indeterminate (n = 1). Neoplasm size range was 15-41 mm. One lesion was stage T1b N0 M0 with the reminder T1a N0 M0. Cryoablation was predominantly performed using a percutaneous approach under US or CT guidance. There were no primary treatment failures and no local recurrences after a follow-up ranging from 1 to 59 mo. One patient developed regional lymph node metastasis 4 years after the procedure and died from cardiovascular accident a few months later. No episodes of cancer-related death were recorded. Overall, there were 2 peri-operative complications: An abdominal wall hematoma and a retroperitoneal hematoma requiring surgical drainage. No significant changes in allograft function were noted before and after treatment. Characteristics and outcome of cryoablation are summarized in Table 4.
Three cases of MWA of a KTx tumour were reported. In their retrospective case series, Gul et al[44] described successful treatment of 1 clear cell (28 mm, Fuhrman grade I-II, T1a N0 M0) and 2 papillary (22 mm, Fuhrman grade I-II, T1a N0 M0 and 31 mm, Fuhrman grade II, T1a N0 M0) RCC in 3 transplant recipients. All the procedures (2 percutaneous and 1 transosseous) were carried out under CT guidance and led to complete tumour ablation. During follow-up (range, 8-61 mo), no primary treatment failures or recurrences were observed and graft function remained consistently stable. One of the patients experienced transient leg pain due to thermal injury to the genitofemoral nerve. Characteristics and outcome of MWA are summarized in Table 4.
Two studies reporting on HIFU for the treatment of renal allograft tumours were identified, reporting 3 AT treatments in 3 patients. In the first report, Chakera et al[27] described how they treated a symptomatic 55 mm clear cell RCC (Fuhrman grade II, T1b N0 M0) in a 58-year old recipient. The procedure was performed percutaneously under US guidance with no intra- or post-operative complications. Despite 3 ablations to the lesion, treatment was not successful and the patient required a partial graftectomy. Percutaneous US-guided HIFU was also used by Di Candio et al[43] to ablate two small (22 mm and 8 mm) papillary RCC (T1a N0 M0). Treatment was effective in both cases with no signs of relapse after 6 years of follow-up. Peri-operative course was uneventful and no loss of graft function was observed. Characteristics and outcome of HIFU are summarized in Table 4.
We found only one study describing IRE[44]. The procedure was carried out under CT guidance via a percutaneous approach to treat an asymptomatic 16 mm clear cell RCC (Fuhrman grade III, T1a N0 M0) arising in a 57-year-old patient. There were no intra- or post-operative complications, graft function remained stable over time, and no signs of recurrence were observed after 34 mo of follow-up. Characteristics and outcome of IRE are summarized in Table 4.
An increased susceptibility to primary malignancies and lymphoproliferative disorders is a well-recognized complication of KTx[2-4]. For renal transplant recipients, cancer currently represents the third leading cause of mortality[45] and overall cancer rates as high as 40% at 20 years have been reported[46]. In comparison to the general population, this group of patients have been shown to have higher incidences of non-melanoma skin cancers[47] and RCC[48]. Most renal neoplasms detected after transplant occur in the native kidneys[48]. However, solid tumours involving the allograft are being increasingly identified[5,7]. Reported prevalence of de novo neoplasms in the transplanted kidney is between 0.2% and 0.5%, depending on the series[33,49,50]. The exact incidence of these tumours is difficult to determine because available data predominantly comes from retrospective registry analyses and refer to RCC. As the majority of the studies included in our systematic review did not report the total number of transplants performed during the same time in which the lesions were treated, incidence or prevalence of the disease could not be estimated. Nevertheless, considering the increased use of kidneys from elderly donors[51], the progressive aging of the population on the transplant waiting list[52], the improvement in long-term recipient and graft survival[45], and the widespread application of systematic imaging-based screening protocols during the post-transplant follow-up[49], it is likely that the incidence of kidney allograft tumours will, in the near future, rise considerably.
Specific risk factors for KTx neoplasms have been poorly investigated[33]. It is generally accepted that well established risk factors for RCC in the native kidney may be responsible for carcinogenesis in the renal allograft[53]. Chronic kidney disease, prolonged renal replacement therapy, and long-term immunosuppression also play a role[48,53,54]. Deceased donor recipients seem to be at higher risk than those receiving a kidney from a living donor, representing approximately 90% of the cases[55]. A possible explanation is that deceased donors are generally older than living donors and therefore more prone to develop malignancies. Differences in cancer screening protocols before organ retrieval may also contribute. Tillou et al[55] showed that among affected patients, there was a disproportionate number of men . An association between primary renal disease (i.e. glomerulopathies and uropathies) and kidney allograft neoplasms has been also suggested but evidence remain weak[55]. It has been demonstrated that the majority of renal allograft tumours originate from donor-derived cells. However, whether these neoplasms are de novo transformations or a transplanted disease is often difficult to discriminate[7,56]. Primary RCC of recipient origin has been also reported[57]. Although not routinely undertaken, discerning between donor-derived and recipient-derived neoplasms may have important implications as different cancer behaviours may be expected suggesting tailored therapeutic strategies.
Time interval between transplantation and diagnosis is variable[7,58]. In our review the considered cases showed a range between 0.1 and 312 mo. In contrast to Penn et al[50] it also suggests that the interval can be extremely long. Such a finding not only confirms the importance of age and duration of immunosuppression as risk factors for tumour development[59] but also supports long-term US-based screening protocols after transplantation[49].
It is commonly believed that neoplasms arising in a transplanted kidney are more aggressive than those originating in the native kidney or in patients not exposed to immunosuppression but significant differences in tumour growth dynamics or metastatic behaviours have not been consistently demonstrated[7]. Like in native kidneys, renal allograft neoplasms are generally asymptomatic and mostly discovered at an early stage during routine surveillance imaging studies or diagnostic work up performed for other reasons[60]. Our analysis focused on small KTx tumours treated by AT. The vast majority of lesions considered amenable to focal ablation were asymptomatic. It is reasonable to expect that symptomatic lesions may more often present at an advanced stage thus limiting therapeutic options[7,61]. This observation further underlines the importance of periodic US evaluation of the allograft for the detection of silent KTx neoplasms[60,62].
US scan undoubtedly represents the cornerstone of kidney allograft imaging not only in the early post-transplant phase but also in the long-term[63]. Cost-effectiveness of annual US screening remains debated but many centres worldwide perform US evaluation of the allograft as a part of their routine follow-up. Such a policy allows detecting transplant tumours at a very early stage and therefore increases the possibility of using conservative treatments like NSS and AT. We found that Doppler-US and contrast-enhanced US (CEUS) were the preferred first line imaging modalities for tumour diagnosis[49,55,60]. Lesions with indeterminate characteristics at US were evaluated using CT scan, MRI or a combination of both, mostly with contrast enhancement. In patients with suboptimal renal function, Doppler-US and CEUS offer several advantages as CT scan and MRI may expose the patient to contrast induced nephropathy or nephrogenic systemic sclerosis. Studies comparing CEUS to contrast-enhanced CT scan for the differentiation between benign and malignant renal tumours have shown encouraging results[64]. In particular, CEUS seems to be superior to Doppler-US and contrast-enhanced CT scan in case of complicated cystic lesions or small solid masses[64].
As for any other neoplasms, accurate staging is paramount for proper treatment planning. Since indication for AT is currently limited to small allograft tumours with no signs of local invasiveness or metastatic disease (AJCC T1a N0 M0 and T1b N0 M0), careful pre-operative evaluation is mandatory. Overall, there is a lack of information regarding staging work up of renal allograft tumours but our review showed that lesions were frequently assessed by contrast-enhanced CT scan or a combination of contrast-enhanced CT scan and MRI with or without contrast dye.
Like tumours arising in the native kidney, renal allograft neoplasms can be benign or malignant. Four main variants could be identified: Clear cell RCC, papillary RCC, chromophobe RCC, and oncocytoma. Clear cell type and papillary carcinomas represent most of the cases. Clear cell type is more often unifocal and aggressive whereas papillary type is generally indolent and multifocal[20,65]. A disproportionate number of papillary carcinoma over clear cell carcinoma has been noticed in kidney allografts compared to native kidneys[33]. In our review papillary type was the most represented lesion treated with AT. This is an important epidemiological data with relevant clinical implications. Due to its multifocality, for many years papillary carcinoma has been considered as an indication for radical nephrectomy or graftectomy. More recently, positive outcomes obtained with NSS have cautiously allowed to extend indications for conservative therapeutic options also to patients with papillary carcinoma[66,67]. We detected several reports in the literature describing successful ablation of papillary lesions in the renal allograft[18,20,23,25,30,38-41,43,44]. Therefore our review further supports the use of AT in recipients with papillary carcinoma as current literature showed similar primary treatment failure, secondary treatment failure, recurrence, graft survival, and disease-free survival for papillary, clear cell, and chromophobe RCC.
Pre-operative histology is crucial for the assessment of solid masses in the transplanted kidney as it provides information on tumour type, grading, and origin thus helping clinicians choose the best therapy. Proper characterization of renal allograft neoplasms also allows to obtain important epidemiological and clinical data that may favour the construction of specific tumour registries and the analysis of specific treatment outcomes. Given the fact that renal allografts are generally located in the iliac fossa, in the retroperitoneum, and in a relatively superficial position, US-guided biopsy is considered the procedure of choice[68].
Current treatment of KTx tumours mostly reflects the evolution observed in the management of neoplasms of the native kidney. Historically, radical nephrectomy and graftectomy have been considered the only acceptable options. Over years, improvements in surgical technique and peri-operative care as well as a better understanding of cancer biology and behaviour have progressively favoured the use of nephron sparing strategies. Current evidence show that for RCC up to 4 cm in maximal diameter, cancer-specific survival at 5 years is 95%[7,69]. The risk of death arising from transplantectomy and return to dialysis is much higher with a reported 5-year survival rate of 34%[6]. Therefore, radical nephrectomy and transplantectomy are now indicated only for malignant lesions with features of advanced local (AJCC stage III) or metastatic (AJCC stage IV) disease, in case of large masses exceeding 7 cm (AJCC stage II), for sarcomatoid RCC, for lesions infiltrating the hilum, and in patients with irreversible kidney failure or a non-functioning allograft. For the remainder (mostly AJCC stage I T1a N0 M0), NSS represents the most widely used therapeutic option. NSS includes several procedures such as enucleation, wedge resection, and polar resection. After partial nephrectomy, excellent oncological outcomes can be achieved with resection margins of 5 mm and for T1a lesions, local recurrence rates of less than 5% have been reported. The risk of new cancer development in the resected kidney is also minimal (< 5%)[7] and successful reoperation with allograft salvage in case of local recurrence or metachronous disease has been described[70]. Given the technical difficulty of the procedures and the risk of intra-operative and post-operative complications, perfect candidates for NSS are relatively healthy uninephric or chronic kidney disease subjects with a small unifocal tumour located in a favourable position[8]. Such an ideal patient is seldom encountered among KTx recipients as most of them present with advanced age, complex comorbidities, increased risk of infection, and impaired healing response. Decise adhesions from previous operation, short vascular pedicles, and increased tissue fragility may represent a surgical challenge in case of NSS limiting the chances of organ mobilization, vasculature control, and adequate tumour resection[9].
In the last decade, for non-transplant patients with contraindications to general anaesthesia, high surgical risk, or in a need for maximum renal function preservation, AT have been increasingly recognized as a valuable alternative to NSS. Compared to NSS, focal ablation offers several advantages: It can be performed under local anaesthesia, is less invasive, allows to spare more renal parenchyma, does not require vascular clamping, and has minimal or absent blood loss. Initial experience showed comparable mid-term oncological outcomes, lower complication rates, and better preservation of renal function than radical nephrectomy and NSS[71]. These encouraging results have favoured the acceptance of AT also in the transplant community. Moreover, specific characteristics of the renal allograft such as superficial location and greater susceptibility to ischemia-reperfusion injury in case of vascular occlusion, make it even more suitable for ablation than the native kidney.
Available AT for the treatment of renal allograft neoplasms are RFA, CA, MWA, HIFU, and IRE. To date, RFA is the most widely used AT in both native and transplanted kidney (78% of the procedures included in our review). It utilizes a high frequency, alternating electrical current to generate heat in the target lesion. The current is transmitted to the tissues through an electrode with a non-insulated tip. Cellular and extracellular ions in which the current flows are forced to follow the same path as the alternating current determining agitation and frictional heating eventually leading to coagulative necrosis. This procedure is particularly indicated for small exophytic lesions distant from the hilum[72,73]. CA is the second most frequently used AT (15% in our analysis) and basically utilizes argon gas to freeze and damage the tumour. Cooled and thermally conductive fluids are transmitted to the lesion via hollow needles. Once the probes are in place, a cryogenic freezing unit removes heat from the target causing ice crystal formation, membrane disruption, cell lysis, apoptosis, and ischemic necrosis due to intravascular coagulation. A theoretical advantage of CA over other AT is the greater selectiveness and therefore the possibility to safely treat parenchymal lesions located in critical areas of the organ[73]. Overall experience with MWA in kidney allografts is limited (3% of the cases). It is a thermal ablation modality using microwaves to generate oscillation of polar molecules within the target tissues with subsequent frictional heating and coagulative necrosis. Since multiple applicators can be utilized simultaneously, MWA allows to treat larger lesions compared to other AT and also to ablate several masses during the same procedure[73]. HIFU represents another thermal ablation modality more recently introduced in clinical practice. Available reports in KTx neoplasms are still scarce (3% of the procedures) but results in native kidneys are promising[74]. This AT uses multiple US beams converging into a focus to produce inertial cavitation, micro streaming, and radiation forces eventually causing localized heating and coagulative necrosis. HIFU does not require needles or probes and as such represents the least invasive technique currently available. It is also extremely selective with an excellent safety profile. The need for adequate acoustic windows to successfully operate remains the major limitation of the technique. Nevertheless, such an issue may be more theoretical than practical in the case of renal allograft lesions as transplanted kidneys are almost always in a superficial plane, relatively distant from sensible organs, and not surrounded by adipose tissue[75,76]. IRE is a non-thermal AT utilizing electrical pulses to generate nanopores in the cell membrane thus leading to irreversible disruption of cell homeostasis and apoptosis[77]. Only one report could be identified in the transplant setting (1%).
As previously mentioned, in our review, papillary RCC was the most frequent neoplasms treated by AT. The outcomes were overall similar for patients with different tumour types. The vast majority of these lesions were less than 4 cm in maximal diameter and staged T1a N0 M0. It is worth noticing that among the 2 tumours exceeding 4 cm (T1b N0 M0), one could not be successfully treated by AT and required partial nephrectomy to achieve complete removal. Available data showed that neoplasms were mostly Fuhrman grade I or II but information on grading score were overall insufficient for proper inferential analysis. Multifocality and unifocality were also seldom reported. Most lesions treated were actually endophytic. This is particularly interesting because focal ablation has been more often restricted to exophytic masses. One of the arguments in favour of AT over radical nephrectomy and NSS is that it is less invasive. Almost all the procedures included in our review were performed using a percutaneous approach. Moreover, patients were mostly treated under local anaesthesia, there were no intra-operative complications, and hospital stay was mostly less than 3 d. Post-operative complication rate was also reassuring with only 2 cases requiring further surgical intervention. Allograft function was preserved in the vast majority of the patients. These results show that AT is safely offered to elderly and frail transplant recipients who may not be suitable for more demanding surgical procedures. It can also offer a valuable alternative to active surveillance in carefully selected candidates[71]. We found that preferred guidance modalities were Doppler-US and CEUS. CEUS has been increasingly recognized as the technique of choice for percutaneous interventional procedures. It can be easily utilized for diagnostic, guidance, and follow-up purposes. There is no exposure to ionizing radiations and particularly in patients with impaired renal function, it has also the advantage of avoiding the administration of toxic contrast agents[64]. Overall, in our review AT was effective and safe. Only 3 primary treatment failures were reported with a single episode of local recurrence. Persisting and relapsing tumours could be all treated with further AT or NSS with excellent oncological outcomes and preservation of allograft function. No cancer-related deaths were observed. Whilst limited by the lack of long-term follow-up these reports are encouraging and suggest that AT can be considered a valuable alternative to radical nephrectomy and NSS not only for critically ill patients but also for the majority of the recipients with a renal allograft neoplasms staged T1a N0 M0. Comparison with NSS supports this point of view. After partial graftectomy, an overall recurrence rate as high as 9% has been reported with higher rates of severe post-operative complications and allograft dysfunction (15%)[7-9,55].
Despite the numerous advantages, AT has some limitations. First of all, percutaneous procedures do not allow to obtain definitive histological diagnosis and staging of the lesion treated. There is also the possibility that pre-operative imaging and ablation itself may miss very small satellite lesions or multifocal neoplasms. Finally, which is the optimal strategy for the assessment of complete tumour ablation and the detection of local recurrence remains debated[78]. In our review, follow-up modalities were very heterogeneous among transplant centres. In most cases, multiple imaging techniques such as US, CT scan, and MRI were used. Such an observation confirms the difficulty in discriminating between necrosis, vital parenchyma, and neoplastic tissue and underlines the importance of strict and diligent surveillance strategies after AT. Especially in difficult cases, protocol ablation site biopsy may help rule out the presence of residual tumours or local recurrences and prompt timely and effective treatment[23].
Details on post-ablation immunosuppression were not routinely reported in the studies included in our review. To date, there is no consensus regarding the best immunosuppressive strategy for cancer prevention and control after KTx. In case of tumours amenable of NSS or AT, the use of a proliferation signal inhibitor such as sirolimus or everolimus may be considered but evidence are still limited[79-81].
To the best of our knowledge, this is the first systematic review on AT for the treatment of renal allograft neoplasms. All principal focal ablation techniques were explored describing treatment-specific and overall outcomes. The main limitation of the review is the impossibility to perform any sort of meta-analysis due to the small case series considered with heterogeneous patients. Nevertheless, our work offers a comprehensive and updated reference which may provide the basis for further studies and help clinicians counselling their patients. In order to improve the quality of further research, systematic use of proper ablation terminology and current reporting standards is recommended[82-84]. As suggested by Su et al[37], patients and neoplasms should be also stratified according to the R.E.N.A.L. nephrometry scoring system, probably the most appropriate tool to describe tumours arising in the transplanted kidney[85].
Results of AT are overall encouraging but long-term follow-up data remain limited. AT is generally offered to all transplant recipients with benign neoplasms or AJCC stage I T1a N0 M0 RCC of the kidney allograft who are not suitable for more aggressive and demanding surgical treatments. The inability to obtain definitive histological diagnosis represents the main limitation of AT. Therefore, strict and diligent follow-up strategies are mandatory. All the AT modalities currently available can be considered a valuable option but tailored treatment may help achieve the best outcomes. Properly designed prospective randomized clinical trials are needed.
Kidney allograft tumours represent a challenging complication of renal transplantation. Optimal treatment should ensure adequate oncological results while preserving as much renal function as possible. For many years, graftectomy has been considered the gold standard. In the last decade, improved surgical techniques and technological advances have favoured the use of nephron-sparing alternatives such as partial nephrectomy and focal ablation. Preliminary reports on ablation treatment of solid masses of the kidney allograft have shown promising results. However, solid evidence supporting widespread application of ablation therapy are lacking and there is still concern in the transplant community regarding efficacy and safety in the long term. To date, no guideline, meta-analysis or systematic review on the topic have been published.
The rarity of the disease and the multiple options available (radiofrequency ablation, cryoablation, microwave ablation, high-intensity focused ultrasound, and irreversible electroporation) make it extremely difficult to assess results of ablation therapy for the treatment of kidney allograft neoplasms. A better insight into this complex topic would help clinicians choose the best treatment and provide the basis for further research.
We performed a systematic review of ablation therapy for the treatment of solid neoplasms of the transplanted kidney. All major ablation techniques were considered. Overall and treatment-specific outcomes were extensively reported in order to offer a comprahensive overview on currently availbale data and remark the need for properly designed clinical trials.
We conducted a systematic review according to the PRISMA 2009 Checklist. PubMed was extensively searched in March 2019 for any papers reporting on ablation therapy of kidney allograft neoplasms. Only English manuscripts were considered. No time limits were applied. Multiple key word combinations were used. Duplicate articles were removed. Remainder were screened out reading titles and abstracts. Manuscripts potentially describing cases of ablation of kidney allograft tumours were assessed in full text. Only original studies reporting on actual cases of ablative treatment of neoplasms in the transplanted kidney were included. Additional search of reference lists was performed. Selected studies were also assessed for methodological quality using a tool based on a modification of the Newcastle Ottawa scale. Data were extracted and transferred to a dedicated database for analysis purpose. Given the nature of the studies and the large heterogeneity of the patients included, we decided not to meta-analyze data. To the best of our knowledge, this is the first systematic review on the topic.
Preliminary search identified 133206 articles. After duplicate were removed (n = 87755), a pool of 45451 manuscripts remained for further evaluation. Reviewing articles by title and abstract, 110 full text papers were selected. Articles not reporting on original cases of ablation of kidney allograft tumours were excluded (n = 82). No additional cases were found through references lists. At the end of the process, 28 studies were included in the systematic review: 12 retrospective case reports, 13 single-centre retrospective non-comparative case series, 2 multi-centre retrospective non-comparative case series, and 1 multi-centre retrospective comparative study. In total, 100 kidney transplant neoplasms in 92 recipients were treated by 100 ablation procedures: 78 radiofrequency ablation, 15 cryoablation, 3 microwave ablation, 3 high-intensity focused ultrasound, and 1 irreversible electroporation. According to our review, incidence of renal allograft neoplasms is approximately 0.2%. Recipient age at the time of tumour diagnosis ranged from 21 to 71 years whereas time between transplant and diagnosis ranged from 0.1 to 312 mo. Most represented lesions were papillary and clear cell renal cell carcinomas. Considered neoplasms were more often endophytic with a maximal tumour diameter ranging from 5 to 55 mm. The vast majority were asymptomatic masses staged T1a N0 M0. Ablation was predominantly performed using a percutaneous route under ultrasound or computed tomography guidance. Overall, retrieved reports showed that ablation therapy was effective with only 3 episodes of primary tratment failures and 1 episode of local recurrence. Safety was also satisfactory. There were no intra-operative complications or cancer-related deaths. Post-operative complications were rare and allograft function was preserved in most of the recipients. Follow-up range was 1-81 mo.
Our systematic review, shows that ablation therapy has been increasingly used as an effective and safe alternative to graftectomy and nephron-sparing surgery in carefully selected recipients with kidney allograft neoplasms. In particular, ablation was successfully offered to all patients with benign tumours or with T1a N0 M0 malignant lesions not suitable for more demanding surgical procedures. Main advantages of ablation therapy were easy feasibility, mini-invasiveness, short intra-operative time, reduced risk of bleeding, low post-operative complication rate, preserved allograft function, and short hospital stay. The inability to obtain definitive histological diagnosis and the difficult follow-up represented the main limitations of ablation therapy.
Overall results of ablation therapy are encouraging but there is still a lack of long-term efficacy and safety data. Current evidence do not allow to safely extend indications to more advanced cancer stages. Radiofrequency ablation is the most widely used ablative modality. Proper comparison between different ablation therapies is limited by the small experience gained with cryoablation, microwave ablation, high-intensity focused ultrasound, and irreversible electroporation. In theory, tailored treatments might help achieve the best outcomes. Properly designed multi-centre prospective randomized clinical trials are needed.
We would like to thank Paola Martino for logistics support.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Trkulja V, Zhang ZH S-Editor: Wang JL L-Editor: Wang TQ E-Editor: Qi LL
| 1. | Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Birkeland SA, Løkkegaard H, Storm HH. Cancer risk in patients on dialysis and after renal transplantation. Lancet. 2000;355:1886-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 222] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Engels EA, Pfeiffer RM, Fraumeni JF, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1094] [Article Influence: 78.1] [Reference Citation Analysis (0)] |
| 4. | Au EH, Chapman JR, Craig JC, Lim WH, Teixeira-Pinto A, Ullah S, McDonald S, Wong G. Overall and Site-Specific Cancer Mortality in Patients on Dialysis and after Kidney Transplant. J Am Soc Nephrol. 2019;30:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 5. | Griffith JJ, Amin KA, Waingankar N, Lerner SM, Delaney V, Ames SA, Badani K, Palese MA, Mehrazin R. Solid Renal Masses in Transplanted Allograft Kidneys: A Closer Look at the Epidemiology and Management. Am J Transplant. 2017;17:2775-2781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Rao PS, Schaubel DE, Jia X, Li S, Port FK, Saran R. Survival on dialysis post-kidney transplant failure: results from the Scientific Registry of Transplant Recipients. Am J Kidney Dis. 2007;49:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Diller R, Senninger N. Treatment options and outcome for renal cell tumors in the transplanted kidney. Int J Artif Organs. 2008;31:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Chambade D, Meria P, Tariel E, Vérine J, De Kerviler E, Peraldi MN, Glotz D, Desgrandchamps F, Mongiat-Artus P. Nephron sparing surgery is a feasible and efficient treatment of T1a renal cell carcinoma in kidney transplant: a prospective series from a single center. J Urol. 2008;180:2106-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Barama A, St-Louis G, Nicolet V, Hadjeres R, Daloze P. Renal cell carcinoma in kidney allografts: a case series from a single center. Am J Transplant. 2005;5:3015-3018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Olweny EO, Park SK, Tan YK, Best SL, Trimmer C, Cadeddu JA. Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol. 2012;61:1156-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 11. | Thompson RH, Atwell T, Schmit G, Lohse CM, Kurup AN, Weisbrod A, Psutka SP, Stewart SB, Callstrom MR, Cheville JC, Boorjian SA, Leibovich BC. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol. 2015;67:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 305] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 12. | Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, Chang A, Clark PE, Davis BJ, Derweesh IH, Giambarresi L, Gervais DA, Hu SL, Lane BR, Leibovich BC, Pierorazio PM. Renal Mass and Localized Renal Cancer: AUA Guideline. J Urol. 2017;198:520-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 870] [Article Influence: 108.8] [Reference Citation Analysis (0)] |
| 13. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269, W64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21613] [Cited by in RCA: 18174] [Article Influence: 1135.9] [Reference Citation Analysis (0)] |
| 14. | Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655-663. [PubMed] |
| 15. | Williamson SR, Taneja K, Cheng L. Renal cell carcinoma staging: pitfalls, challenges, and updates. Histopathology. 2019;74:18-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60-63. [PubMed] [DOI] [Full Text] |
| 17. | Shingleton WB, Sewell PE. Percutaneous cryoablation of renal cell carcinoma in a transplanted kidney. BJU Int. 2002;90:137-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Charboneau JW, O'Loughlin MT, Milliner DS, Engen DE. Sonographically guided percutaneous radio frequency ablation of a renal cell carcinoma in a transplanted kidney. J Ultrasound Med. 2002;21:1299-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Baughman SM, Sexton WJ, Glanton CW, Dalrymple NC, Bishoff JT. Computerized tomography guided radio frequency ablation of a renal cell carcinoma within a renal allograft. J Urol. 2004;172:1262-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Roy C, El Ghali S, Buy X, Lindner V, Gangi A. Papillary renal cell carcinoma in allograft kidney. Eur Radiol. 2005;15:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Goeman L, Joniau S, Oyen R, Van Poppel H. Percutaneous ultrasound-guided radiofrequency ablation of recurrent renal cell carcinoma in renal allograft after partial nephrectomy. Urology. 2006;67:199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Hruby GW, Fine JK, Landman J. Ultrasound-guided percutaneous ablation of a renal mass in a renal allograft. Urology. 2006;68:891.e5-891.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Aron M, Hegarty NJ, Remer E, O'Malley C, Goldfarb D, Kaouk JH. Percutaneous radiofrequency ablation of tumor in transplanted kidney. Urology. 2007;69:778.e5-778.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Matevossian E, Novotny A, Vogelsang B, Mehler J, Stangl M, Thorban S, Dobritz M. Noninvasive therapy of incidental de novo renal cell carcinoma in a kidney allograft 12 years after transplantation: report of a case and review of literature. Transplant Proc. 2008;40:915-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Veltri A, Grosso M, Castagneri F, Garetto I, Sacchetto P, Tosetti I, Stratta P, Terrone C, Fava C. Radiofrequency thermal ablation of small tumors in transplanted kidneys: an evolving nephron-sparing option. J Vasc Interv Radiol. 2009;20:674-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Sanchez K, Barr RG. Contrast-enhanced ultrasound detection and treatment guidance in a renal transplant patient with renal cell carcinoma. Ultrasound Q. 2009;25:171-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Chakera A, Leslie T, Roberts I, O'Callaghan CA, Cranston D. A lucky fall? Case report. Transplant Proc. 2010;42:3883-3886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Elkentaoui H, Robert G, Pasticier G, Bernhard JC, Couzi L, Merville P, Ravaud A, Ballanger P, Ferrière JM, Wallerand H. Therapeutic management of de novo urological malignancy in renal transplant recipients: the experience of the French Department of Urology and Kidney Transplantation from Bordeaux. Urology. 2010;75:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Olivani A, Iaria M, Missale G, Capocasale E, Biasini E, Mazzoni MP, Lombardelli L, Luzi E, Frattini A, Pelosi G. Percutaneous ultrasound-guided radiofrequency ablation of an allograft renal cell carcinoma: a case report. Transplant Proc. 2011;43:3997-3999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Cornelis F, Buy X, André M, Oyen R, Bouffard-Vercelli J, Blandino A, Auriol J, Correas JM, Pluvinage A, Freeman S, Solomon SB, Grenier N. De novo renal tumors arising in kidney transplants: midterm outcome after percutaneous thermal ablation. Radiology. 2011;260:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Leveridge M, Musquera M, Evans A, Cardella C, Pei Y, Jewett M, Robinette M, Finelli A. Renal cell carcinoma in the native and allograft kidneys of renal transplant recipients. J Urol. 2011;186:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Ploussard G, Chambade D, Meria P, Gaudez F, Tariel E, Verine J, De Bazelaire C, Peraldi MN, Glotz D, Desgrandchamps F, Mongiat-Artus P. Biopsy-confirmed de novo renal cell carcinoma (RCC) in renal grafts: a single-centre management experience in a 2396 recipient cohort. BJU Int. 2012;109:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Tillou X, Doerfler A, Collon S, Kleinclauss F, Patard JJ, Badet L, Barrou B, Audet M, Bensadoun H, Berthoux E, Bigot P, Boutin JM, Bouzguenda Y, Chambade D, Codas R, Dantal J, Deturmeny J, Devonec M, Dugardin F, Ferrière JM, Erauso A, Feuillu B, Gigante M, Guy L, Karam G, Lebret T, Neuzillet Y, Legendre C, Perez T, Rerolle JP, Salomon L, Sallusto F, Sénéchal C, Terrier N, Thuret R, Verhoest G, Petit J; “Comité de Transplantation de l’Association Française d’Urologie (CTAFU)”. De novo kidney graft tumors: results from a multicentric retrospective national study. Am J Transplant. 2012;12:3308-3315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 34. | Swords DC, Al-Geizawi SM, Farney AC, Rogers J, Burkart JM, Assimos DG, Stratta RJ. Treatment options for renal cell carcinoma in renal allografts: a case series from a single institution. Clin Transplant. 2013;27:E199-E205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Végső G, Toronyi É, Deák PÁ, Doros A, Langer RM. Detection and management of renal cell carcinoma in the renal allograft. Int Urol Nephrol. 2013;45:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Silvestri T, Stacul F, Bertolotto M, Artero M, Siracusano S. Percutaneous cryoablation of a renal cell carcinoma in a transplanted kidney. Can J Urol. 2014;21:7390-7392. [PubMed] |
| 37. | Su MZ, Campbell NA, Lau HM. Management of renal masses in transplant allografts at an Australian kidney-pancreas transplant unit. Transplantation. 2014;97:654-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Christensen SF, Hansen JM. Donor Kidney With Renal Cell Carcinoma Successfully Treated With Radiofrequency Ablation: A Case Report. Transplant Proc. 2015;47:3031-3033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Hernández-Socorro CR, Henríquez-Palop F, Santana-Toledo L, Gallego-Samper R, Rodríguez-Pérez JC. Radiofrequency ablation as an alternative therapy for renal neoplasms in graft recipients. A preliminary study. Nefrologia. 2015;35:514-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 40. | Guleryuz K, Doerfler A, Codas R, Coffin G, Hubert J, Lechevallier E, Tillou X; Renal Transplantation Committee of the French Urological Association (CTAFU). A national study of kidney graft tumor treatments: Toward ablative therapy. Surgery. 2016;160:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Cool DW, Kachura JR. Radiofrequency Ablation of T1a Renal Cell Carcinomas within Renal Transplant Allografts: Oncologic Outcomes and Graft Viability. J Vasc Interv Radiol. 2017;28:1658-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Iezzi R, Posa A, Romagnoli J, Salerno MP, Carchesio F, Veltri G, Spagnoletti G, Citterio F, Manfredi R. Radiofrequency thermal ablation of renal graft neoplasms: Case series and literature review. Clin Transplant. 2018;32:e13432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Di Candio G, Porcelli F, Campatelli A, Guadagni S, Vistoli F, Morelli L. High-Intensity Focused Ultrasonography and Radiofrequency Ablation of Renal Cell Carcinoma Arisen in Transplanted Kidneys: Single-Center Experience With Long-Term Follow-Up and Review of Literature. J Ultrasound Med. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Gul ZG, Griffith JJ, Welch C, Fischman A, Palese MA, Badani KK, Mehrazin R. Focal Ablative Therapy for Renal Cell Carcinoma in Transplant Allograft Kidneys. Urology. 2019;125:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Favi E, Salerno MP, Romagnoli J, Castagneto M, Citterio F. Significant improvement in patient survival after renal transplantation in the last decade. Transplant Proc. 2011;43:285-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Lutz J, Heemann U. Tumours after kidney transplantation. Curr Opin Urol. 2003;13:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 47. | Fania L, Abeni D, Esposito I, Spagnoletti G, Citterio F, Romagnoli J, Castriota M, Ricci F, Moro F, Perino F, Mazzanti C, De Simone C, Peris K. Behavioural and demographic factors associated with occurrence of non-melanoma skin cancer in organ transplant recipients. G Ital Dermatol Venereol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. 2005;80:S254-S264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 412] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 49. | Rouprêt M, Peraldi MN, Thaunat O, Chrétien Y, Thiounn N, Dufour B, Kreis H, Méjean A. Renal cell carcinoma of the grafted kidney: how to improve screening and graft tracking. Transplantation. 2004;77:146-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Penn I. Primary kidney tumors before and after renal transplantation. Transplantation. 1995;59:480-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 195] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 51. | Pérez-Sáez MJ, Montero N, Redondo-Pachón D, Crespo M, Pascual J. Strategies for an Expanded Use of Kidneys From Elderly Donors. Transplantation. 2017;101:727-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 52. | Roodnat JI, Zietse R, Mulder PG, Rischen-Vos J, van Gelder T, IJzermans JN, Weimar W. The vanishing importance of age in renal transplantation. Transplantation. 1999;67:576-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1056] [Cited by in RCA: 997] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 54. | Sassa N, Hattori R, Tsuzuki T, Watarai Y, Fukatsu A, Katsuno S, Nishikimi T, Fujita T, Ohmae K, Gotoh M. Renal cell carcinomas in haemodialysis patients: does haemodialysis duration influence pathological cell types and prognosis? Nephrol Dial Transplant. 2011;26:1677-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Tillou X, Guleryuz K, Collon S, Doerfler A. Renal cell carcinoma in functional renal graft: Toward ablative treatments. Transplant Rev (Orlando). 2016;30:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Desai R, Collett D, Watson CJ, Johnson P, Evans T, Neuberger J. Cancer transmission from organ donors-unavoidable but low risk. Transplantation. 2012;94:1200-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 57. | Boix R, Sanz C, Mora M, Quer A, Beyer K, Musulen E, González C, Bayona S, Saladié JM, Ariza A. Primary renal cell carcinoma in a transplanted kidney: genetic evidence of recipient origin. Transplantation. 2009;87:1057-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Walton TJ, McCulloch TA, Bishop MC. Aggressive renal cell carcinoma in a 27-year-old kidney transplant. Nephrol Dial Transplant. 2005;20:1018-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Sprangers B, Nair V, Launay-Vacher V, Riella LV, Jhaveri KD. Risk factors associated with post-kidney transplant malignancies: an article from the Cancer-Kidney International Network. Clin Kidney J. 2018;11:315-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 60. | Viart L, Surga N, Collon S, Jaureguy M, Elalouf V, Tillou X. The high rate of de novo graft carcinomas in renal transplant recipients. Am J Nephrol. 2013;37:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 61. | Patard JJ, Leray E, Rodriguez A, Rioux-Leclercq N, Guillé F, Lobel B. Correlation between symptom graduation, tumor characteristics and survival in renal cell carcinoma. Eur Urol. 2003;44:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 62. | Moudouni SM, Tligui M, Doublet JD, Haab F, Gattegno B, Thibault P. Nephron-sparing surgery for de novo renal cell carcinoma in allograft kidneys. Transplantation. 2005;80:865-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Sharfuddin A. Renal relevant radiology: imaging in kidney transplantation. Clin J Am Soc Nephrol. 2014;9:416-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Tranquart F, Correas JM, Martegani A, Greppi B, Bokor D. [Feasability of real time contrast enhanced ultrasound in renal disease]. J Radiol. 2004;85:31-36. [PubMed] |
| 65. | Delahunt B, Eble JN. Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol. 1997;10:537-544. [PubMed] |
| 66. | Mejean A, Hopirtean V, Bazin JP, Larousserie F, Benoit H, Chrétien Y, Thiounn N, Dufour B. Prognostic factors for the survival of patients with papillary renal cell carcinoma: meaning of histological typing and multifocality. J Urol. 2003;170:764-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 67. | Tillou X, Guleryuz K, Doerfler A, Bensadoun H, Chambade D, Codas R, Devonec M, Dugardin F, Erauso A, Hubert J, Karam G, Salomon L, Sénéchal C, Salusto F, Terrier N, Timsit MO, Thuret R, Verhoest G, Kleinclauss F; members of the Renal Transplantation Committee of the French Urological Association (CTAFU). Nephron sparing surgery for De Novo kidney graft tumor: results from a multicenter national study. Am J Transplant. 2014;14:2120-2125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 68. | Preda A, Van Dijk LC, Van Oostaijen JA, Pattynama PM. Complication rate and diagnostic yield of 515 consecutive ultrasound-guided biopsies of renal allografts and native kidneys using a 14-gauge Biopty gun. Eur Radiol. 2003;13:527-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 69. | Campbell S, Lane BR, Wein AJ, Kavoussi LR, Campbell MF. Malignant renal tumors. Wein AJ, Kavoussi LR, Campbell MF. Campbell-Walsh Urology. Philadelphia: Elsevier Saunders 2012; 1412-1474. |
| 70. | Johnson A, Sudarshan S, Liu J, Linehan WM, Pinto PA, Bratslavsky G. Feasibility and outcomes of repeat partial nephrectomy. J Urol. 2008;180:89-93; discussion 93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Sanchez A, Feldman AS, Hakimi AA. Current Management of Small Renal Masses, Including Patient Selection, Renal Tumor Biopsy, Active Surveillance, and Thermal Ablation. J Clin Oncol. 2018;JCO2018792341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 72. | Lui KW, Gervais DA, Arellano RA, Mueller PR. Radiofrequency ablation of renal cell carcinoma. Clin Radiol. 2003;58:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 73. | Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee FT, Brace CL. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation--what should you use and why? Radiographics. 2014;34:1344-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 74. | Ritchie RW, Leslie T, Phillips R, Wu F, Illing R, ter Haar G, Protheroe A, Cranston D. Extracorporeal high intensity focused ultrasound for renal tumours: a 3-year follow-up. BJU Int. 2010;106:1004-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 75. | Zhou YF. High intensity focused ultrasound in clinical tumor ablation. World J Clin Oncol. 2011;2:8-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 466] [Cited by in RCA: 333] [Article Influence: 23.8] [Reference Citation Analysis (3)] |
| 76. | Al-Bataineh O, Jenne J, Huber P. Clinical and future applications of high intensity focused ultrasound in cancer. Cancer Treat Rev. 2012;38:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 77. | Thomson KR, Cheung W, Ellis SJ, Federman D, Kavnoudias H, Loader-Oliver D, Roberts S, Evans P, Ball C, Haydon A. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol. 2011;22:611-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 329] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 78. | Meloni MF, Bertolotto M, Alberzoni C, Lazzaroni S, Filice C, Livraghi T, Ferraioli G. Follow-up after percutaneous radiofrequency ablation of renal cell carcinoma: contrast-enhanced sonography versus contrast-enhanced CT or MRI. AJR Am J Roentgenol. 2008;191:1233-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Krisl JC, Doan VP. Chemotherapy and Transplantation: The Role of Immunosuppression in Malignancy and a Review of Antineoplastic Agents in Solid Organ Transplant Recipients. Am J Transplant. 2017;17:1974-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 80. | Hickman LA, Sawinski D, Guzzo T, Locke JE. Urologic malignancies in kidney transplantation. Am J Transplant. 2018;18:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 81. | Tedesco-Silva H, Del Carmen Rial M, Cruz Santiago J, Mazzali M, Pacheco-Silva A, Torres R. Optimizing the clinical utility of sirolimus-based immunosuppression for kidney transplantation. Clin Transplant. 2019;33:e13464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:S199-S202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1143] [Cited by in RCA: 1340] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 83. | Clark TW, Millward SF, Gervais DA, Goldberg SN, Grassi CJ, Kinney TB, Phillips DA, Sacks D, Cardella JF; Technology Assessment Committee of the Society of Interventional Radiology. Reporting standards for percutaneous thermal ablation of renal cell carcinoma. J Vasc Interv Radiol. 2009;20:S409-S416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 84. | Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, Gervais DA, Gillams AR, Kane RA, Lee FT, Livraghi T, McGahan J, Phillips DA, Rhim H, Silverman SG, Solbiati L, Vogl TJ, Wood BJ, Vedantham S, Sacks D; Society of Interventional Radiology Technology Assessment Committee and the International Working Group on Image-guided Tumor Ablation. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20:S377-S390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 325] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 85. | Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1335] [Cited by in RCA: 1640] [Article Influence: 102.5] [Reference Citation Analysis (0)] |