Published online Aug 26, 2019. doi: 10.12998/wjcc.v7.i16.2401
Peer-review started: February 15, 2019
First decision: May 31, 2019
Revised: June 25, 2019
Accepted: July 27, 2019
Article in press: July 27, 2019
Published online: August 26, 2019
Processing time: 192 Days and 9.8 Hours
Multiple renal artery aneurysms (RAAs) involving multiple branches in a solitary kidney are rare and present a major challenge to surgeons. Ex vivo or in situ repair combined with renal artery revascularization is the classical procedure for these complicated cases, which are not suitable for endovascular repair. The choice of bypass graft remains controversial because of the risk of aneurysmal degeneration for autologous graft.
A 39-year-old female patient presented with left lumbar pain for more than 3 mo. Computed tomography angiography showed congenital absence of the right kidney and three left RAAs involving multiple distal branches. This patient met the criteria for surgical repair due to symptoms of threatened rupture. According to the anatomy and location of multiple RAAs, ex vivo revascularization with saphenous vein graft (SVG) was performed. At the 3-year follow-up, computed tomography angiography demonstrated the aneurysmal degeneration of the Y-shaped SVG. The patient remained asymptomatic and follow-up ultrasound showed no continuous growth of SVG aneurysm.
SVG aneurysm in RAA revascularization causes us to reflect on the choice of graft, especially for solitary kidney patients.
Core tip: This rare case of complicated left renal artery aneurysms (RAAs) with absence of right kidney presented a major challenge for the surgeon. From a technical aspect, most RAAs are treated by endovascular procedures, and such complicated surgical repair could give surgeons more confidence with complex renal artery revascularization. Although saphenous vein graft is considered the first choice for auto-renal bypass graft, the risk of restenosis and aneurysmal degeneration remains unresolved. For the RAAs without evidence of inflammation, prosthetic graft may be the alternative choice for patients.
- Citation: Chen XY, Zhao JC, Huang B, Yuan D, Yang Y. Ex vivo revascularization of renal artery aneurysms in a patient with solitary kidney: A case report. World J Clin Cases 2019; 7(16): 2401-2405
- URL: https://www.wjgnet.com/2307-8960/full/v7/i16/2401.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i16.2401
Renal artery aneurysm (RAA) is uncommon with an estimated incidence around 0.1% in the general population. Although the incidence is low, once ruptured, the mortality can reach 80%. Isolated RAA is dominant, and multiple RAAs are seldom reported[1,2]. Treatment of RAA includes surgical repair with revascularization of the RA by ex vivo or in situ repair with or without cold preservation. As the development of percutaneous techniques, endovascular repair with coils and stents has increasingly been used[3-5]. This case of triple left RAAs with absence of the right kidney and involvement of multiple branches is extremely rare, and it was a major challenge for the vascular surgeon. We here report the details of the procedure and long-term follow-up results.
A 39-year-old female patient presented to the clinic of our hospital complaining of left lumbar pain for > 3 mo.
The patient’s symptoms started 3 mo ago with left lumbar pain, which had worsened in the previous 2 wk.
The patient had no previous medical history.
The patient had no personal and family history.
After admission to our department, the patient’s temperature was 36.7 °C, heart rate 73 beats/min, respiratory rate 16 breaths/min, blood pressure 130/70 mmHg, and oxygen saturation in room air 98%. Physical examination revealed percussion tenderness over the left kidney region. No abdominal or rebound tenderness was detected.
Blood analysis revealed normal white blood cell counts, neutrophils, hematocrit, and platelet count. Prothrombin and partial thromboplastin times were normal. Serum C-reactive protein, erythrocyte sedimentation rate, and other immunological tests were all negative. Creatine was 69 μmol/L (normal range 44-133 μmol/L), and estimated glomerular filtration rate was slightly decreased at 80 mL/min/1.73 m2 (normal range > 90 mL/min/1.73 m2). Electrocardiogram and chest X-ray were also normal.
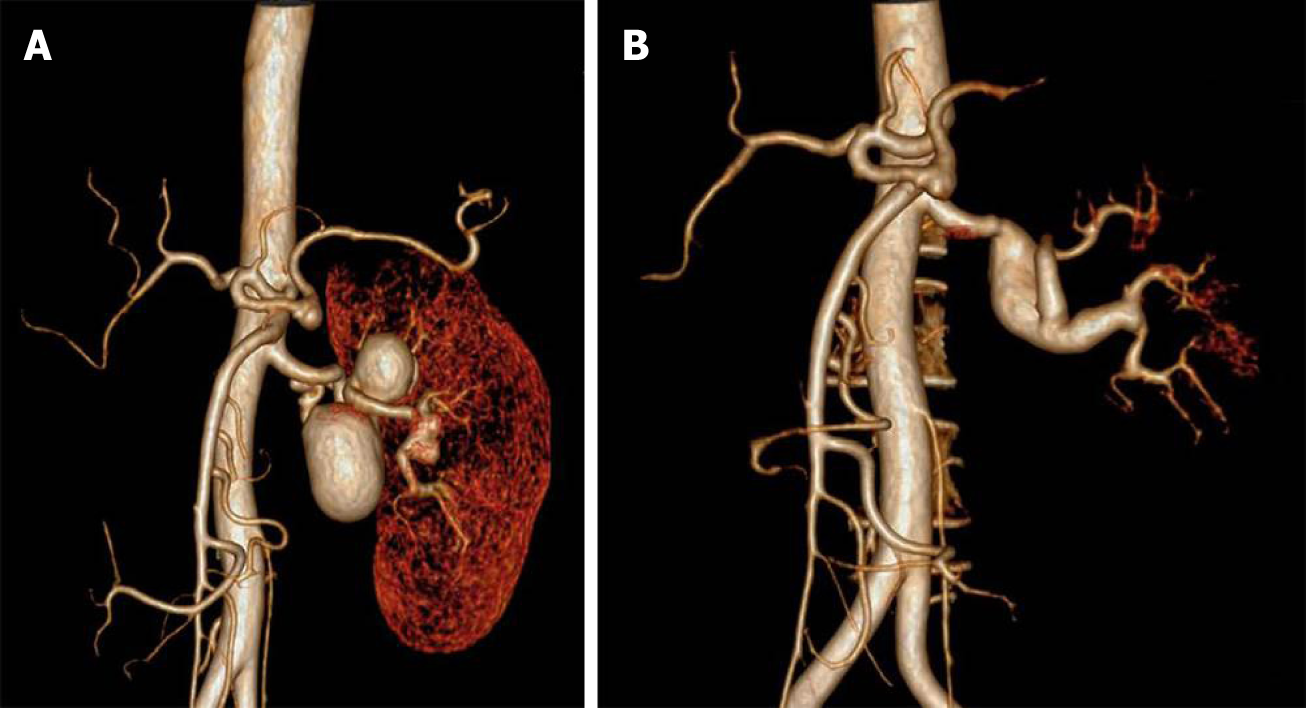
Renography demonstrated that renal index was 38.06% (normal range > 45%). Computed tomography angiography (CTA) showed congenital absence of the right kidney and three left RAAs. The first two aneurysms were located on the twisted main trunk of the left RA with a size of 3 cm and 4 cm, respectively, with a branch originating from the second aneurysm. Another distal small aneurysm of 1.8 cm was located on the distal bifurcation involving two branches (Figure 1A).
The final diagnosis of the present case was threatened rupture of left triple RAAs and congenital absence of right kidney.
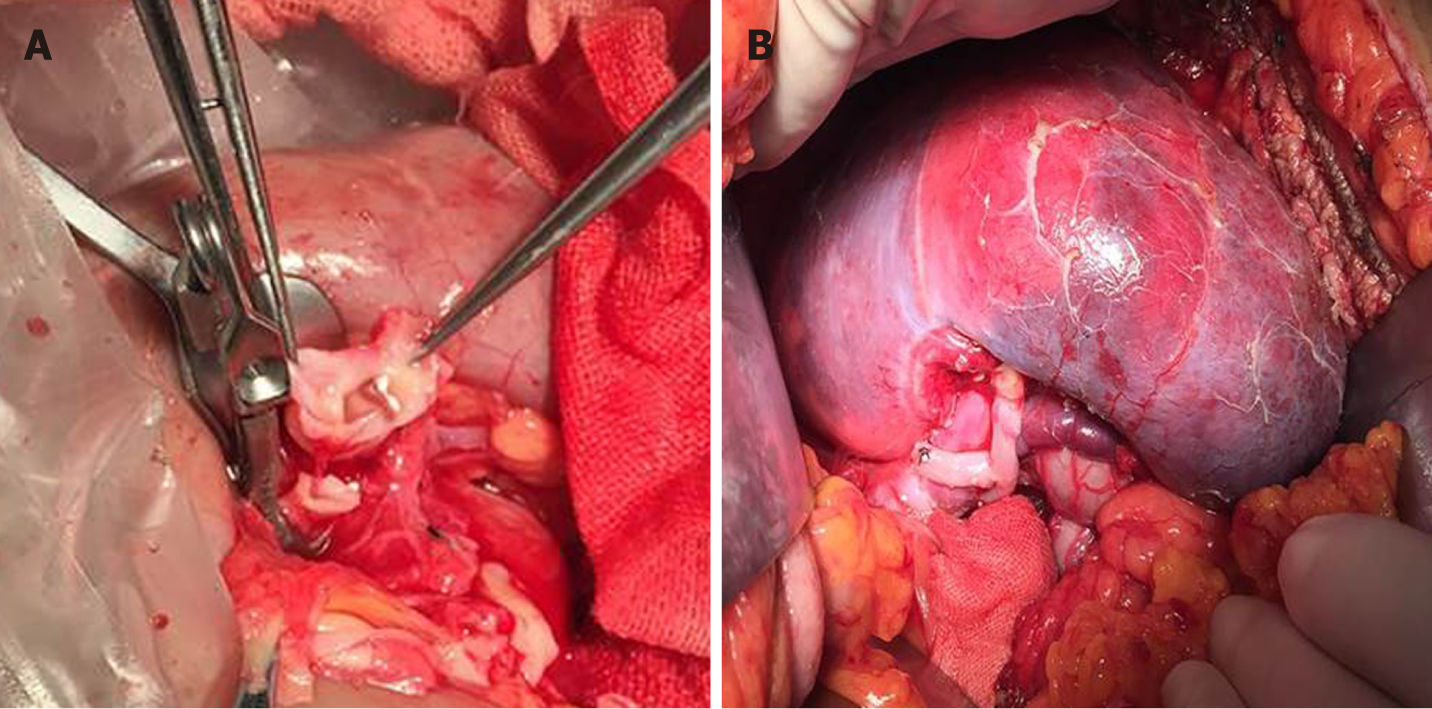
Left triple RAAs resection and ex vivo revascularization with saphenous vein graft (SVG) were performed. Surgical exposure was achieved with a wide left subcostal incision via a transperitoneal approach. As multiple distal branches were involved in the aneurysms, ex vivo repair was considered. Full mobilization and dissection of the left kidney, RAAs, and proximal and distal branches of the RA were performed. The renal pedicle and ureter were mobilized to the pelvic brim. After systemic heparinization (unfractionated heparin 0.5 mg/kg), the main left RA and vein were divided. The left kidney was then placed in ice slush with cold perfusion (4 °C Ringer’s solution). After resection of the distal aneurysm, two residual distal branches were conjoined as a patch, and distal anastomosis was performed end-to-end between the patch and a reversed SVG (Figure 2A). Another branch originating from the second aneurysm was anastomosed end-to-side to the lateral wall of the SVG (Figure 2B). After branch reconstruction, the left kidney was put back into the orthotopic renal fossa. The proximal SVG was anastomosed end-to-end to the proximal main RA, and the renal vein was anastomosed with the original orifice of the inferior vena cava. Intraoperative renal ultrasound identified patency of the anastomosis and distal branches. Whole blocking time for renal vessels was 80 min.
The patient had an uneventful postoperative clinical course and was discharged from hospital 5 d after the operation. Three-year follow-up CTA demonstrated aneurysmal degeneration of the SVG (with a maximum graft diameter of 2.2 cm), and all distal branches were clearly visible (Figure 1B). We carried out further ultrasound follow-up every 6 mo that revealed no continuous growth of SVG aneurysm, and the patient remained asymptomatic. Update renography showed that the renal index of the left kidney increased to 72.06%.
Multiple RAAs involving multiple branches in a solitary kidney are rare and present a major challenge to surgeons. Open surgical repair has been the predominant method for treatment of these lesions; however, currently, endovascular techniques have offered less invasive treatment options in selected cases[4-6].
Currently accepted indications for RAA intervention include size > 2 cm; women within childbearing age; symptoms such as pain, hematuria, and medically refractory hypertension, including that associated with functionally important RA stenosis, thromboembolism, dissection, and rupture[5]. Several approaches are adopted for revascularization of RAA including in situ or ex vivo surgical repair and endovascular therapy.
It is reported truncal aneurysms can be treated by covered stent and intrarenal aneurysms by coil embolization. However, with RAAs involving multiple distal branches, current endovascular techniques do not warrant complete exclusion without renal infarction[7-10]. In the present case, endovascular repair was designed as isolation of the first two RAAs with covered stents, and the last small aneurysm was left behind as the size did not reach the indication for intervention. However, as a branch originated from the second aneurysm, isolation of the first two aneurysms could have blocked the blood flow of this branch, which may have caused partial infarction of the left kidney. Although the diameter of the third RAA involving distal branches did not reach the indication for intervention, we could not leave the risk of future aneurysm development as the patient was young, and it would be much more difficult to deal with the third RAA. The preliminary results of the endovascular treatment are not very informative. For the above reasons, surgical repair was performed on this patient.
It is reported that ex vivo repair ensures good protection of the renal parenchyma. In contrast, ex vivo surgical repair of RAA has the advantage of facilitating reconstruction of distal branches, especially those with multiple branches involved[10,11]. Several studies have already reported a satisfactory result of ex vivo surgical repair with low morbidity and patency rates of 82%–99%[12,13]. To date, there is no report of randomized controlled trial comparing ex vivo and in situ repair of RAAs. Considering the reconstruction of multiple distal branches, and in situ surgical repair is more challenging, ex vivo repair was performed for this patient.
For the choice of bypass graft, the saphenous vein is the most common conduit for revascularization. Most current studies have preferred to use SVG with good durability and patency. Follow-up patency (mean, 33 mo; range, 1–118 mo) was determined for 64 (91%) RA reconstructions. Product-limit estimate of primary patency at 48 mo was 96%[13,14]. Although the aneurysmal degeneration of SVG is widely reported in coronary artery disease, it is less frequently reported in the RAAs with revascularization by SVG, especially in noninflammatory RA disease. Besides SVG, branched and unbranched internal iliac artery autografts have been used as bypass grafts for the RA, and no aneurysmal degeneration of the internal iliac artery graft has been reported. To prevent risk of SVG aneurysmal degeneration, a tubular SVG supported by external Dacron mesh was adopted, presented to be a suitable graft material for renal reconstruction in pediatric population. With an average follow-up of 4.3 years, no SVG aneurysm was detected[15]. For RAAs without evidence of inflammation, considering the risk of aneurysmal degeneration of SVG, prosthetic grafts may be an alternative, as no aneurysmal degeneration was present in our patient with a solitary kidney, although more stable long-term results are needed.
Three-year follow-up CTA revealed aneurysmal degeneration of the SVG, with a maximum diameter of 2.1 cm. Because there was no growth of the aneurysm during follow-up under ultrasound surveillance, we chose to continue observing the size change.
This rare case of complicated RAAs with absence of the right kidney presented a major challenge to the surgeon. Successful results give us more confidence for ex vivo surgical repair of complicated RAA. Aneurysmal degeneration of SVG in RAA revascularization causes us to reflect on the choice of graft, especially for solitary kidney patients who need more stable long-term results.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Yan SL, Ueda H S-Editor: Wang JL L-Editor: Filipodia E-Editor: Liu JH
| 1. | Gandini R, Morosetti D, Chiocchi M, Chiaravalloti A, Citraro D, Loreni G, DA Ros V, Salvatori E, Simonetti G. Long-term follow-up of endovascular treatment of renal artery aneurysms with covered stent deployment. J Cardiovasc Surg (Torino). 2016;57:625-633. [PubMed] |
| 2. | Buck DB, Curran T, McCallum JC, Darling J, Mamtani R, van Herwaarden JA, Moll FL, Schermerhorn ML. Management and outcomes of isolated renal artery aneurysms in the endovascular era. J Vasc Surg. 2016;63:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Sousa J, Mansilha A. Endovascular Treatment of Symptomatic Renal Artery Aneurysm with Hostile Anatomy. Eur J Vasc Endovasc Surg. 2017;53:843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Orion KC, Abularrage CJ. Renal artery aneurysms: movement toward endovascular repair. Semin Vasc Surg. 2013;26:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | González J, Esteban M, Andrés G, Linares E, Martínez-Salamanca JI. Renal artery aneurysms. Curr Urol Rep. 2014;15:376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Morita K, Seki T, Iwami D, Sasaki H, Fukuzawa N, Nonomura K. Long-term outcome of single institutional experience with conservative and surgical management for renal artery aneurysm. Transplant Proc. 2012;44:1795-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Pride YB, Nguyen MC, Garcia LA. Management of a renal artery aneurysm with coil embolization. J Invasive Cardiol. 2008;20:470-472. [PubMed] |
| 8. | Nassiri N, Huntress LA. Stent-Assisted Coil Embolization of a Symptomatic Renal Artery Aneurysm at a Bifurcation Point. Ann Vasc Surg. 2017;42:299.e11-299.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Rodriguez-Rapale VA, Martinez-Trabal JL. Hilar Renal Artery Aneurysm Repair Using Coil Embolization and Covered Stent. Vasc Endovascular Surg. 2019;53:82-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Wetstein PJ, Clark ME, Cafasso DE, Golarz SR, Ayubi FS, Kellicut DC. Surgical Repair of Abdominal Aortic and Renal Artery Aneurysms in Takayasu's Arteritis. Hawaii J Med Public Health. 2016;75:4-7. [PubMed] |
| 11. | Tsilimparis N, Reeves JG, Dayama A, DPerez SD, Debus ES, Ricotta JJ 2nd. Endovascular vs open repair of renal artery aneurysms: outcomes of repair and long-term renal function. J Am Coll Surg. 2013;217:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Duprey A, Chavent B, Meyer-Bisch V, Varin T, Albertini JN, Favre JP, Barral X, Ricco JB. Editor's Choice - Ex vivo Renal Artery Repair with Kidney Autotransplantation for Renal Artery Branch Aneurysms: Long-term Results of Sixty-seven Procedures. Eur J Vasc Endovasc Surg. 2016;51:872-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Klausner JQ, Lawrence PF, Harlander-Locke MP, Coleman DM, Stanley JC, Fujimura N; Vascular Low-Frequency Disease Consortium. The contemporary management of renal artery aneurysms. J Vasc Surg. 2015;61:978-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Duran M, Hausmann DF, Grabitz K, Schelzig H, Simon F, Sagban TA. Reconstruction for renal artery aneurysms using the tailoring technique. J Vasc Surg. 2017;65:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Berkowitz HD, O'Neill JA. Renovascular hypertension in children. Surgical repair with special reference to the use of reinforced vein grafts. J Vasc Surg. 1989;9:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |