Published online Aug 26, 2019. doi: 10.12998/wjcc.v7.i16.2346
Peer-review started: January 28, 2019
First decision: May 31, 2019
Revised: June 18, 2019
Accepted: July 27, 2019
Article in press: July 27, 2019
Published online: August 26, 2019
Processing time: 211 Days and 11.9 Hours
Surgical treatment for large carotid body tumor (CBT), particularly the Shamblin III type, is challenging and rarely reported.
In July 2014, a 63-year-old woman presented to our hospital with a large CBT (130 mm × 60 mm × 70 mm). The lesion was hypervascular, spanned from the first to the seventh cervical vertebra, and adhered to the right common carotid artery (CCA), internal carotid artery (ICA) and external carotid artery (ECA). The resection was carried out in a hybrid operating theatre. First, we used Onyx gel to embolize the feeding artery. An ICA balloon was used to prevent gel entry into the ICA. After shrinkage and hardening of the CBT, we quickly resected the CBT as well as a part of the ECA that adhered to the CBT. A vascular shunt was inserted between CCA and ICA, and the part where the ICA was cut off from the CCA was directly sutured. A follow-up at four years later showed no neurological damage.
For large hypervascular CBT, embolization of the feeding artery prior to resection is helpful. The hybrid operating theatre is the ideal platform to carry out such operations.
Core tip: Carotid body tumor of giant Shamblin III type is very rare, and the treatment for which is a big challenge for a surgeon. Combination of interventional embolization and surgical resection in the hybrid operating room is an effective method to safely and completely remove carotid body tumor lesions.
- Citation: Li MQ, Zhao Y, Sun HY, Yang XY. Large carotid body tumor successfully resected in hybrid operating theatre: A case report. World J Clin Cases 2019; 7(16): 2346-2351
- URL: https://www.wjgnet.com/2307-8960/full/v7/i16/2346.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i16.2346
Carotid body tumor (CBT) is the most common paraganglioma in the head and neck[1]. CBT typically originates from glomus caroticum in carotid bifurcation and consists of chemoreceptor cells of primitive neural crest origin[2-4]. CBT can be categorized into three types: Sporadic, familial and hyperplastic[1].
Surgical resection is the standard treatment for CBT but carried substantial risk for large CBT, including cranial nerve injury and stroke[5]. Intraoperative embolism has been used to successfully remove large CBT previously considered not resectable[6]. Hybrid operating rooms are equipped with digital subtraction angiography (DSA) and near-infrared indocyanine green video angiography, and thus could readily identify severe complications in vascular surgery[7-9].
Here, we report a case of large CBT successfully resected after Onyx gel embolization of the feeding arteries in a hybrid operating theatre.
In July 2014, a 63-year-old right-handed woman presented with a progressively enlarging mass over the past five years and emerging numbness of the right hand and heel.
Patient’s symptoms started two months ago with recurrent numbness of the right hand and heel.
She had osteoarthritis of the knee for twenty years and hypertension for four years.
The mass was painless, non-moveable, and located between the right lower jaw and the right clavicle. No vascular murmur was heard over the mass upon auscultation. She had no dysphagia or voice hoarseness. No signs of hypoglossal neuropathy were detected.
Laboratory blood tests were normal.
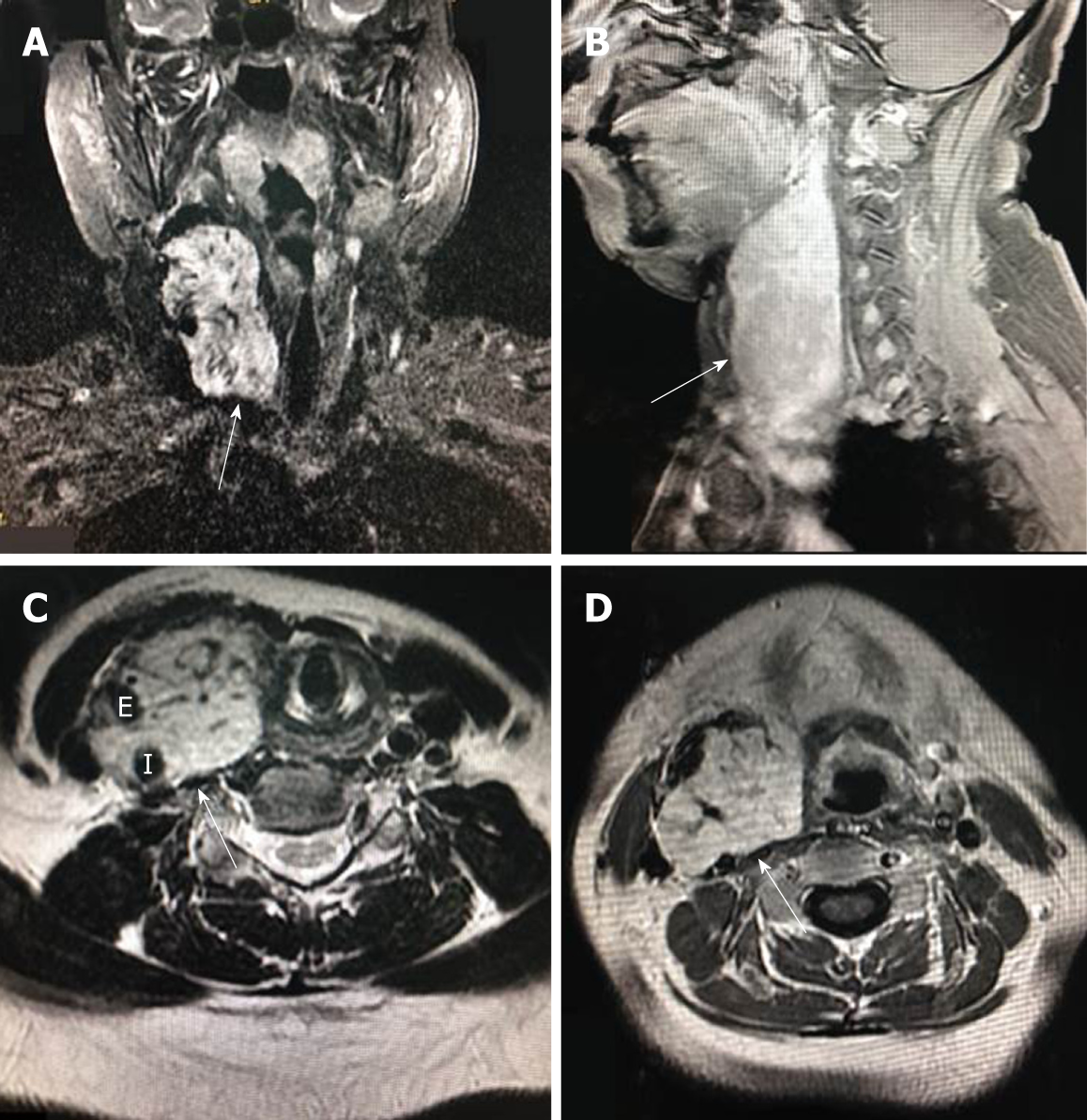
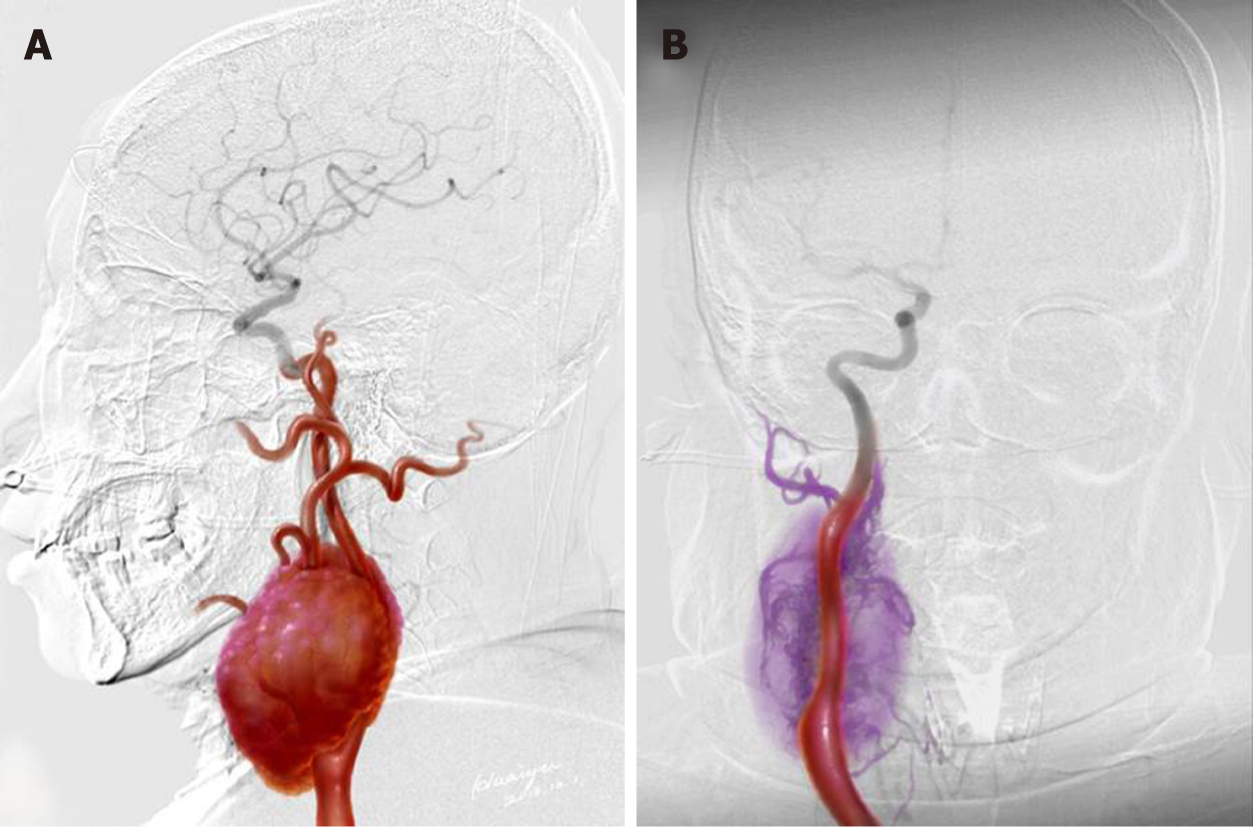
Magnetic resonance imaging (MRI) revealed a 130 mm × 60 mm × 70 mm mass with enhancement (Figure 1A and 1B). The mass spanned from the first to the seventh cervical vertebra, crossed over carotid bifurcation, and seemed to adhere to the right common carotid artery (CCA), right internal carotid artery (ICA), and right external carotid artery (ECA) (Figures 1C, D and Figure 2A).
The final diagnosis of the presented case is carotid body tumor; this is based on the clinical examination and postoperative pathology.
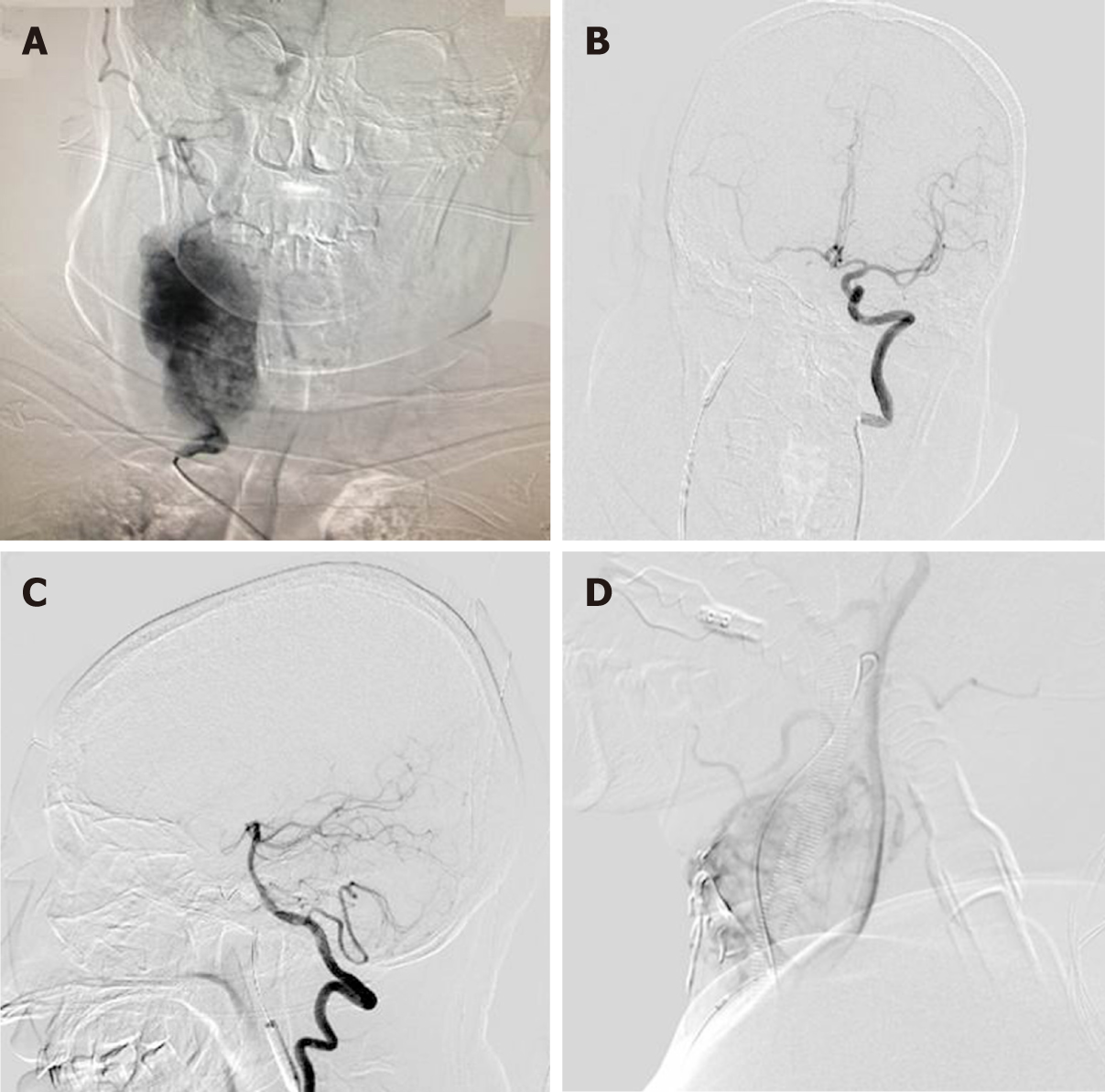
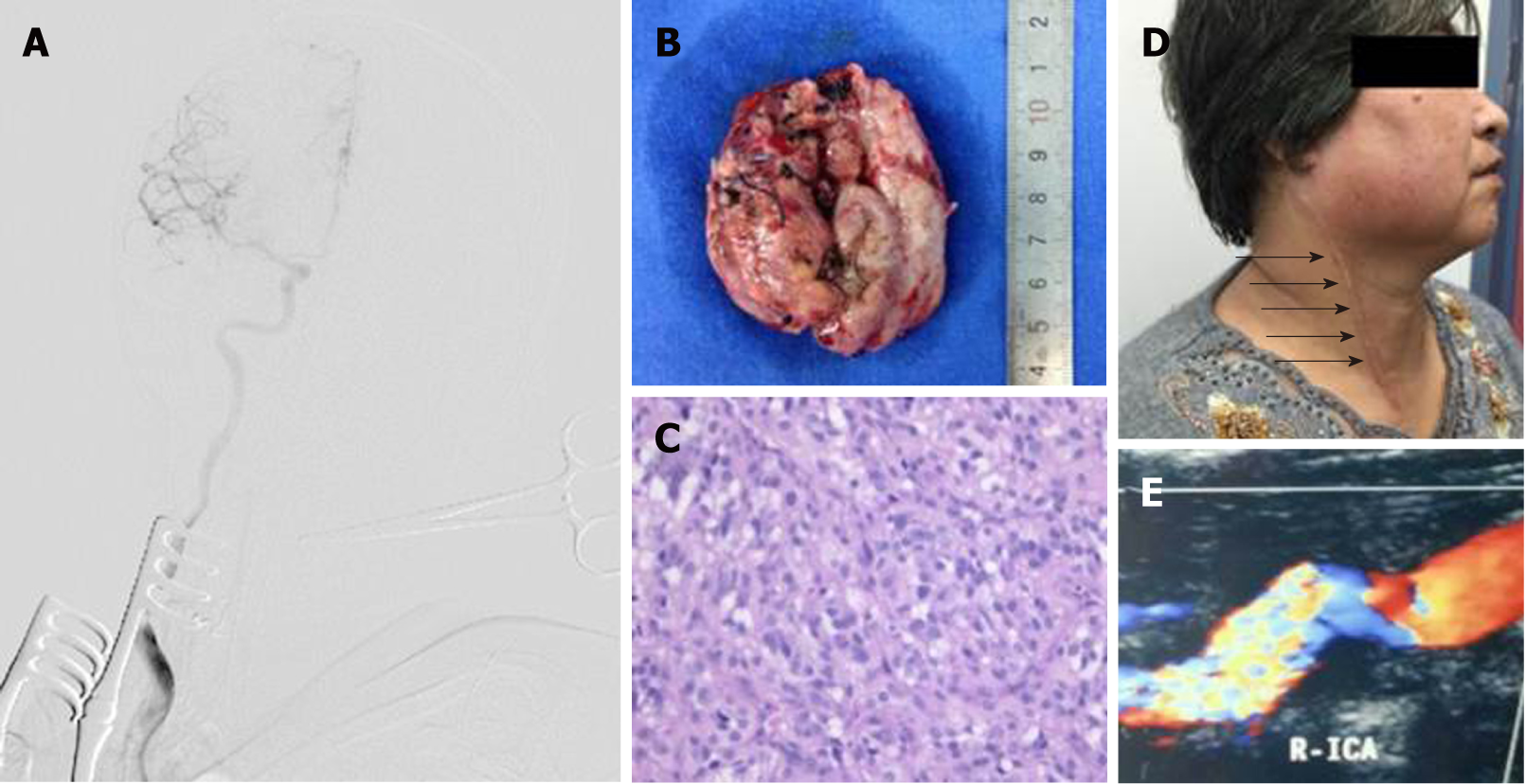
Surgical resection was conducted in a hybrid operating room, with a goal to completely resect the tumor (Figure 2B). DSA indicated that the right external carotid artery (ECA) was the major source of blood supply to the lesion (Figure 3A). A balloon test occlusion (BTO) was performed under general anesthesia. Upon angiography of the left ICA angiography, the right anterior cerebral artery and right middle cerebral artery were visualized. Twenty minutes after balloon occlusion, the patient’s neurological reflex was normal. BTO revealed poor compensation of the posterior communicating artery (PCoA) (Figure 3B and C). Onyx gel was used to embolize the feeding artery superior thyroid artery (STA) (Figure 3D). The ICA balloon was inflated to prevent gel entry into the right ICA. The procedure resulted in significant decrease in blood supply (Figure 3D) and the tumor began to shrink and harden. The balloon was deflated and removed from ICA, and surgical resection started. After isolation of the CBT, the blood supply from the right CCA, ICA, and ECA was blocked. The distal end of the right ECA was ligated, and the (including the part that adhered to the right ECA) was removed. At this point, the blood flow had been blocked for over 30 min. We restored blood flow of the carotid artery and put a vascular shunt between ICA and CCA. The part where the ICA was cut from the CCA was sutured. Intraoperative angiography at this time revealed patency of the ICA and its branches (Figure 4A).
Figure 4B shows the removed tumor. Post-operative pathological examination confirmed paraganglioma (Figure 4C). Two months after the surgery, numbness of the right hand and heel disappeared. Ultrasound examination showed that CCA and RICA were unobstructed (Figure 4D). Upon the last follow-up visit four years later, the patient had no recurrence and no any neurological abnormalities (Figure 4E).
To the best of our knowledge, the report represents the largest CBT that was successfully resected under interventional embolization in a hybrid operating room.
The CBT in the current case was very close to the base of the skull. As a result, the risk of significant blood loss and cranial nerve injuries during operation is high. A previous study showed that, for every 1-cm decrease in DTBOS, intraoperative blood loss (> 250 mL) increases by 1.8 times (95%CI: 1.25-2.55), and the cranial nerve injury increases by 1.5 times (95%CI: 1.19-1.92)[10]. To reduce the intraoperative bleeding, we used Onyx gel to embolize the feeding artery. The right CCA, ICA and ECA were also blocked. We believe that these procedures are important for complete resection of the tumor.
Based on Shamblin's classification[4], CBT is classified into three types. Type I: Tumor is small and confined to the bifurcation of carotid artery, and there is little adhesion to the carotid artery; Type II: Tumor is large and partly surrounds the carotid artery with certain degree of adhesion to the carotid artery; and Type III: The tumor is large and completely envelops the carotid artery. CBT with higher Shamblin grade tends to have more severe neurological complications upon the surgery[11], mostly cranial nerve injuries[12,13]. The tumor, in this case, was Shamblin type III, and unusually large, but neurological complications did not happen, possibly due to feeding artery embolization, blocking of the CCA, ICA and ECA, and experience of the surgeons.
It is noteworthy to point out that the management of large CBT should be individualized since the tumor could be located in varying positions and may invade different tissues[14,15]. Also, an interdisciplinary approach is needed.
For large hypervascular CBT, embolization of the feeding artery in a hybrid operating theatre could be helpful.
We thank the index patient for the consent to publish this manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gheita TA, Vaudo G S-Editor: Wang JL L-Editor: A E-Editor: Liu JH
| 1. | Sajid MS, Hamilton G, Baker DM; Joint Vascular Research Group. A multicenter review of carotid body tumour management. Eur J Vasc Endovasc Surg. 2007;34:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Davila VJ, Chang JM, Stone WM, Fowl RJ, Bower TC, Hinni ML, Money SR. Current surgical management of carotid body tumors. J Vasc Surg. 2016;64:1703-1710. [PubMed] |
| 3. | Georgiadis GS, Lazarides MK, Tsalkidis A, Argyropoulou P, Giatromanolaki A. Carotid body tumor in a 13-year-old child: Case report and review of the literature. J Vasc Surg. 2008;47:874-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Shamblin WR, ReMine WH, Sheps SG, Harrison EG. Carotid body tumor (chemodectoma). Clinicopathologic analysis of ninety cases. Am J Surg. 1971;122:732-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 463] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | San Norberto EM, Taylor JH, Carrera S, Vaquero C. Intraoperative embolization with poloxamer 407 during surgical resection of a carotid body tumor. J Vasc Surg. 2012;56:1782-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Fandino J, Taussky P, Marbacher S, Muroi C, Diepers M, Fathi AR, Remonda L. The concept of a hybrid operating room: applications in cerebrovascular surgery. Acta Neurochir Suppl. 2013;115:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Chiang VL, Gailloud P, Murphy KJ, Rigamonti D, Tamargo RJ. Routine intraoperative angiography during aneurysm surgery. J Neurosurg. 2002;96:988-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Klopfenstein JD, Spetzler RF, Kim LJ, Feiz-Erfan I, Han PP, Zabramski JM, Porter RW, Albuquerque FC, McDougall CG, Fiorella DJ. Comparison of routine and selective use of intraoperative angiography during aneurysm surgery: a prospective assessment. J Neurosurg. 2004;100:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V. Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery. 2003;52:132-9; discussion 139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 153] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Kim GY, Lawrence PF, Moridzadeh RS, Zimmerman K, Munoz A, Luna-Ortiz K, Oderich GS, de Francisco J, Ospina J, Huertas S, de Souza LR, Bower TC, Farley S, Gelabert HA, Kret MR, Harris EJ, De Caridi G, Spinelli F, Smeds MR, Liapis CD, Kakisis J, Papapetrou AP, Debus ES, Behrendt CA, Kleinspehn E, Horton JD, Mussa FF, Cheng SWK, Morasch MD, Rasheed K, Bennett ME, Bismuth J, Lumsden AB, Abularrage CJ, Farber A. New predictors of complications in carotid body tumor resection. J Vasc Surg. 2017;65:1673-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | Sen I, Stephen E, Malepathi K, Agarwal S, Shyamkumar NK, Mammen S. Neurological complications in carotid body tumors: a 6-year single-center experience. J Vasc Surg. 2013;57:64S-68S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Lim JY, Kim J, Kim SH, Lee S, Lim YC, Kim JW, Choi EC. Surgical treatment of carotid body paragangliomas: outcomes and complications according to the shamblin classification. Clin Exp Otorhinolaryngol. 2010;3:91-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Westerband A, Hunter GC, Cintora I, Coulthard SW, Hinni ML, Gentile AT, Devine J, Mills JL. Current trends in the detection and management of carotid body tumors. J Vasc Surg. 1998;28:84-92; discussion 92-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Hurtado-Lopez LM, Fink-Josephi G, Ramos-Méndez L, Dena-Espinoza E. Nonresectable carotid body tumor: hybrid surgical procedure to achieve complete and safe resection. Head Neck. 2008;30:1646-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Bobadilla-Rosado LO, Garcia-Alva R, Anaya-Ayala JE, Peralta-Vazquez C, Hernandez-Sotelo K, Luna L, Cuen-Ojeda C, Hinojosa CA. Surgical Management of Bilateral Carotid Body Tumors. Ann Vasc Surg. 2019;57:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |