Published online Aug 26, 2019. doi: 10.12998/wjcc.v7.i16.2316
Peer-review started: May 8, 2019
First decision: May 31, 2019
Revised: June 18, 2019
Accepted: July 20, 2019
Article in press: July 20, 2019
Published online: August 26, 2019
Processing time: 124 Days and 6.2 Hours
Refractory pouchitis is a common cause of pouch failure, which may require surgical excision of the pouch or permanent diversion. We aimed to show the effect of vedolizumab on treatment of the patient with refractory pouchitis.
A 32-year-old male with pancolonic ulcerative colitis since the age of 25 with primary failure of infliximab and mesalamine and intolerance of azathioprine, underwent a total proctocolectomy with ileal pouch-anal anastomosis in 2012. He developed chronic diarrhea in 2014, which was watery, 30 per day and accompanied with blood and mucus affecting his quality of life.
Vedolizumab is safe and effective in the management of anti-tumor necrosis factor alpha refractory pouchitis.
Core tip: Vedolizumab, a humanized immunoglobulin G1 monoclonal antibody to α4β7 integrin, has been shown to moderate gut lymphocyte trafficking with an efficacy in treatment of both Crohn’s disease and ulcerative colitis. In our patient who had two different anti-tumor necrosis factor refractory pouchitis, the gut-specific immune modulation mediated by vedolizumab treatment resulted in good responses. This case is important because vedolizumab is the novel therapy for refractory pouchitis. However, further large and prospective studies are needed for efficacy and the underlying mechanisms of efficacy of vedolizumab in treatment of refractory pouchitis.
- Citation: Cakir OO. Effectiveness of vedolizumab treatment in two different anti-tumor necrosis factor alpha refractory pouchitis: A case report. World J Clin Cases 2019; 7(16): 2316-2321
- URL: https://www.wjgnet.com/2307-8960/full/v7/i16/2316.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i16.2316
Refractory pouchitis is a common cause of pouch failure, which may require surgical excision of the pouch or permanent diversion. Vedolizumab, a humanized immunoglobulin G1 monoclonal antibody to α4β7 integrin, has been shown to moderate gut lymphocyte trafficking with an efficacy in treatment of both Crohn's disease and ulcerative colitis (UC)[1,2]. Although tumor necrosis factor-alpha (TNF-α) inhibitors have been reported to be effective as treatment for pouchitis[3], there is little data regarding the use of vedolizumab in refractory pouchitis[4]. The effect of vedolizumab treatment on chronic antibiotic refractory pouchitis is very limited. Chronic antibiotic refractory pouchitis is a challenging complication in patients with UC who undergo proctocolectomy with ileal pouch-anal anastomosis. Chronic antibiotic refractory pouchitis occurs when patients do not respond to a 2-wk course of ciprofloxacin, metronidazole or rifaximin for pouchitis[5].
We report on a 32-year-old male with pancolonic UC.
A 32-year-old male with pancolonic UC since the age of 25 with primary failure of infliximab and mesalamine and intolerance of azathioprine, underwent a total proctocolectomy with ileal pouch-anal anastomosis in 2012.
He developed chronic diarrhea in 2014, which was watery, 30 per day and accompanied with blood and mucus affecting his quality of life. He could not work. He lost a lot of weight. He had fallen from 55 kg to 43 kg during pouchitis. His body mass index was 15.2 kg/m2. He used meselamine 3 g orally, steroid intermittently, lavman and loperamide orally three times daily.
His family history was unremarkable.
His abdominal physical examination was normal.
Laboratory work-up revealed erythrocyte sedimentation rate of 56 mm/h and C-reactive protein of 3.6 mg/dL with no liver function abnormalities. Autoimmune markers including IgG4, anti-nuclear antibody and anti-mitochondrial antibody were negative. His blood tests for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus antibodies were negative. Stool studies for Clostridium difficile, viruses and bacteria were negative. Blood tests for Epstein-Barr virus and cytomegalovirus antibodies were negative.
An ileoscopy and pouchoscopy were performed that demonstrated normal proximal ileal mucosa, but there were diffuse edema, erythema and nodularity and multiple superficial and deep ulcers in the pouch. His pouchitis disease activity index score was 16. Biopsies obtained were negative for cytomegalovirus. An upper endoscopy was done at the same time to evaluate diarrhea, and it was normal. Duodenal biopsy was negative for the presence of celiac disease. Serum antibodies for celiac disease including anti-gliadin antibodies, endomysial antibodies and anti-transglutaminase antibodies were negative. Therefore, gluten restricted diet was not given to the patient. His chest X-ray was normal. Purified protein derivative skin test was 0 mm.
Chronic pouchitis.
He was prescribed metronidazole 500 mg orally three times daily and ciprofloxacin 500 mg orally two times daily. But his symptoms did not improve. Then we added rifaximin 550 mg orally three times daily. We continued meselamine 3000 mg orally two times rectally and loperamide three times daily. He also used probiotics. He continued to have diarrhea with blood and mucus 20 to 30 times per day. Then adalimumab was started at 160 mg, 80 mg and 40 mg subcutaneously at 0, 2, and every 2 wk, respectively. He reported improvement of diarrhea without blood 10 to 15 per day the first week of adalimumab treatment. However, this response decreased within 4 wk, and the diarrhea and weight loss increased. His pouchoscopy was the same as before treatment at 6 mo after the beginning of treatment. Therefore, we stopped adalimumab. We tested the patient again for other etiologies like infections that were negative. Finally, we decided to start vedolizumab. The patient was given 300 mg parenterally at 0, 2, and 6 wk then every 8 wk.
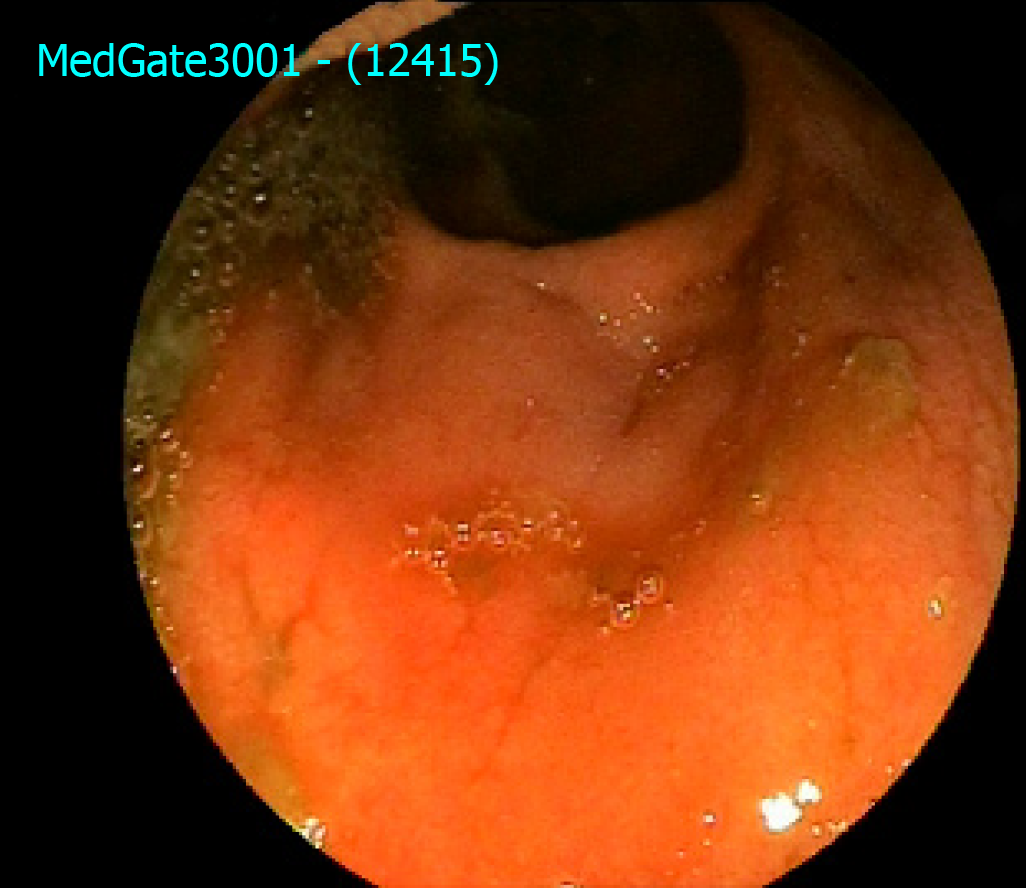
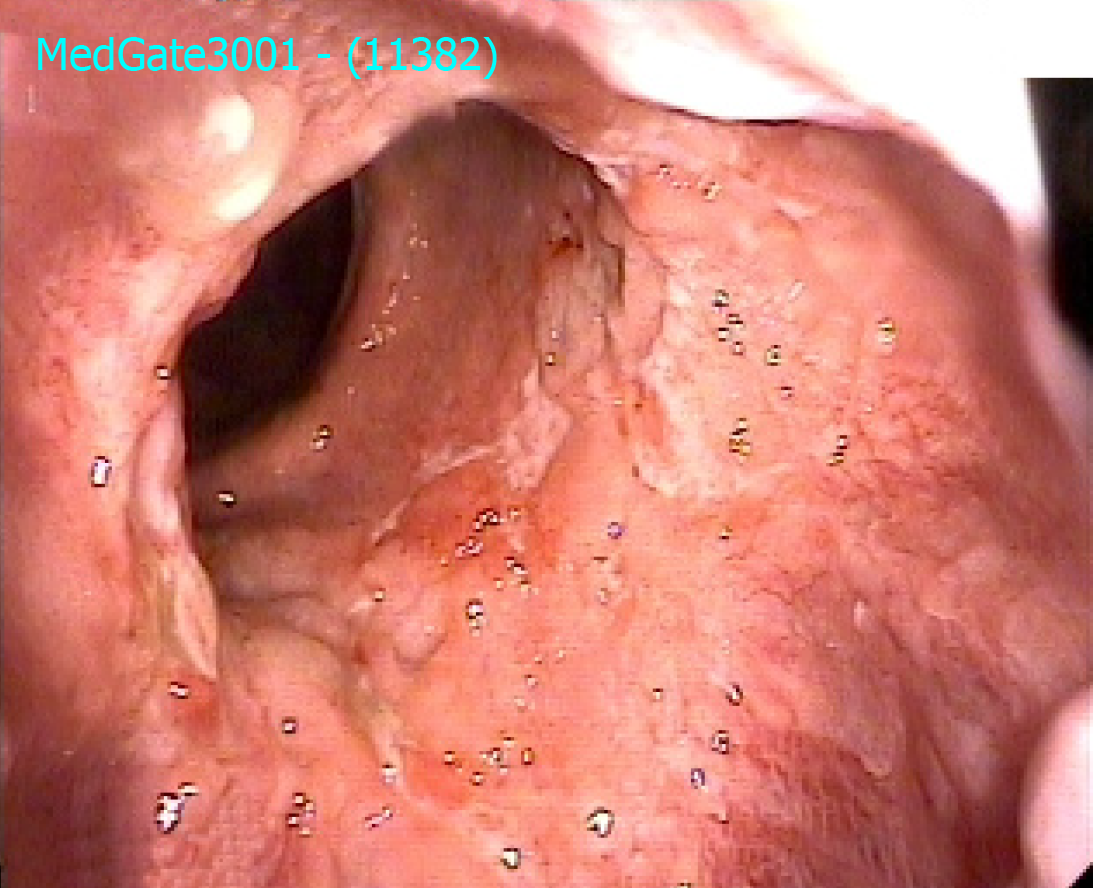
He reported improvement in clinical symptoms at 4 wk for frequency of diarrhea (six to eight per day) without blood and mucus. He did not have any abdominal complaints. A pouchoscopy at 6 wk and 15 wk after beginning vedolizumab demonstrated that there were less ulcers after 6 wk, and there was only one small superficial ulcer after 15 wk (Figure 1). A pouchoscopy before beginning vedolizumab treatment is shown in Figure 2. His laboratory tests including C-reactive protein, erythrocyte sedimentation rate and liver test were normal. He gained almost 9 kg during vedolizumab treatment, and his quality of life improved (he started to work again).
There is only one retrospective study on the efficacy of vedolizumab for refractory pouchitis of the ileo-anal pouch in the literature[6]. This study suggested that vedolizumab is safe and effective for treatment of refractory pouchitis. The other studies found in the literature are case presentations and case series. These presentations showed us vedolizumab was a good choice for refractory pouchitis[7-11]. The effects of vedolizumab for treatment of pouchitis is summarized in Table 1[8-13]. We differentiated our case from other cases in the literature by taking an effective clinical and endoscopic response with vedolizumab treatment in two different anti-TNF alpha refractory pouchitis.
| Country and reference | Number of patients | Age and gender | Features of inflammatory bowel disease | Outcomes |
| United States and reference 8 | 1 | 41-year-old female | She had pouchitis; 2 years later IPAA | Improvement in clinical symptoms and decreased frequency of bowel movements to four to six per day without blood or mucus were reported with 6 wk of vedolizumab treatment. There were no side effects |
| Italy and reference 9 | 1 | 33-year-old male | Anti-TNF-refractory chronic pouchitis and concomitant PSC | 3 mo after ileostomy closure, chronic pouchitis occurred, refractory to antibiotics and anti-TNF. Thus, vedolizumab was started, leading to a marked improvement in clinical symptoms, which was maintained to the end of follow up (wk 34). There were no side effects |
| Germany and reference 10 | 20 | 12 male, 8 female; The median age was 22.5 years old | All of the patients were diagnosed with pouchitis | Improvement of clinical symptoms, the Oresland score and the PDAI score. There were no side effects |
| Greece and reference 11 | 1 | 22-year-old female | She was first diagnosed with pouchitis 1 year after surgery. Administered infliximab followed by adalimumab, both of which she discontinued after an early severe allergic reaction | Vedolizumab was subsequently initiated, together with a single course of antibiotics, and the patient experienced improvement in clinical symptoms and laboratory results with no documented relapse since then. A new pouchoscopy at wk 33 showed significant improvement |
| Greece and reference 11 | 1 | 22-year-old female | She was first diagnosed with pouchitis 1 year after surgery. Administered infliximab followed by adalimumab, both of which she discontinued after an early severe allergic reaction | Vedolizumab was subsequently initiated, together with a single course of antibiotics, and the patient experienced improvement in clinical symptoms and laboratory results with no documented relapse since then. A new pouchoscopy at wk 33 showed significant improvement |
| United States and reference 12 | 12 | 9 female, 3 male; The mean age was 41 years old | All of the patients had active pouchitis. Five patients (41.7%) used mesalamine, six (50.0%) took budesonide and four (33.3%) took prednisone prior to using vedolizumab. Eight (66.7%) had used anti-TNF agents prior to vedolizumab use | Eight (66.7%) patients demonstrated significant reduction in mPDAI symptom subscores before and after vedolizumab therapy |
| Portugal and reference 13 | 1 | 20-year-old female | She was diagnosed with pouchitis and a severe symptomatic autoimmune hemolytic anemia 1 year after IPAA | Patient reported symptom improvement at wk 12 and a pouchoscopy revealed only mucosal edema after 6 mo of therapy. Her inflammatory markers and hemoglobin normalized on repeat testing, allowing steroid withdrawal |
Vedolizumab, a monoclonal antibody, selectively blocks gut lymphocyte trafficking by interacting with α4β7 heterodimer[1]. There is severe infiltration of the mucosa by both innate and adaptive immune cells in active pouchitis. An increased proportion of mucosal dendritic cells expressing integrin β7 in patients with pouch inflammation has been shown[14]. The integrin signaling in the pathogenesis of this clinical condition of pouchitis may have a pathogenic role. Therefore, blockade of α4β7 integrin with vedolizumab treatment might represent a promising therapeutic strategy for this clinical condition[14].
Vedolizumab has been shown to be beneficial for the treatment of chronic antibiotic-refractory pouchitis[15,16]. After 3 mo of therapy with vedolizumab in patients with refractory pouchitis, the small case series of four patients had symptomatic and endoscopic improvements[17].
Vedolizumab may be a new choice as a treatment option in patients with refractory pouchitis who showed no improvement with steroids and other biological therapies such as anti-TNFs. Future studies may show when to start vedolizumab and the advantages of vedolizumab therapy in patients with refractory pouchitis.
In our patient who had anti-TNF refractory pouchitis, the gut-specific immune modulation mediated by vedolizumab treatment resulted in good responses. Further large and prospective studies are needed for the efficacy and the underlying mechanisms of efficacy of vedolizumab in treatment of refractory pouchitis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rocha R, Chiba T S-Editor: Dou Y L-Editor: Filipodia E-Editor: Wu YXJ
| 1. | Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S, Fox I, Milch C, Sankoh S, Wyant T, Xu J, Parikh A; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1576] [Cited by in RCA: 1830] [Article Influence: 152.5] [Reference Citation Analysis (1)] |
| 2. | Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, Fox I, Rosario M, Sankoh S, Xu J, Stephens K, Milch C, Parikh A; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1539] [Article Influence: 128.3] [Reference Citation Analysis (1)] |
| 3. | Herfarth HH, Long MD, Isaacs KL. Use of Biologics in Pouchitis: A Systematic Review. J Clin Gastroenterol. 2015;49:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Philpott J, Ashburn J, Shen B. Efficacy of Vedolizumab in Patients with Antibiotic and Anti-tumor Necrosis Alpha Refractory Pouchitis. Inflamm Bowel Dis. 2017;23:E5-E6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Shen B. Acute and chronic pouchitis--pathogenesis, diagnosis and treatment. Nat Rev Gastroenterol Hepatol. 2012;9:323-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Gregory M, Weaver KN, Hoversten P, Hicks SB, Patel D, Ciorba MA, Gutierrez AM, Beniwal-Patel P, Palam S, Syal G, Herfarth HH, Christophi G, Raffals L, Barnes EL, Deepak P. Efficacy of Vedolizumab for Refractory Pouchitis of the Ileo-anal Pouch: Results From a Multicenter US Cohort. Inflamm Bowel Dis. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Schmid M, Frick JS, Malek N, Goetz M. Successful treatment of pouchitis with Vedolizumab, but not fecal microbiota transfer (FMT), after proctocolectomy in ulcerative colitis. Int J Colorectal Dis. 2017;32:597-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Mir F, Yousef MH, Partyka EK, Tahan V. Successful treatment of chronic refractory pouchitis with vedolizumab. Int J Colorectal Dis. 2017;32:1517-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Coletta M, Paroni M, Caprioli F. Successful Treatment With Vedolizumab in a Patient With Chronic Refractory Pouchitis and Primary Sclerosing Cholangitis. J Crohns Colitis. 2017;11:1507-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Bär F, Kühbacher T, Dietrich NA, Krause T, Stallmach A, Teich N, Schreiber S, Walldorf J, Schmelz R, Büning C, Fellermann K, Büning J, Helwig U; German IBD Study Group. Vedolizumab in the treatment of chronic, antibiotic-dependent or refractory pouchitis. Aliment Pharmacol Ther. 2018;47:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Orfanoudaki E, Foteinogiannopoulou K, Koutroubakis IE. Use of vedolizumab in a patient with chronic and refractory pouchitis. Ann Gastroenterol. 2018;31:379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Khan F, Gao XH, Singh A, Philpott JR, Shen B. Vedolizumab in the treatment of Crohn's disease of the pouch. Gastroenterol Rep (Oxf). 2018;6:184-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Martins D, Ministro P, Silva A. Refractory Chronic Pouchitis and Autoimmune Hemolytic Anemia Successfully Treated with Vedolizumab. GE Port J Gastroenterol. 2018;25:340-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Landy J, Al-Hassi HO, Ronde E, English NR, Mann ER, Bernardo D, Ciclitira PJ, Clark SK, Knight SC, Hart AL. Innate immune factors in the development and maintenance of pouchitis. Inflamm Bowel Dis. 2014;20:1942-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Fazio VW, Kiran RP, Remzi FH, Coffey JC, Heneghan HM, Kirat HT, Manilich E, Shen B, Martin ST. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg. 2013;257:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 520] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 16. | Shen B, Lashner B. Can we immunogenotypically and immunophenotypically profile patients who are at risk for pouchitis? Am J Gastroenterol. 2004;99:442-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Shen B, Achkar JP, Lashner BA, Ormsby AH, Remzi FH, Bevins CL, Brzezinski A, Petras RE, Fazio VW. Endoscopic and histologic evaluation together with symptom assessment are required to diagnose pouchitis. Gastroenterology. 2001;121:261-267. [PubMed] |