Published online Aug 26, 2019. doi: 10.12998/wjcc.v7.i16.2309
Peer-review started: April 15, 2019
First decision: May 31, 2019
Revised: July 9, 2019
Accepted: July 27, 2019
Article in press: July 27, 2019
Published online: August 26, 2019
Processing time: 134 Days and 8.2 Hours
Immunoglobulin G4-related disease (IgG4-RD) is an immune-mediated condition that consisted of disorders that share particular clinical, serologic and pathologic properties. The common presentation of disease includes tumor-like swelling of involved organs and the histopathological findings are a lymphoplasmacytic infiltrate enriched with IgG4-positive plasma cells, and a variable degree of fibrosis that has a characteristic "storiform" pattern in biopsy specimens of tumor-like masses. Major presentations of this disease, which often affects more than one organ, include autoimmune pancreatitis, salivary gland disease (sialadenitis), orbital disease and retroperitoneal fibrosis. The steroid treatment is essential for the treatment of the disease however, other immunosuppressive drugs including cyclophosphamide or rituximab could be an option in resistant cases.
Herein, we reported a 34-year-old woman whom previously had diagnosed with asthma, rheumatoid arthritis and Sjögren’s syndrome (SS) referred our nephrology department due to acute kidney failure development at the last rheumatology visit. After kidney biopsy she has been diagnosed with IgG4-RD and tubuluointerstitial nephritis. She had been accepted resistant to steroid, mycophenolate mofetil, methotrexate and azathioprine therapies due to receiving in last two years. She refused to receive cyclophosphamide due to potential gonadotoxicity of the drug. Thus, rituximab therapy was considered. She received 1000 mg infusion, 15 d apart and 6 mo later it has been administered same protocol. After one year from the last rituximab dose serum creatinine decreased from 4.4 mg/dL to 1.6 mg/dL, erythrocyte sedimentation rate decreased from 109 mm/h to 13 mm/h [reference range (RR) 0-20], and C-reactive protein decreased from 55.6 mg/L to 5 mg/L (RR 0–6). All pathologic lymph nodes and masses were also disappeared.
Patients with IgG4-RD usually misdiagnosed with rheumatologic diseases including systemic lupus erythematous or SS and also they were screened for the presence of malignancy. Rituximab could be an important treatment option in cases with steroid resistant tubulointerstitial nephritis in IgG4-RD.
Core tip: Immunoglobulin G4-related disease is an immune-mediated condition that mimics several rheumatologic disorders and malignancies. The diagnosis can be provided by detecting lymphoplasmacytic infiltration with storiform fibrosis in the histopathological examination of the affected organ. However, steroid is the mainstay treatment agent, rituximab should be addressed in resistant cases.
- Citation: Eroglu E, Sipahioglu MH, Senel S, Ertas SK, Savas S, Ozturk F, Kocyigit I, Tokgoz B, Oymak O. Successful treatment of tubulointerstitial nephritis in immunoglobulin G4-related disease with rituximab: A case report. World J Clin Cases 2019; 7(16): 2309-2315
- URL: https://www.wjgnet.com/2307-8960/full/v7/i16/2309.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i16.2309
Immunoglobulin G4-related disease (IgG4-RD) is a recently recognized systemic fibro-inflammatory condition that mimics several autoimmune, malignant, and rheumatological diseases[1]. The hallmarks of the disease are swelling of affected tissue(s)/organ(s) caused by a lymphoplasmacytic infiltrate enriched in IgG4-positive plasma cells, usually accompanied by fibrosis and an increased number of eosinophils[2]. Serum IgG4 levels are elevated in about two-thirds of these patients. In addition, a minority of patients have normal serum IgG4 levels despite the presence of the typical IgG4-RD-related histopathological changes[3,4].
IgG4-RD can involve one or multiple organs. Patients often present with subacute development of mass/masses or diffuse enlargement of the affected organ(s). Lymphadenopathy is an important finding, and asthma or allergy is present in approximately 40% of IgG4-RD patients. One of the most common conditions in these patients is autoimmune pancreatitis. This condition often presents as a pancreatic mass or painless obstructive jaundice and, often which can mimics pancreatic cancer. Moreover, patients often present with lacrimal and salivary gland involvement (IgG4-related Mikulicz’s disease), which was once thought to be a subset of Sjögren’s syndrome (SS). Orbital diseases, including orbital masses and retroperitoneal fibrosis, are other clinical presentations observed in IgG4-RD patients. Depending upon the multiorgan nature of the condition, several clinical phenotypes have recently been identified. These phenotypes include four main groups: (1) Pancreatic hepatobiliary disease; (2) Retroperitoneal fibrosis and/or aortitis; (3) Head-and neck-limited disease; and (4) Classic Mikulicz syndrome with systemic involvement. Renal involvement in patients with IgG4-RD is rarely seen. Only individual case series have been reported in the literature and the most frequent finding in these patients is tubulointerstitial nephritis (TIN)[5-7].
In this case report, we present a case previously misdiagnosed as SS because of the presence of sialadenitis. The patient was referred to our nephrology department because of development of acute kidney dysfunction that had been detected during her last rheumatology visit. Histopathological examination of the kidney biopsy revealed TIN with fibrosis. Serum IgG4 levels were predominantly increased, and the patient was diagnosed with IgG4-RD. Steroid therapy failed to lead to remission; however, complete remission achieved as a result of rituximab administration.
A 34-year-old female patient was admitted to the rheumatology department in July 2017 with complaints of nausea, joint pain, and swelling in the neck.
Her medical history was notable for asthma, rheumatoid arthritis (RA), SS, and sialadenitis.
She had been undergoing follow-up for RA and SS in the rheumatology department since 2015. Her last follow-up was in November 2016, and laboratory results revealed a rheumatoid factor of 273 IU/mL [reference range (RR) 0-20], anti-cyclic citrullinated peptide (anti-CCP) of < 1.5 U/mL (RR 1.5-1.93), creatinine of 0.74 mg/dL (RR 0.5-0.9 mg/dL), erythrocyte sedimentation rate (ESR) of 109 mm/h (RR 0-20), and C-reactive protein (CRP) of 55.6 mg/L (RR 0–6).
Upon initial physical examination, a 2-3 cm palpable cervical lymphadenomegaly was detected. She had clear lungs and normal heart sounds with no murmurs or gallops upon auscultation. Her current medication upon admission included methotrexate (15 mg once weekly injection), methylprednisolone (16 mg once daily), calcium carbonate (2500 mg) plus cholecalciferol/vitamin D3 (880 IU once daily).
Laboratory results revealed several abnormal findings: Hemoglobin: 10.8 g/dL (RR 14-18 g/dL); blood urea nitrogen 33 mg/dL (RR 8-23 mg/dL); creatinine 4.4 mg/dL (RR 0.5-0.9 mg/dl); K: 5.6 mmol/L (3.5-5.1 mmol/L); phosphorus 4.6 mmol/L (RR 2.5-4.5 mmol/L); uric acid: 8.4 mg/dL (RR 2.4-5.7 mg/dL); ferritin: 221.8 ng/mL (RR 15-150 ng/mL); ESR: 84 mm/h (RR 0-20); and CRP: 23 mg/L (RR 0-6 mg/L). All laboratory results are depicted in Table 1. The patient was referred to the nephrology department. A urine stick test revealed one positive protein. Urine microscopy was negative for casts. The 24-h urine protein level was 0.8 g. Anti-nuclear antibody, anti-double-stranded DNA (dsDNA), anti-glomerular basement membrane, and antineutrophil cytoplasmic antibodies profiles were all negative. The C3 level was 60 mg/dL (RR 90-180 mg/dL), and the C4 level was 15.3 (RR 10-40).
| Parameter | Value | Normal Range |
| WBC (103/µL) | 6.63 | 4.8-10.7 |
| Hemoglobin (g/dL) | 10.8 | 12-16 |
| Platelet (103/µL) | 286 | 130-400 |
| MCV (fl) | 92 | 80-100 |
| Glucose (mg/dL) | 98 | 82-115 |
| BUN (mg/dL) | 33.5 | 8-23 |
| Creatinine (mg/dL) | 4.4 | 0.5-0.9 |
| Calcium (mg/dL) | 9.6 | 8.8-10.2 |
| Phosphorus (mg/dL) | 4.6 | 2.5-4.5 |
| Sodium (mmol/L) | 139 | 136-145 |
| Potassium (mmol/L) | 5.6 | 3.5-5.1 |
| Uric acid(mg/dL) | 8.4 | 2.4-5.7 |
| Total bilirubin (mg/dL) | 0.51 | 0.2-1.2 |
| Direct bilirubin (mg/dL) | 0.16 | 0-0.3 |
| GGT (U/L) | 11 | 6-42 |
| LDH (U/L) | 141 | 135-214 |
| AST (U/L) | 9 | 0-32 |
| ALT (U/L) | 12 | 0-33 |
| ALP (U/L) | 46 | 35-105 |
| Total protein (g/dL) | 8.3 | 6.4-8.3 |
| Albumin (g/dL) | 3.82 | 3.5-5 |
| Iron (µg/dL) | 80 | 70-180 |
| Iron binding (µg/dL) | 320 | 225-480 |
| Ferritin (ng/mL) | 221.8 | 15-150 |
| Folic acid (ng/mL) | 4.5 | 3.89-26.8 |
| Vitamin B12 (pg/mL) | 386.6 | 197-771 |
| ESR (mm/h) | 84 | 0-20 |
| CRP (mg/L) | 23 | 0-6 |
| iPTH (pg/mL) | 35 | 15-65 |
Hypereosinophilia was detected on a peripheral blood smear (eosinophil count was 3.99 × 103/L; RR is 0-0.2 × 103/L), eosinophil percentage was 37.3% (RR 0.9%-2.9%), and the IgG4 level was 2602 mg/dL (RR 3-201 mg/dL).
Renal ultrasonography indicated enlarged bilateral kidneys. The right and left kidneys had long axes of 159 and 181 mm, respectively. Bilateral renal echoes were significantly decreased, and hypoechoic areas were observed in both kidneys. Among the enlarged area, the largest one was 54 mm × 42 mm in the lower middle section of the left kidney.
Computed tomography (CT) revealed nodular lesions in the liver, a pancreatic mass, cervical and mediastinal lymph nodes of 2 to 3 cm in diameter.
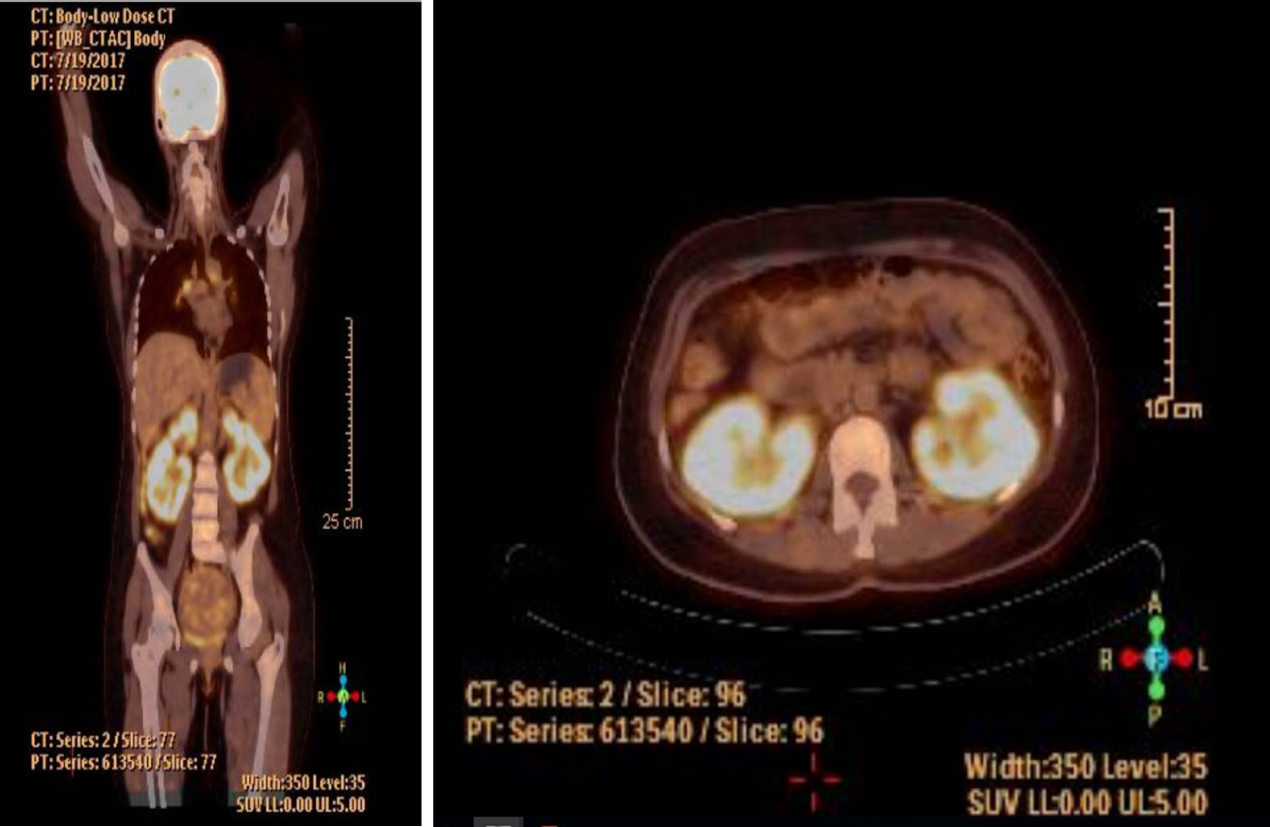
Positron emission tomography/CT (PET-CT) screening was used based on suspicion of metastatic malignancy or lymphoma. It has been revealed that cervical and mediastinal lymph nodes had high metabolic activity. Infiltrative soft tissue masses were observed ranging from 2 to 4 cm in size in both kidneys. These masses have showed intense hypermetabolic activity in the cortical areas of the kidney parenchyma (standardized uptake value max: 8.01) (Figure 1).
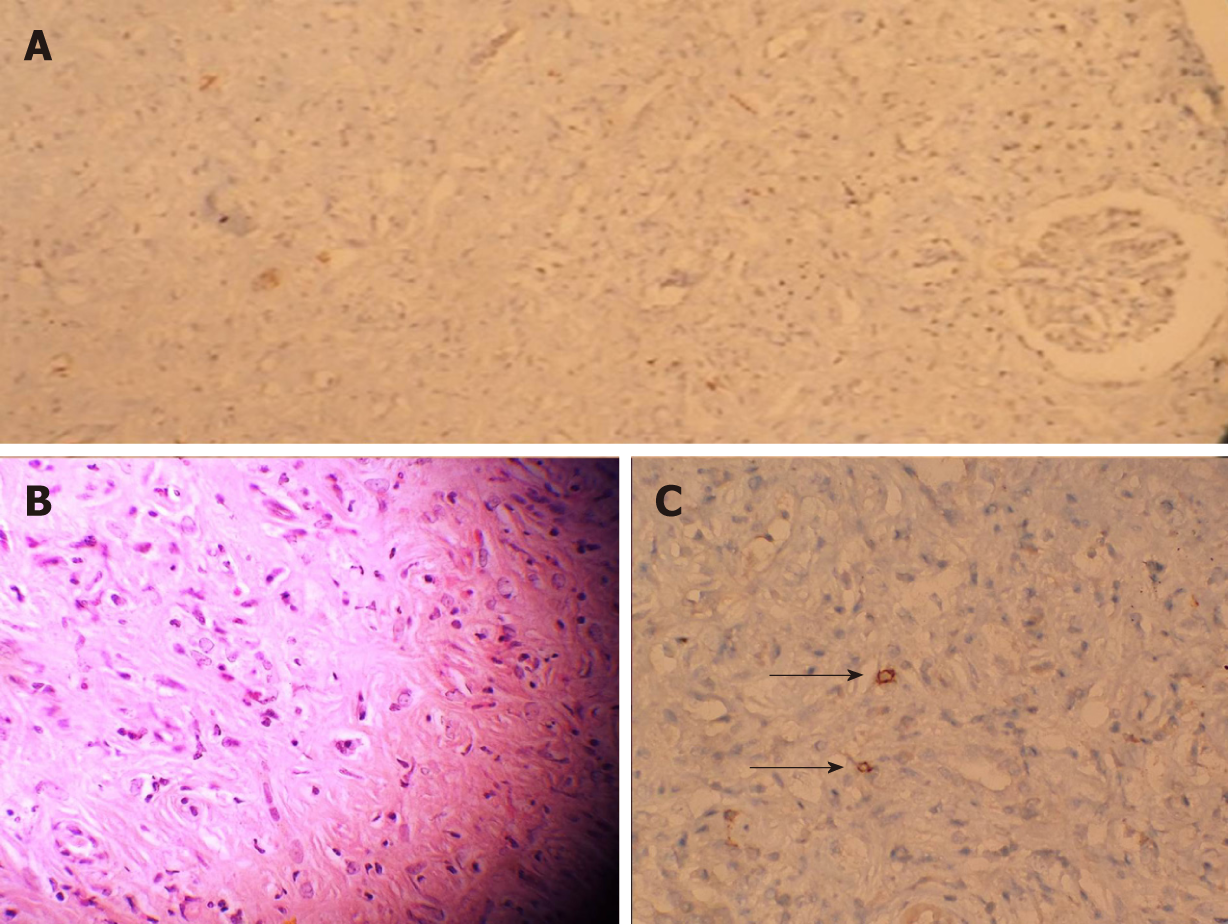
A kidney biopsy was performed. The biopsy showed intense, connective tissue infiltration between the glomerular and tubular structures. Lymphoplasmacytic and eosinophilic cell infiltrations were observed. IgG4-stained cells were detected on the biopsy specimen (Figure 2).
The patient was diagnosed with TIN and IgG4-RD.
A two-hundred fifty milligram intravenous (iv) pulse of methylprednisolone treatment was administered for three days. Afterwards 1 mg/kg (64 mg) oral methylprednisolone initiated and continued. After ten weeks with steroid treatment, her creatinine level was 3.5 mg/dL in October 2017. Steroid resistance was considered and steroid dose was tapered gradually. She had been treated with mycophenolate mofetil, methotrexate and azathioprine previously in last two years and she had been accepted resistant to these therapies. She refused to receive cyclophosphamide due to her child-bearing potential. Thus, rituximab therapy was considered. The 2 infusions of 1000 mg, 15 d apart. After six months dose was repeated due to partial response in May 2018. Total dose of rituximab reached to 4000 mg.
Her last visit was in May 2019. After one year of rituximab therapy serum creatinine level decreased from 4.4 mg/dL to 1.6 mg/dL, ESR decreased from 109 mm/h to 13 mm/h (RR 0-20), and CRP decreased from 55.6 mg/L to 5 mg/L (RR 0–6). She has no complaint. All pathologic lymph nodes and masses were also disappeared. Clinical and laboratory remission was achieved. The steroid therapy withdrawal was achieved six months ago.
The diagnosis of IgG4-RD is often challenging due to a complex presentation as this condition mimics malignancies and autoimmune rheumatic diseases. Herein, we reported a case of IgG4-RD and TIN that was diagnosed by kidney biopsy. IgG4-RD rarely involves the kidneys, but the finding of kidney involvement has been described in some case series and in individual case reports. The most common renal-related finding is TIN. However, it has been reported that many of the affected IgG4-RD patients with kidney disease are middle-aged and older men, and our particular case involved a middle-aged woman[1,8]. Lymphoplasmacytic infiltration of the renal interstitium and presence of fibrosis were distinct features to distinguish IgG4RD-TIN from other TIN cases as has been shown in our case. Immunohistochemistry staining of renal biopsy specimen have revealed increased numbers of IgG4-positive plasma cells supported the diagnosis of IgG4RD-TIN. On the other hand, nodular lesions in kidneys mimicking metastatic malignancy is a very rare condition as in this case[2,9]. Kawano et al[10] recently reported a series of 41 Japanese IgG4-RD patients identified between 2004 and 2011 who presented with histopathological findings consistent with kidney involvement. Twenty-nine patients underwent CT scans, and the most common radiological findings were multiple low-density lesions with subsequent diffuse bilateral renal swelling as the second most common involvement. A solitary hypovascular parenchymal nodule was detected in just one patient in that study. More recently, Bianchi et al. reported an IgG4-RD case that presented with a solid mass in kidney, which mimicked a malignancy[9]. Our case also showed both bilateral enlarged kidneys with multiple lesions in both kidneys. This is important because establishing a differential diagnosis between IgG4-RD and malignancy is challenging. It has been well-established that conventional imaging techniques (ultrasound, CT, magnetic resonance imaging, and PET/CT) have limited efficiency in diagnosing IgG4-RD[11]. A biopsy from the affected organ remains the cornerstone for the IgG4-RD diagnosis[6].
However, the optimal treatment for IgG4-RD has not yet been well defined yet, an international consensus guideline has recently been published[12]. The treatment recommendations consist of several issues: (1) Glucocorticoids are the first-line drugs for remission induction in all patients with active, IgG4-RD, unless contraindications to steroids are present; (2) After successful achievement of induction therapy, certain patients will need maintenance therapy; and (3) Retreatment with glucocorticoids is indicated in some patients in whom relapse following successful remission induction was observed. Otherwise, some experts recommend the initiation of steroid-sparing immunosuppressive agents from treatment start due to the ultimate failure of steroid therapy and potential toxicities associated with long-term steroid treatment. Steroids and cyclophosphamide combinations have recently been reported to cause a low risk of relapse in these patients[13]. The risk of infertility could also be a barrier to this treatment combination, especially in women of child-bearing potential as in our case.
It has been shown that B-cell depletion leads to the targeted and quick reduction of serum IgG4 concentrations, with relative preservation of the concentrations of other immunoglobulins and immunoglobulin subclasses. Authors also speculate that rituximab achieves its effects in IgG4-RD by depleting the pool of B lymphocytes that replenish short-lived IgG4-secreting plasma cells[14-16]. Thus, rituximab was considered as a promising option for these cases. When we review the literature, the efficacy of B-cell depletion with rituximab in patients with that is resistant to steroids and other therapies has been recently demonstrated. Khosroshahi et al[14,15] have been treated successfully total 14 patients with IgG4-RD in a two different case series in the literature. They showed that almost all patients had clinical and laboratory response to rituximab. Only a few patients (4 of 14) have received second infusion six months after first infusion. More recently a retrospective study has been conducted in France with 156 patients with IgG4-RD and 33 patients have been treated with rituximab. Clinical response was noted in 29/31 (93.5%) symptomatic patients. Maintenance therapy (second dose six months after initial dose before occurrence of a relapse) with rituximab have been associated with longer relapse-free survival (41 vs 21 mo; P = 0.02) comparing to patients whom had been administered single rituximab dose[16]. They concluded that rituximab might be a novel treatment option for both induction and maintenance therapy in these patients. However, rituximab has not been evaluated in a randomized trial in patients with IgG4-RD, and its use for this disease might be evaluated for off-label use by drug control agencies.
In this unique case, we showed that rituximab therapy was successful in IgG4-RD and TIN treatment especially resistant to steroid and other therapies. Future randomized trials with larger patients are needed to establish the usage of rituximab in these patients.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elzawawy A, Tanaka H S-Editor: Dou Y L-Editor: A E-Editor: Liu JH
| 1. | Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1856] [Cited by in RCA: 1863] [Article Influence: 143.3] [Reference Citation Analysis (83)] |
| 2. | Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385:1460-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 847] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 3. | Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis. 2015;74:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 353] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 4. | Wallace ZS, Deshpande V, Mattoo H, Mahajan VS, Kulikova M, Pillai S, Stone JH. IgG4-Related Disease: Clinical and Laboratory Features in One Hundred Twenty-Five Patients. Arthritis Rheumatol. 2015;67:2466-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 457] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 5. | Uchida K, Masamune A, Shimosegawa T, Okazaki K. Prevalence of IgG4-Related Disease in Japan Based on Nationwide Survey in 2009. Int J Rheumatol. 2012;2012:358371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Salvadori M, Tsalouchos A. Immunoglobulin G4-related kidney diseases: An updated review. World J Nephrol. 2018;7:29-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 7. | Wallace ZS, Zhang Y, Perugino CA, Naden R, Choi HK, Stone JH; ACR/EULAR IgG4-RD Classification Criteria Committee. Clinical phenotypes of IgG4-related disease: an analysis of two international cross-sectional cohorts. Ann Rheum Dis. 2019;78:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 259] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 8. | Saeki T, Nishi S, Imai N, Ito T, Yamazaki H, Kawano M, Yamamoto M, Takahashi H, Matsui S, Nakada S, Origuchi T, Hirabayashi A, Homma N, Tsubata Y, Takata T, Wada Y, Saito A, Fukase S, Ishioka K, Miyazaki K, Masaki Y, Umehara H, Sugai S, Narita I. Clinicopathological characteristics of patients with IgG4-related tubulointerstitial nephritis. Kidney Int. 2010;78:1016-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 277] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 9. | Bianchi D, Topazio L, Gaziev G, Iacovelli V, Bove P, Mauriello A, Finazzi Agrò E. IgG4-Related Kidney Disease: Report of a Case Presenting as a Renal Mass. Case Rep Surg. 2017;2017:9690218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Kawano M, Saeki T, Nakashima H, Nishi S, Yamaguchi Y, Hisano S, Yamanaka N, Inoue D, Yamamoto M, Takahashi H, Nomura H, Taguchi T, Umehara H, Makino H, Saito T. Proposal for diagnostic criteria for IgG4-related kidney disease. Clin Exp Nephrol. 2011;15:615-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 289] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 11. | Horger M, Lamprecht HG, Bares R, Spira D, Schmalzing M, Claussen CD, Adam P. Systemic IgG4-related sclerosing disease: spectrum of imaging findings and differential diagnosis. AJR Am J Roentgenol. 2012;199:W276-W282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Khosroshahi A, Wallace ZS, Crowe JL, Akamizu T, Azumi A, Carruthers MN, Chari ST, Della-Torre E, Frulloni L, Goto H, Hart PA, Kamisawa T, Kawa S, Kawano M, Kim MH, Kodama Y, Kubota K, Lerch MM, Löhr M, Masaki Y, Matsui S, Mimori T, Nakamura S, Nakazawa T, Ohara H, Okazaki K, Ryu JH, Saeki T, Schleinitz N, Shimatsu A, Shimosegawa T, Takahashi H, Takahira M, Tanaka A, Topazian M, Umehara H, Webster GJ, Witzig TE, Yamamoto M, Zhang W, Chiba T, Stone JH; Second International Symposium on IgG4-Related Disease. International Consensus Guidance Statement on the Management and Treatment of IgG4-Related Disease. Arthritis Rheumatol. 2015;67:1688-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 670] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 13. | Yunyun F, Yu C, Panpan Z, Hua C, Di W, Lidan Z, Linyi P, Li W, Qingjun W, Xuan Z, Yan Z, Xiaofeng Z, Fengchun Z, Wen Z. Efficacy of Cyclophosphamide treatment for immunoglobulin G4-related disease with addition of glucocorticoids. Sci Rep. 2017;7:6195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Khosroshahi A, Bloch DB, Deshpande V, Stone JH. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum. 2010;62:1755-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 407] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 15. | Khosroshahi A, Carruthers MN, Deshpande V, Unizony S, Bloch DB, Stone JH. Rituximab for the treatment of IgG4-related disease: lessons from 10 consecutive patients. Medicine (Baltimore). 2012;91:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 340] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 16. | Ebbo M, Grados A, Samson M, Groh M, Loundou A, Rigolet A, Terrier B, Guillaud C, Carra-Dallière C, Renou F, Pozdzik A, Labauge P, Palat S, Berthelot JM, Pennaforte JL, Wynckel A, Lebas C, Le Gouellec N, Quémeneur T, Dahan K, Carbonnel F, Leroux G, Perlat A, Mathian A, Cacoub P, Hachulla E, Costedoat-Chalumeau N, Harlé JR, Schleinitz N. Long-term efficacy and safety of rituximab in IgG4-related disease: Data from a French nationwide study of thirty-three patients. PLoS One. 2017;12:e0183844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |