Published online Aug 26, 2019. doi: 10.12998/wjcc.v7.i16.2302
Peer-review started: March 25, 2019
First decision: June 4, 2019
Revised: June 29, 2019
Accepted: July 27, 2019
Article in press: July 27, 2019
Published online: August 26, 2019
Processing time: 158 Days and 4 Hours
Primary malignant melanoma of the biliary tract (MBT) is a rare condition whose diagnosis requires excluding a primary origin in another location. This paper reviews the most important characteristics of MBT cases published in the literature and reports a new case. The patient reported here is the first case of primary malignant melanoma of the biliary tract with pulmonary metastasis treated with immunotherapy. This patient remains disease-free 36 mo after the treatment of metastatic lung lesions.
A 51-year-old man was admitted to the gastrointestinal department to study obstructive jaundice of a 1 wk clinical course. Magnetic resonance cholangiopancreatography revealed dilatation of the intrahepatic biliary tract and stenosis of the common hepatic duct. Given the suspicion of biliary tract neoplasia, cholecystectomy and resection of the common hepatic duct were performed with hepatic jejunostomy free of complications. Anatomo-pathological diagnosis was melanoma. After intervention, the patient was referred to the Department of Medical Oncology, where a primary origin was excluded in the skin, mucosa, and eyes. This confirmed diagnosis of primary biliary tract melanoma. Computed tomography was performed 12 mo after the procedure revealed several subcentimetric lung nodules. Wedge resection was performed. After confirming the diagnosis of pulmonary metastasis of primary melanoma of the biliary tract, the patient was started on immunotherapy with nivolumab. Tolerance to treatment was excellent. The patient remains disease-free 36 mo after the treatment of metastatic lung lesions.
The patient reported here is the first case of primary malignant melanoma of the biliary tract with lung metastases successfully treated with immunotherapy.
Core tip: Primary malignant melanoma of the biliary tract is a very rare entity that mainly affects men (men/women ratio 12:2) and has an average age of presentation of 47 years (range: 26-67). Cases usually present as abdominal pain and jaundice. Lesions are usually black, polypoid, and have endoluminal growth. In cases of localised disease, the treatment of choice is surgery. Mortality because of this disease was 50% after average follow-up of 37 mo (range: 4-204 mo). Here, for the first time, we describe a case of primary malignant melanoma of the biliary tract with pulmonary metastases, successfully treated with immunotherapy.
- Citation: Cameselle-García S, Pérez JLF, Areses MC, Castro JDF, Mosquera-Reboredo J, García-Mata J. Primary malignant melanoma of the biliary tract: A case report and literature review. World J Clin Cases 2019; 7(16): 2302-2308
- URL: https://www.wjgnet.com/2307-8960/full/v7/i16/2302.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i16.2302
Melanoma is a malignant tumour arising from melanocytes whose origin is the neural crest[1]. This is a very aggressive neoplasia with a high capacity for remote dissemination[2]. In the last few years there has been a major increase in the incidence of this neoplasia[3,4] due to early diagnosis and overdiagnosis of benign lesions[5,6], whereby mortality remains virtually stable[4,7,8]. Its location in the mucosa is less common and associated with a delay in diagnosis2. Location in the biliary tract in the form of primary tumour is exceptional with only 13 cases reported in the literature[9-20]. Increased knowledge of molecular biology has enabled more development of treatments for this disease[2,4]. This paper reviews the most important features of primary malignant melanoma of the biliary tract (MBT) cases using the PubMed database (up to January 2019) and reports the first patient treated with immunotherapy.
A 51-year-old man with a personal history of smoking (one pack/d) and high blood pressure. The patient was admitted to the gastrointestinal department to study obstructive jaundice of a 1 wk clinical course.
Initial analysis revealed: total bilirubin 13.9 mg/dL, GGT 900 U/L, and AF 324 U/L.
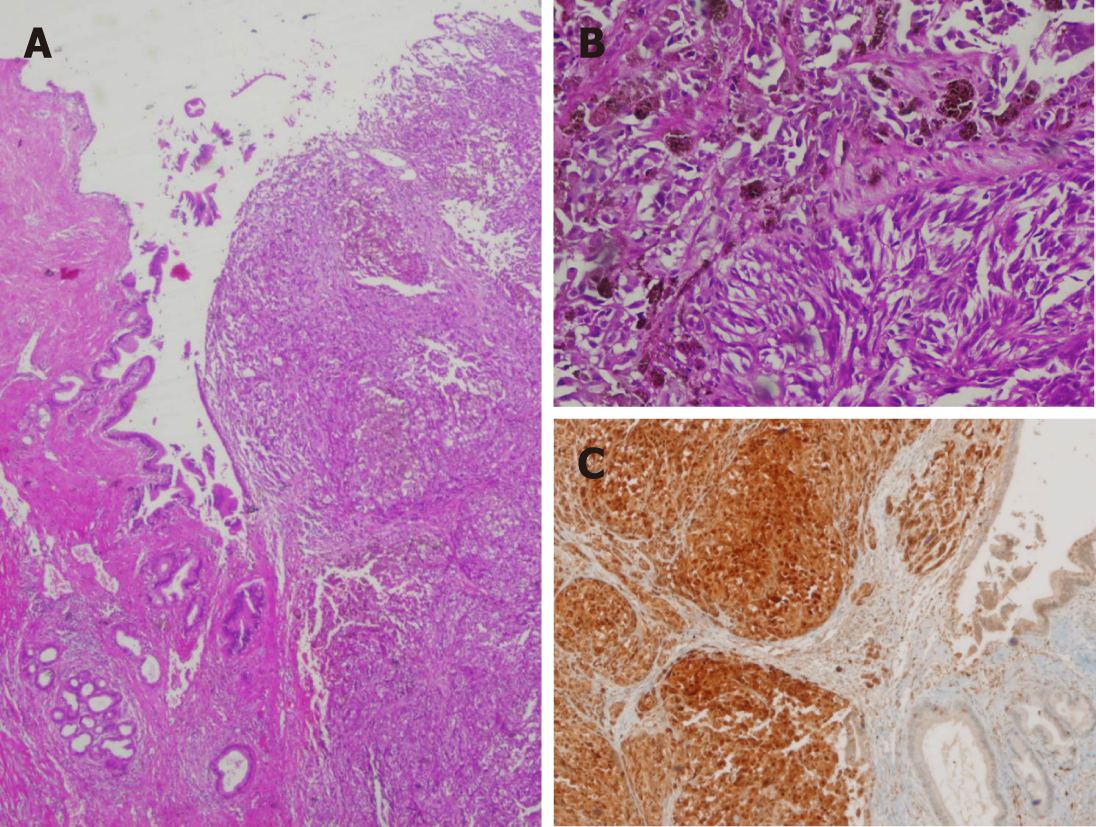
Magnetic resonance cholangiopancreatography (MRC) revealed dilatation of the intrahepatic biliary tract and stenosis of the common hepatic duct. Cytological samples obtained by endoscopic retrograde cholangiopancreatography were negative for malignancy (non-representative samples). Abdominal computed tomography (CT) confirmed the findings of the MRC and was negative for distant metastasis. Given the suspicion of biliary tract neoplasia, cholecystectomy and resection of the common hepatic conduct were performed with hepatic jejunostomy free of complications. Anatomopathological examination, including the immunohistochemical study, revealed a malignant melanoma measuring 1 cm in diameter (Figure 1). A total of two adenopathies were analysed on a perichole-cystic and peri-portal level; these did not reveal metastasis.
After intervention, the patient was referred to the Department of Medical Oncology, where primary origin was excluded in the skin, mucosa, and eyes. This confirmed diagnosis of primary biliary tract melanoma.
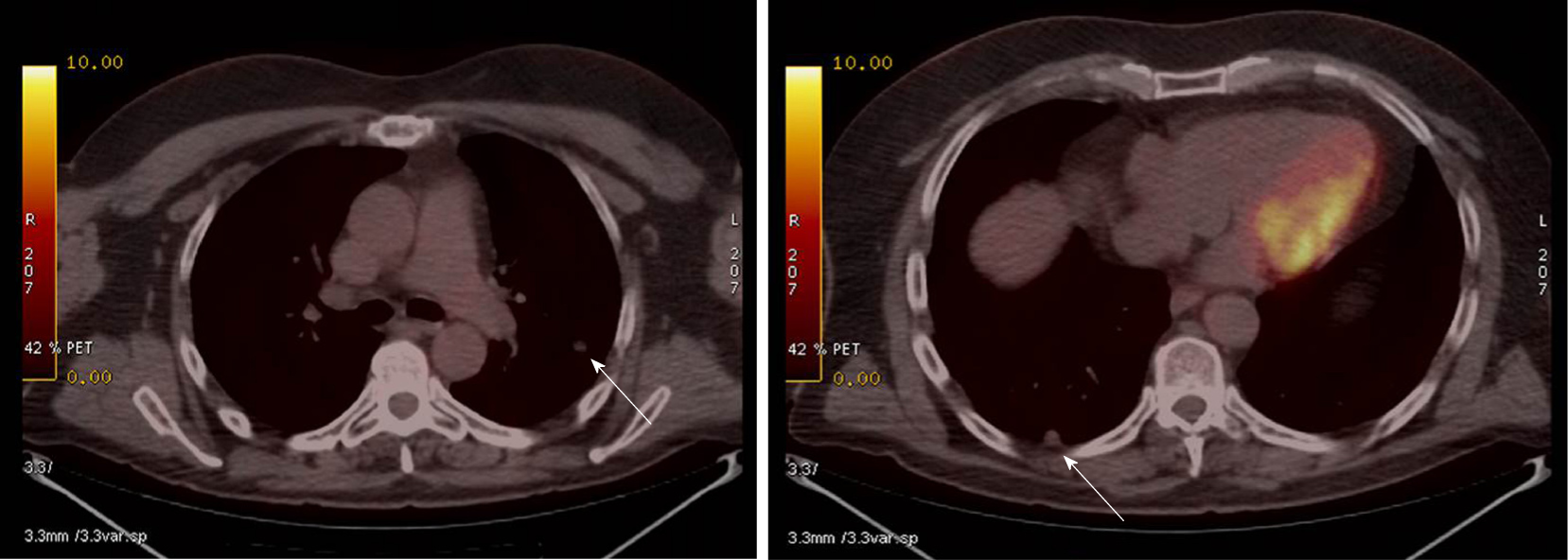
CT performed 12 mo after the procedure revealed several subcentimetric lung nodules in the posterior and apical segments of the left upper lobe (LUL), in the anterior and superior segments of the left lower lobe (LLL), and in the posterior-basal region of the right lower lobe (RLL). These nodules revealed pathological increased uptake on positron emission tomography-CT (Figure 2). Wedge resection was performed on the LUL and LLL free of complications. Second, atypical segmentectomy was performed in the RLL. Anatomopathological study confirmed the diagnosis of lung metastasis from melanoma (positive for HMB45, vimentin, and S100 protein but negative for cytokeratin in the immunohistochemical studies). BRAF gene mutation in tumour tissue was studied by real-time polymerase chain reaction (Cobas 4800 BRAF V600 Mutation Test; Roche Diagnostics, Indianapolis, IN, United States); this proved to be negative.
Pulmonary metastasis from primary melanoma of the biliary tract.
After confirming the diagnosis of pulmonary metastasis from primary malignant melanoma of the biliary tract, the patient started treatment with intravenous nivolumab at a dose of 3 mg/kg every 2 wk.
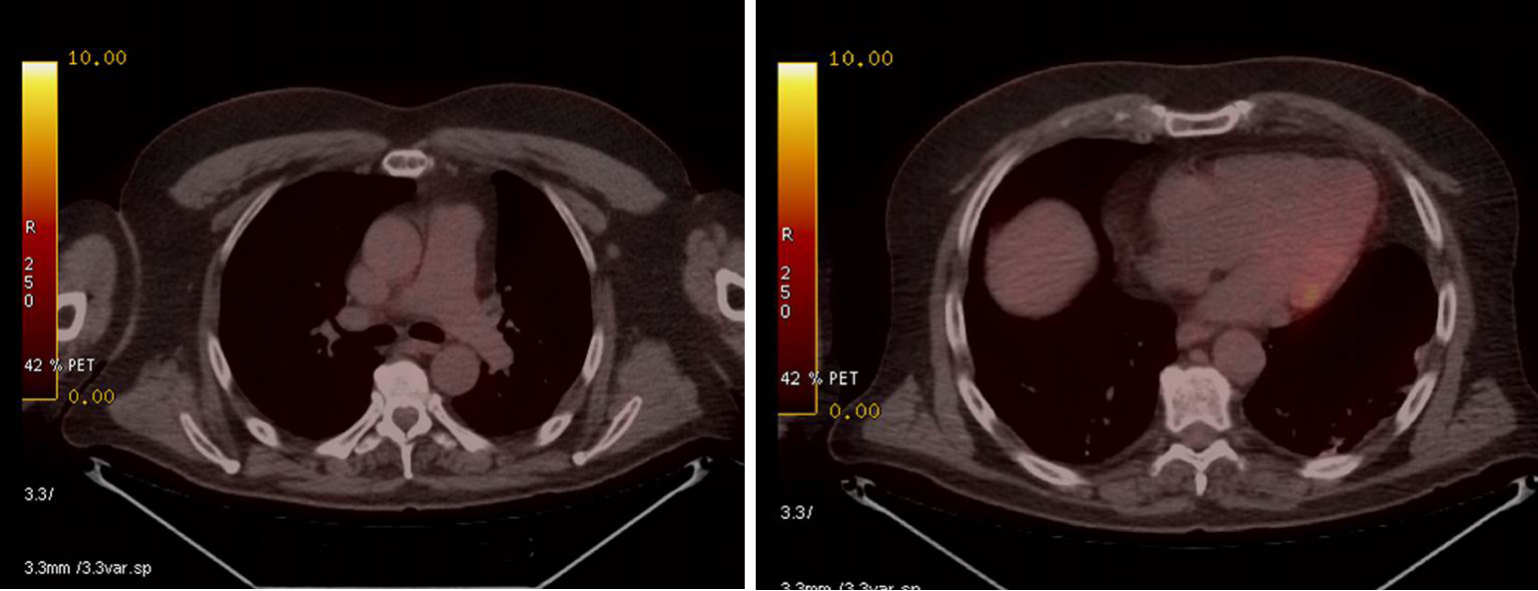
Positron emission tomography-CT studies performed 4 mo from starting nivolumab, subsequently every 6 mo and recently at 36 mo from pulmonary surgery, revealed a prolonged complete response (Figure 3). Tolerance to treatment was excellent; no immunomediated toxicity presented.
Melanoma is considered a multifactorial disease that arises from interactions between genetic susceptibility and environmental exposure[2,4]. Ultraviolet radiation (UVR) is the most significant modifiable risk factor. Both UVA-A and UVR-B have genotoxic effects either because of generation of oxygen radicals or abnormality in the nucleotide sequence, among others[21]. Melanomas are mainly classified according to their location and relationship to solar exposure[2]. Types of melanoma that arise on the skin exposed to sun are: Melanoma with superficial extension/melanoma with low cumulative solar damage, lentigo-malignant melanoma/melanoma with significant solar damage, and desmoplastic melanoma[2]. Melanomas that arise on skin protected from the sun or free of aetiological association UVR are: spitz melanoma, acral melanoma, melanoma of mucosa, melanoma that arises in congenital naevi, melanoma that arises in blue naevus, and uveal melanoma[2]. The four most common histological subtypes of melanoma are superficial spreading melanoma, lentigo maligna melanoma, acrallentiginous melanoma, and nodular melanoma[2].
Although these are very useful classifications in clinical practice, new classifications are being developed based on genetic abnormalities, which have therapeutic indications. In general, melanoma is a tumour with a high mutational load[2,22]. Mutations are common in driver genes such as BRAF and RAS[2,23]. Those melanomas that appear in exposed areas have more abnormalities in the genes BRAF, N-RAS, and PTEN. However, it appears that those not exposed to UVR (melanoma of mucosa and acral melanoma) have a higher number of chromosomal abnormalities (amplifications and deletions) and gene amplification, essentially of CDK4 and CCND1[2,23]. Therefore, it appears that melanomas that arise in areas not exposed to radiation would have a higher mutational load, and therefore, predict a greater response to immuno-therapy[22].
The most common melanoma location is on the skin, mainly the trunk (43.5%) and the limbs (33.9%)[2,7]. Melanoma is a highly aggressive tumour that can disseminate to any part of the body, although metastases are more common in the lymph nodes, liver, lungs, and brain. Only 2% to 4% of patients present metastasis in the gastrointestinal system, mainly in the bowel, colon, and stomach[24]. There are unusual cases of solitary metastasis of melanoma to the gallbladder[25] and asymptomatic metastasis has been reported in the gallbladder and biliary tract in up to 4% to 20% of autopsies performed on patients with metastatic melanoma[26,27]. Therefore, another primary origin in the event of existence of melanoma in the biliary tract should be ruled out. Along these lines, Ricci et al[28] proposed five criteria that could provide guidance around diagnosis of primary melanoma in the biliary tract: (1) Absence of previous melanoma; (2) Absence of melanoma in other locations; (3) Single solitary lesion; (4) Polyploid or papillary form; and (5) Existence of biliary obstructive clinical symptoms.
After reviewing the literature and including this case, there are only 14 cases of MBT reported to date (Table 1). MBT is a very rare entity that mainly affects men (men/women ratio 12:2) and has an average age of presentation of 47 years (range: 26-67). The most common form of presentation is abdominal pain together with jaundice and at times a general syndrome. In cases with macroscopic data lesions were black, polyploid, and with endoluminal growth, which conditioned obstructive clinical symptoms associated with clinical presentation. Although only three patients were revealed to have metastatic disease at diagnosis, up to seven revealed remote course after the procedure. The most common site of metastatic dissemination documented was the liver, although other locations were the brain, lung, mesentery, and pelvis. In all cases of localised disease, the treatment of choice was surgery. Radical surgery was performed in those with good “performance status”; duodenopancreatectomy was the most common intervention. Only two patients were not candidates for surgery due to their general condition and/or disease extension at the time of diagnosis.
| Ref. | Age/sex | Clinical presentation | Tumour location | Metastasis at presentation | Treatment | Outcome |
| Zaide[9] | 47/M | Jaundice | Proximal CHD | No | Cholecystectomy and resection of CHD | DFS (6 mo) |
| Carstens et al[10] | 30/M | Jaundice; Abdominal pain | Distal CBD | No | Whipple resection | PD (liver); Expired (6 mo) |
| Deugnier et al[11] | 34/F | Jaundice; Abdominal pain | LHD | No | Left hepatectomy with partial right hepatic and CBD resection | PD (skin and lymph nodes); Expired (18 mo) |
| Zhang et al[12] | 58/M | Jaundice | CBD and gallbladder | No | Whipple resection | PD; Expired (48 mo)[13] |
| Washburn et al[13] | 43/M | Jaundice; Abdominal pain; Loss of weight | CHD and RHD | No | Right hepatic lobectomy and cholecystectomy | DFS (11 mo) |
| Washburn et al[13] | 45/M | Jaundice; Loss of weight | Distal CBD and ampulla | No | Whipple resection | DFS (72 mo) |
| Wagner et al[14] | 48/M | Jaundice and acute cholecystitis | CBD | No | Whipple resection | PD (widespread metastases); Expired (9 mo) |
| González et al[15] | 67/F | Jaundice; Abdominal pain | Proximal CHD | No | Extrahepatic BD resection | DFS (17 yr) |
| Bejarano et al[16] | 47/M | Jaundice; Abdominal pain; Loss of weight | Distal CBD | Yes (gallbladder) | Chemotherapy; (dacarbacin + IFN2B, Carboplatin | PD (stomach, spleen, lymph nodes, subcutaneous kidney); Expired (23 mo) |
| Hoshi et al[17] | 55/M | Jaundice | CHD | Unknown | Bile duct resection | PD; Expired (4 mo) |
| Agrawal et al[18] | 26/M | Abdominal pain | Intrahepatic biliary tract | No | Hepatic segmentectomy (II-III) | DFS (72 mo) |
| Smith et al[19] | 55/M | Jaundice | Distal CBD | No | Whipple resection | DFS (12 mo) |
| Addepally et al[20] | 52/M | Jaundice; Abdominal pain; Loss of weight | CHD | No | BCS | PD (liver, lymph nodes, mesentery and brain) Expired (3 mo) |
| Present case | 51/M | Jaundice | CHD | No | Cholecystectomy and CHD resection PD (lung): Lung segmentectomy + nivolumab | PD (lung, 12 mo); OS (31 mo) |
In the last few years, immunotherapy has changed the paradigms for treatment of metastatic melanoma. Currently, first-line treatment of metastatic melanoma or non-resectable wild-type BRAF is considered, which demonstrates benefits on overall survival, progression-free survival, response rates, and duration of response[29]. While most immunotherapy clinical trials only include malignant melanomas of the skin, this case exemplifies that the excellent response of malignant melanoma to this therapy is not limited exclusively to primary malignant melanomas of the skin.
Until a few years ago, malignant melanoma was considered one of the most aggressive tumours and there were hardly any effective systemic treatment options. The overall survival of a patient with metastatic melanoma was less than 1 year. However, knowledge of melanoma’s molecular biology has meant a spectacular change in regard to treatment and survival of melanoma patients. Contrary to what was believed, a greater mutational load has been revealed in those melanomas that appear in sites not exposed to UV[2]. This has meant a greater efficacy of immunotherapy[30]. Here, for the first time, we describe a case of primary biliary malignant melanoma with pulmonary metastases, successfully treated with immunotherapy.
nformed consent statement: Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Luo JC, Rungsakulkij N, Sugimoto M S-Editor: Gong ZM L-Editor: Filipodia E-Editor: Liu JH
| 1. | Le Douarin NM. Cell migrations in embryos. Cell. 1984;38:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | LElder DE, Massi D, Scolyer RA, Willemze R (editors). WHO classifications of the skin tumours. 4th edition. Lyon: IARC. 2018;. |
| 3. | Geller AC, Clapp RW, Sober AJ, Gonsalves L, Mueller L, Christiansen CL, Shaikh W, Miller DR. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol. 2013;31:4172-4178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Rastrelli M, Tropea S, Rossi CR, Alaibac M. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014;28:1005-1011. [PubMed] |
| 5. | Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1141] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 6. | Esserman LJ, Thompson IM, Reid B, Nelson P, Ransohoff DF, Welch HG, Hwang S, Berry DA, Kinzler KW, Black WC, Bissell M, Parnes H, Srivastava S. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol. 2014;15:e234-e242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 373] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 7. | Marks R. Epidemiology of melanoma. Clin Exp Dermatol. 2000;25:459-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 188] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Hopkins ZH, Moreno C, Carlisle R, Secrest AM. Melanoma prognosis in the United States: Identifying barriers for improved care. J Am Acad Dermatol. 2019;80:1256-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Carstens HB, Ghazi C, Carnighan RH, Brewer MS. Primary malignant melanoma of the common bile duct. Hum Pathol. 1986;17:1282-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Deugnier Y, Turlin B, Léhry D, Pennarun JR, Verger P, Launois B, Ramée MP. Malignant melanoma of the hepatic and common bile ducts. A case report and review of the literature. Arch Pathol Lab Med. 1991;115:915-917. [PubMed] |
| 12. | Zhang ZD, Myles J, Pai RP, Howard JM. Malignant melanoma of the biliary tract: a case report. Surgery. 1991;109:323-328. [PubMed] |
| 13. | Washburn WK, Noda S, Lewis WD, Jenkins RL. Primary malignant melanoma of the biliary tract. Liver Transpl Surg. 1995;1:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Wagner MS, Shoup M, Pickleman J, Yong S. Primary malignant melanoma of the common bile duct: a case report and review of the literature. Arch Pathol Lab Med. 2000;124:419-422. [PubMed] |
| 15. | González QH, Medina-Franco H, Aldrete JS. [Melanoma of the bile ducts. Report of a case and review of the literature]. Rev Gastroenterol Mex. 2001;66:150-152. [PubMed] |
| 16. | Bejarano González N, García Moforte N, Darnell Martín A, Dinarès Fernández MC, Laporte Roselló E, Navarro Soto S. Primary malignant melanoma of the common bile duct: a case report and literature review. Gastroenterol Hepatol. 2005;28:382-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Hoshi K, Saito Y, Aznai R, Tanno H. A case of primary malignant melanoma of the common bile duct. Jpn J Gastroenterol Surg. 2006;39:317-322. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Agrawal D, Tannous GC, Chak A. Primary malignant melanoma of the hepatic duct: a case report. Gastrointest Endosc. 2010;72:845-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Smith NE, Taube JM, Warczynski TM, Collier KD, Pawlik TM. Primary biliary tract melanoma: Report of a case and review of the literature. Int J Surg Case Rep. 2012;3:441-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Addepally NS, Klair JS, Lai K, Aduli F, Girotra M. Primary Bile Duct Melanoma Causing Obstructive Jaundice. ACG Case Rep J. 2016;3:e128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Bisevac JP, Djukic M, Stanojevic I, Stevanovic I, Mijuskovic Z, Djuric A, Gobeljic B, Banovic T, Vojvodic D. Association Between Oxidative Stress and Melanoma Progression. J Med Biochem. 2018;37:12-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Maleki Vareki S. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J Immunother Cancer. 2018;6:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 333] [Cited by in RCA: 384] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 23. | Rabbie R, Ferguson P, Molina-Aguilar C, Adams DJ, Robles-Espinoza CD. Melanoma subtypes: genomic profiles, prognostic molecular markers and therapeutic possibilities. J Pathol. 2019;247:539-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 24. | Meyers MO, Frey DJ, Levine EA. Pancreaticoduodenectomy for melanoma metastatic to the duodenum: a case report and review of the literature. Am Surg. 1998;64:1174-1176. [PubMed] |
| 25. | Riva G, Villanova M, Eccher A, Luchini C, Motta F, Bernasconi R, Barbareschi M. Metastatic malignant melanoma to the gallbladder. Case report and review of the literature. Pathologica. 2018;110:68-71. [PubMed] |
| 26. | Langley RG, Bailey EM, Sober AJ. Acute cholecystitis from metastatic melanoma to the gall-bladder in a patient with a low-risk melanoma. Br J Dermatol. 1997;136:279-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Cellerino P, Corsi F, Morandi E, Foschi D, Trabucchi E. Metastatic melanoma of the gallbladder. Eur J Surg Oncol. 2000;26:815-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Ricci R, Maggiano N, Martini M, Mulé AM, Pierconti F, Capelli A, Larocca LM. Primary malignant melanoma of the gallbladder in dysplastic naevus syndrome. Virchows Arch. 2001;438:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbé C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3964] [Cited by in RCA: 4330] [Article Influence: 433.0] [Reference Citation Analysis (0)] |
| 30. | Dummer R, Hauschild A, Lindenblatt N, Pentheroudakis G, Keilholz U; ESMO Guidelines Committee. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v126-v132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 277] [Article Influence: 27.7] [Reference Citation Analysis (0)] |