Published online Aug 26, 2019. doi: 10.12998/wjcc.v7.i16.2143
Peer-review started: May 18, 2019
First decision: June 11, 2019
Revised: June 21, 2019
Accepted: July 20, 2019
Article in press: July 20, 2019
Published online: August 26, 2019
Processing time: 100 Days and 11.8 Hours
Colorectal cancer (CRC) is one of the main reasons of tumor-related deaths worldwide. At present, the main treatment is surgery, but the results are unsatisfactory, and the prognosis is poor. The majority of patients die due to liver or lung metastasis or recurrence. In recent years, great progress has been made in the field of tumor gene therapy, providing a new treatment for combating CRC. As oncolytic viruses selectively replicate almost exclusively in the cytoplasm of tumor cells and do not require integration into the host genome, they are safer, more effective and more attractive as oncolytic agents. Newcastle disease virus (NDV) is a natural RNA oncolytic virus. After NDV selectively infects tumor cells, the immune response induced by NDV’s envelope protein and intracellular factors can effectively kill the tumor without affecting normal cells. Reverse genetic techniques make NDV a vector for gene therapy. Arming the virus by inserting various exogenous genes or using NDV in combination with immunotherapy can also improve the anti-CRC capacity of NDV, and good results have been achieved in animal models and clinical treatment trials. This article reviews the molecular biological characteristics and oncolytic mechanism of NDV and discusses in vitro and in vivo experiments on NDV anti-CRC capacity and clinical treatment. In conclusion, NDV is an excellent candidate for cancer treatment, but more preclinical studies and clinical trials are needed to ensure its safety and efficacy.
Core tip: At present, the main treatment for colorectal cancer are surgical treatment and chemotherapy, but the majority of patients die due to liver or lung metastasis or recurrence. Therefore, there is an urgent need to find more effective treatment strategies to reduce mortality. Newcastle disease virus can selectively infect tumor cells and can also improve the ability of Newcastle disease virus to resist colorectal cancer by constructing an autologous tumor vaccine. Clinical treatment tests have shown a good therapeutic effect and its potential to become a new treatment for colorectal cancer.
- Citation: Song H, Zhong LP, He J, Huang Y, Zhao YX. Application of Newcastle disease virus in the treatment of colorectal cancer. World J Clin Cases 2019; 7(16): 2143-2154
- URL: https://www.wjgnet.com/2307-8960/full/v7/i16/2143.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i16.2143
Colorectal cancer (CRC) is one of the main causes of global cancer death, and its incidence continues to rise[1]. At present, surgery and chemotherapy are still the main treatments for CRC. About one-third of patients with CRC will have metastatic tumors of the liver or lung, making their 5-year overall survival rate only 50%[2]. Similar to many other malignancies, CRC is a heterogeneous disease, therefore optimizing treatment and reducing related mortality is the main challenge. In the current treatment of tumors, in addition to traditional surgery, radiotherapy and chemotherapy, immunotherapy has become a very hot field, which mainly includes oncolytic virus[3], tumor vaccine[4], targeted therapy[5], immune cell therapy[6] and immunological checkpoint inhibitors[7]. With the development of genetic engineering and the application of molecular biology, the treatment of CRC with oncolytic virus has been rapidly developed in recent decades, and genetically modified viruses have been used to evaluate the efficacy of anti-CRC in vitro and in vivo.
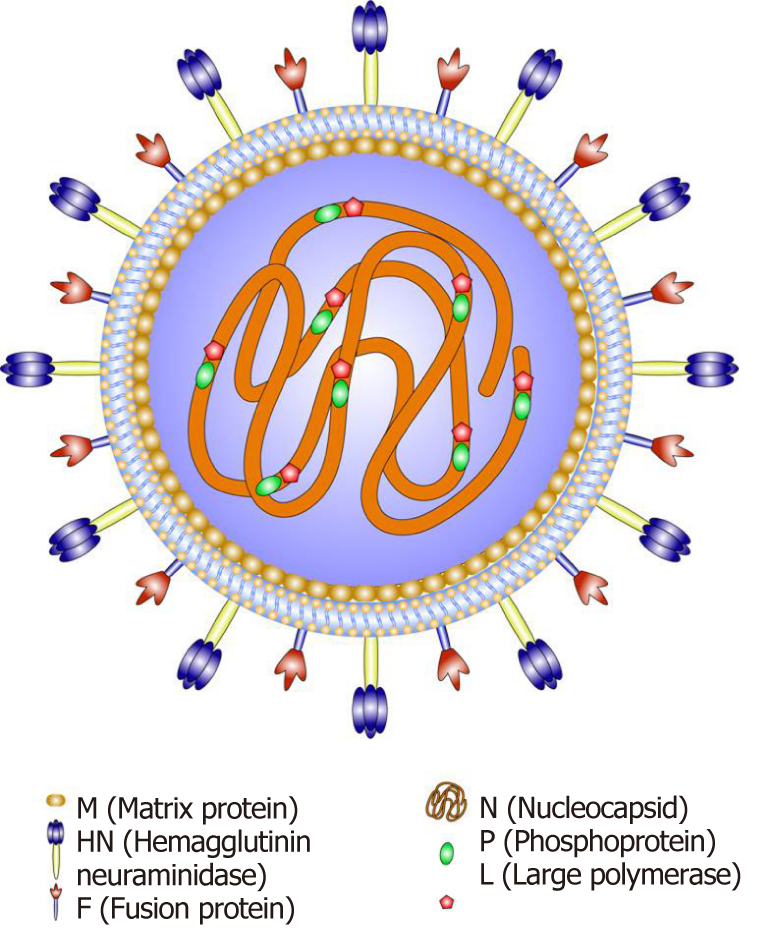
NDV is a highly infectious avian pathogen[8] that is an avian paramyxovirus type I virus, and a member of the genus Avulavirus in the family Paramyxoviridae[9]. NDV is a bilayered, lipid-coated RNA virus of approximately 100-300 nm with a predominantly spherical morphology. The genome of NDV is a nonsegmented negative-sense, single-stranded RNA [ssRNA(-)] molecule consisting of 15186 nucleotides containing six open reading frames encoding six structural proteins: nuclear protein (NP), phosphoprotein (P), large polymerase protein (L), matrix protein (M), hemagglutinin-neuraminidase (HN) and fusion protein (F). Among its structural proteins, NP, P and L combine with the viral RNA to form the ribonucleoprotein complex, which is responsible for replication of the virus[10] (Figure 1). M comprises a layer underneath the viral membrane that is involved in the assembly and budding of the virus. HN and F are present as oligomers, which together with the lipid bilayer membrane of the host constitute the outer envelope of the virus and are involved in entry of the virus into a cell. F is typically present as the inactive polypeptide F0; cleavage produces the mature membrane-anchored F1 and the membrane-distal F2 domain, resulting in an infectious virus[11].
The HN protein of NDV can trigger a conformational change in the F protein through receptor sialic acid-mediated endocytosis to release the fusion peptide and promote fusion of the virus with the cell membrane and allow the ribonucleoprotein complex to enter the cytoplasm of a host cell[12]. The genome replicates in the cytoplasm[13]: (1) The genomic ssRNA(-) is transcribed into messenger RNA in the cytoplasm for translation into different structural proteins; (2) The antigenome copy, or ssRNA(+), is used as the template for viral genome amplification, and subsequent budding releases virus progeny[14]; and (3) HN can scavenge sialic acid residues and promote spread of the virus in infected tissues. Selective replication of the virus results in host cell lysis only in tumor cells[15,16,17] with replication that is 10000 times faster than that in most normal human cells. Viral replication is terminated via the defense mechanisms of interferon (IFN) in normal cells[18]. In contrast, tumor cells usually have a weak type I IFN response and are also less sensitive to type I IFN receptor-mediated signaling; therefore, use of NDV in cancer patients is safe. The frameshift variant protein V is formed during transcription of the P gene. V participates in the antiviral reaction in avian cells, whereby the inhibition of IFN induction by NDV is suppressed by reduced stimulation of IFN-β through degradation of signal transduction and activator of transcription 1 via interactions with avian cell proteins[19]. This response occurs because the immune escape mechanism functions only in birds and not in mammalian cells. Therefore, it appears that the V protein reduces the range of NDV hosts.
NDVs are usually divided into three types according to pathogenicity and virulence: Velogenic strains (virulent strains, strongly toxic), mesogenic strains (poisonous strains, moderately toxic), or lentogenic strains (attenuated strains, poorly toxic or nontoxic)[20]. These viruses are also divided into two groups based on the degree of their influence on tumors: (1) Oncolytic strains that form syncytia in tumor cells and have viral oncolytic activity either in vitro or in vivo; and (2) Nononcolytic strains that inhibit tumor growth and can increase survival, though their killing effect on human tumor cells is still unclear. In general, the site of F protein cleavage mainly determines the virulence of NDV. The F-cleavage site of velogenic and mesogenic strains usually has the polybasic amino acid structure 112R/G/KR-Q/KK/RR↓F117, which is recognized and cut by Folin-like proteases (RXK/RR), inducing deadly respiratory diseases (such as chicken mites) in birds[21]; examples include MTH-68/H, PV701 and Beaudette C. In contrast, lentogenic strains have the single amino acid motif 112GR/KQGR↓L117, which is cleaved by extracellular trypsin-like proteases; thus, replication is limited to specific tissues, and these strains are currently used. For the production of vaccines[22], such as NDV-LaSota and NDV-HUJ, Heiden et al[23] constructed a recombinant attenuated strain of NDV clone-30. When the F-cleavage site was altered, the intracerebral pathogenicity index was enhanced 1.2-fold. Overall, NDV virulence is related to the site of F cleavage and is regulated by multiple factors.
Although NDV is dangerous to many birds, its pathogenicity in humans is weak. Furthermore, most people are seropositive for NDV, and thus the immunogenicity that NDV may cause can be ignored. Under natural conditions, highly virulent NDV strains may infect humans but cause only mild fever, cough and other flu-like symptoms.
The cells of various human tumors, such as liver cancer[24], glioblastoma[25] and lymphoma[26], have been shown to be sensitive to NDV. In addition, because NDV RNA transcription and translation are not related to cell proliferation, the virus can target tumor stem cells, dormant tumor cells, and X-ray-irradiated vaccine tumor cells[27].
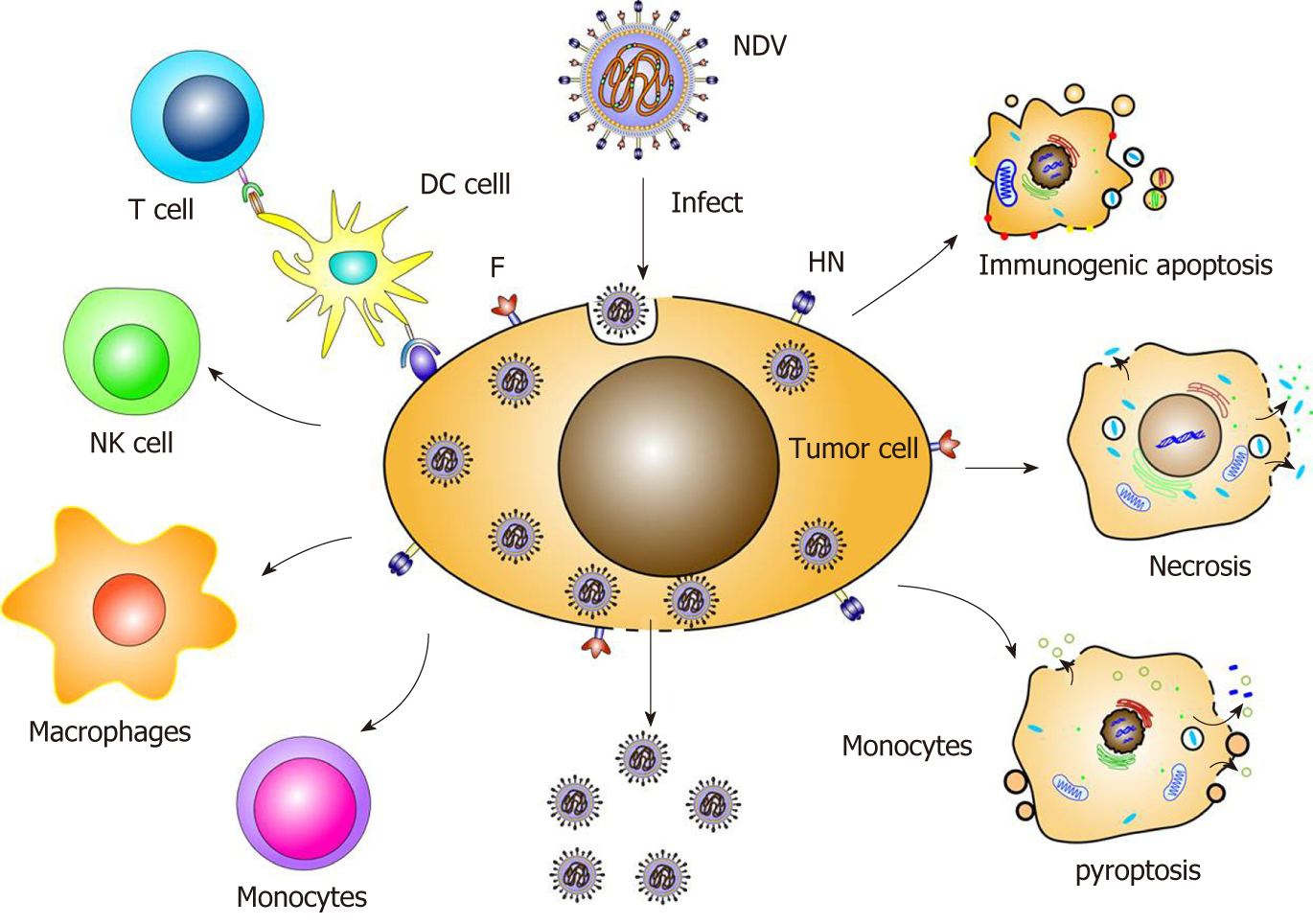
The oncolytic mechanism of NDV mainly include the following aspects. First, the virus selectively infects and replicates in tumor cells. Second, indirect effects of the innate and adaptive immune responses of the host immune system act against the virus, involving natural killer (NK) cells and cytotoxic T lymphocytes targeting the antigen[28,29]. Third, the envelope protein also participates in the oncolytic effect. Fourth, the apoptotic pathway promotes the oncolytic effect. Fifth, the virus induces immunogenic death, necrosis and autophagy (Figure 2).
Studies have found that a variety of proteins of NDV can impact infected cells, and protein sequence characterization also revealed that M, L and F have a BH-3 domain that shows homology with the proapoptotic factor Bcl-2[30]. Among these proteins, HN is an important immunogenic protein and virulence factor. Indeed, it has been reported that despite weaker efficacy than the parental NDV AF2240 strain, HN gene expression alone was able to induce apoptosis in human breast cancer MCF-7 cells[31]. HN induces release of type I IFN from human peripheral blood mononuclear cells and up-regulates expression of tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)[32]. He et al[33] constructed a novel oncolytic adenovirus containing the human telomerase reverse transcriptase (hTERT) promoter and expressing the HN protein of NDV (Ad-hTERTp-E1a-HN) that selectively inhibits esophageal cancer EC-109 cells and inhibits tumor growth in mice. In addition, studies have indicated that the M protein of NDV AF2240 strain binds to Bax through its BH-3 domain to promote the transfer of Bax from the cytoplasm to the mitochondrial membrane, thereby activating the intrinsic apoptotic pathway[34].
NDV stimulates the body's immune system to produce a variety of cytokines with anti-tumor activity, such as various IFNs or TNF. Type I IFN exerts a direct antitumor effect by targeting tumor cells and tumor stem cells and can indirectly stimulate immune system activity to assist in tumor killing. The recombinant NDV obtained by inserting the influenza virus NS1 gene, which antagonizes the IFN system in mammalian cells, into the NDV Hitchner-B1 genome promoted apoptosis in human tumor cell lines and the B16 melanoma mouse model with enhanced oncolytic effects[35].
Some studies have shown that the oncolytic effect of NDV is related to the various apoptotic pathways of cells, and the apoptosis induced by NDV requires viral replication and expression of apoptotic proteins.
The exogenous apoptosis pathway is mainly mediated by the death receptor Fas and its ligands FasL and TRAIL. For example, due to overexpression of Fas, the recombinant attenuated strain rNDV-B1/Fas exhibited enhanced oncolytic ability in vitro and in vivo, which resulted in an earlier and stronger apoptotic response in infected cells[36]. Compared to the wild-type virus, Bai's recombinant virus, LaSota-TRAIL[37], increased expression of TRAIL by 3-fold. In a mouse experiment, the LaSota-TRAIL group displayed significantly increased survival and decreased tumor recurrence. Other studies have indicated that due to overexpression of the inhibitor of apoptosis protein Livin in tissues of stage III melanoma, advanced melanoma is more susceptible to the NDV-HUJ virus than is an early-stage tumor[38]. Up-regulation of the apoptosis inhibitory protein survivin prolongs the viability of human breast cancer-resistant cells infected with the NDV AF2240 strain and increases viral protein synthesis as well as viral replication[39].
One immunotherapy mechanism of oncolytic viruses is immunogenic cell death (ICD)[40]. The manner in which NDV induces ICD in tumor cells includes immunogenic apoptosis, necrosis and pyroptosis, with termination of protein synthesis and subsequent exposure to calreticulin, heat shock proteins and the viral proteins HN and F.
Under endoplasmic reticulum stress, accumulation of unfolded proteins or misfolded proteins in the endoplasmic reticulum can cause the unfolded protein response, a specific response in NDV-infected cells. Activation of the unfolded protein response triggers caspase 12-induced cell death. Moreover, Cheng et al[41] demonstrated that the structural proteins NP and P induce autophagy through the endoplasmic reticulum stress pathway.
The corresponding pattern recognition receptors of innate immune cells include cytoplasmic RIG-1, PKR, TLR and NKp46. The pathogen-associated molecular pattern of NDV involves the virus 5’-adenosine triphosphate leader RNA, dsRNA and HN protein. Pattern recognition receptors trigger a variety of immune responses, including induction of type I IFN responses, promotion of immune cell activation and release of immune factors. In a mouse in situ glioma model, NDV virus therapy and molecules such as calreticulin, heat shock proteins and high mobility group protein-1 were found to induce ICD, stimulating specific immune T cells. Additionally, interaction between RIG-1 and RNA simultaneously activated type I IFN and induced IL-1β production. The double-stranded RNA of NDV induces expression of TLR-3, FN-α and heat shock proteins, which promotes tumor cell apoptosis and inhibits tumor growth by enhancing the immune system response. NKp46 of NK cells recognizes viral HN proteins and transmits cytotoxicity-inducing signals, increasing production of IFN-α and TRAIL. After NDV infection of monocytes and NK cells, IL-2, IFN-γ, GM-CSF and TNF-α are produced to promote tumor-killing activity through TRAIL[42,43].
Viral infection of tumors can promote an immunosuppressive environment by inducing immune cytokines and chemokines (RANTES and IP-10)[44]. Although cytokines and chemokines recruit and activate neutrophils, NK cells, macrophages and CD4+ and CD8+ T lymphocytes, contributing to viral clearance, these molecules also alter immunosuppression. HN molecules on the surface of infected tumor cells increase the cell adhesion strength of lymphocytes and T cells for T cell costimulation. As the first responder of innate immunity, neutrophils cause the release of the chemokines CXCL1, CCL2 and CXCL10, thereby mediating immunogenic cell death[45].
After NDV infection of cells, the accumulation of HN and F proteins on the host cell surface promotes the formation of cell syncytia and lead to cell-to-cell fusion, which ultimately triggers necrosis, syncytium disintegration, content release and an inflammatory response. Cell necrosis is also activated by caspase 8 through the cellular TLR and the TNF family.
The HN and F proteins of NDV rapidly induce syncytium formation and initiate stable autophagy fluxes in lung adenocarcinoma cells (A549), synergistically inducing autophagosome fusion with lysosomes for cell degradation[46]. Autophagy is beneficial for viral replication in the early stages of NDV infection of tumor cells and lengthens the cell life cycle by regulating apoptosis. Mitochondrial autophagy has been reported to promote oncolytic NDV replication by breaking the intrinsic apoptosis regulation pathway in lung adenocarcinoma cells[47]. Several ongoing trials are evaluating the impact of autophagy on human tumor therapy[48]. For example, Hu et al[49] found that NDV-infected U251 cells can promote autophagy to degrade lung cancer cells. The autophagy regulators chloroquine and rapamycin significantly enhanced the oncolytic effect of NDV on A549 cells in mice[50]. This finding provides a new idea for exploring the antitumor strategy utilized by autophagy regulators and NDV.
NDV treatment in different metastatic tumor models has revealed a blockade effect on tumor migration and invasion. Studies have shown that the NDV strain AF2240 can reduce the migration ability of breast cancer cells by directly inducing a decrease in cell proliferation[51]. In addition, oral squamous cell carcinoma was infected with the NDV D90 strain, and a correlation between apoptosis induction and cell migration was observed[52]. Studies have shown that simultaneous injection of NDV, extracellular matrix-degrading enzymes, collagenase and heparanase into a tumor can increase spread of the virus in tumor tissue, thereby enhancing the oncolytic effect[53]. However, because proteolytic enzymes are involved in tumor metastasis and their inhibitors have been used to suppress metastatic tumors, it is not known whether application of virus loading of a matrix metalloproteinase is feasible in the clinic.
The NDV strains currently used in mouse experimental models and clinical tests include pathogenic (MTH-68/H, Ulster and PV701) and nonpathogenic (Hitchner-B1, LaSota, 73-T and HUJ) strains[54]. NDV has been shown to have potent anti-tumor effects against colon tumors[55], hepatocellular carcinoma[56] and melanoma[57].
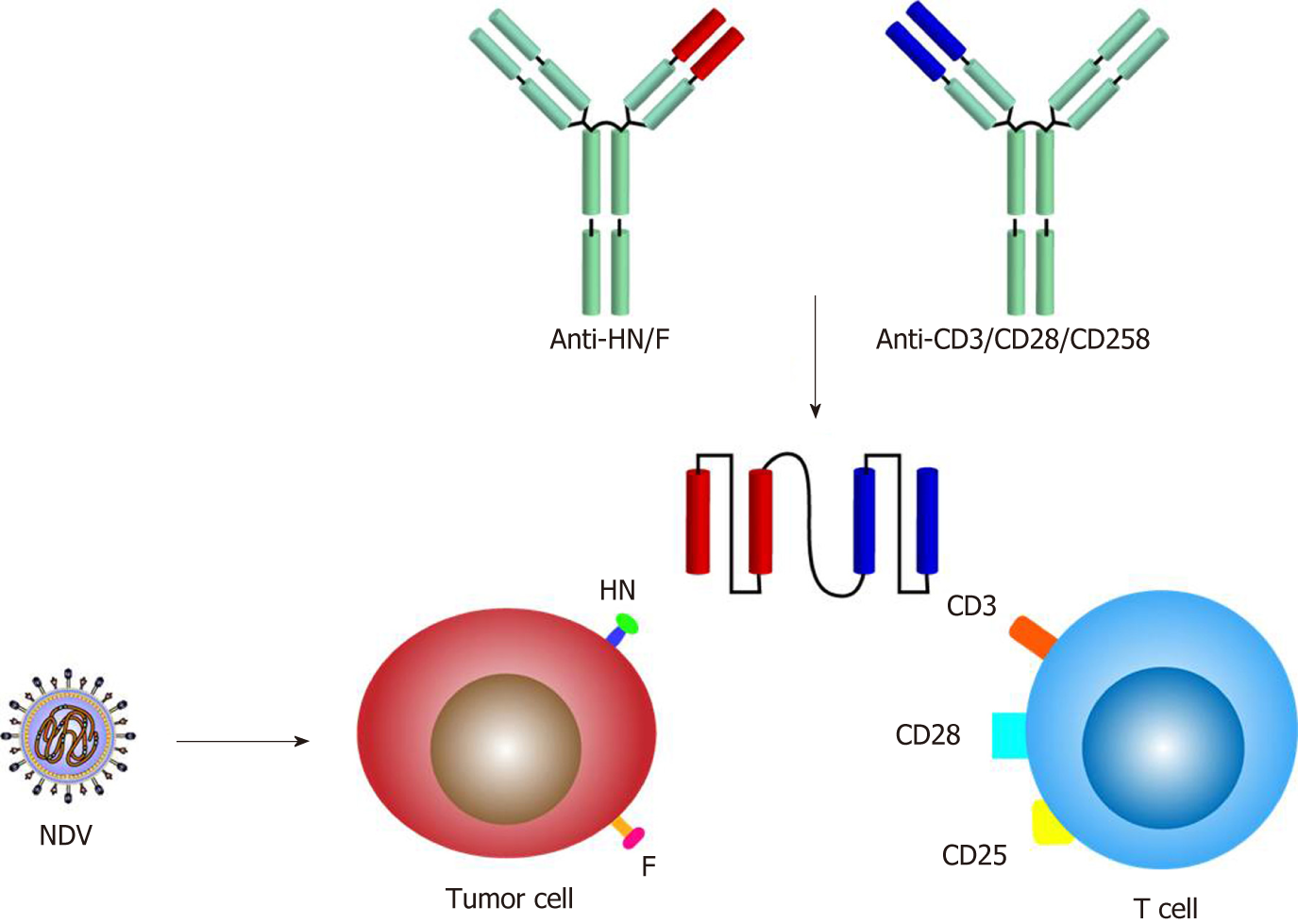
The appeal of viral vectors is related to their broad host cell range and high expression levels of foreign genes. Typically, viral vectors with a DNA or RNA genome can be loaded with foreign genes of different lengths; for example, most vectors allow insertion of 6-8 kb, including extensive therapeutic genes[58]. Recently, reverse genetics technology has been applied to help produce recombinant NDV (rNDV) from nonsegmented negative-strand RNA-cloned cDNA to enhance oncolysis[59], as follows: Mutating the F gene[60]; inserting a gene encoding a cytokine, such as IL-2[61], IL-15[62] or IL-7[63], to enhance the immunostimulatory effect; simultaneous insertion of two cytokines synergistically to increase antitumor effects[64]; and inserting a bispecific antibody consisting of a single-chain variable region that can simultaneously target virus and immune cells. Concerning T cells, the targets can be CD3, CD25 and CD28, whereas the target of NDV can be F or HN[65] (Figure 3).
Chia et al[66] incubated with human NDV strain AF2240 in vitro with human CRC cell lines (SW620, ddd-1, Dks8, HCT116p53+/+, HCT116p53-/- and HT29). Cell death occurred in 70-90% of cells after 96 hours of culture, providing a basis for in vivo experiments. In one study, a nude mouse model of human colon cancer SW620 cells was established, and the anti-tumor effect was studied by intratumoral and intravenous injection using NDV Mukteshwar strain. The results showed that tumor growth in mice was inhibited by 43% and 40%, respectively, and the survival time was prolonged[55].
In the subcutaneous model of BALB/c mice bearing CT26 colon cancer, NDV Ulster strain was injected in and around the tumor after tumor inoculation, and the tumor was completely relieved by the 40th day. The long-term survival rate was 70%[67]. When NDV was used for local treatment of liver metastases of MTH-68/HCT26 colon cancer cells, it was observed that tumor growth was significantly inhibited, the survival time of mice was prolonged, and there was no toxic side effect on mice[68]. Ockert et al[69] constructed a tumor-bearing mouse model of human colon cancer (SW620, HT29 and MM17387) and injected intratumoral and intraperitoneal injections with NDV 73-T strain. The results showed that multi-dose NDV injection significantly inhibited tumor growth in mice compared with single dose, and the inhibition rate reached 77-96%.
Vigil et al[70] treated the constructed CT26 colon cancer mouse model with the NDV recombinant strain rNDV/F3aa-IL-2 expressing IL-2. Compared with the wild-type control virus, the recombinant virus can significantly reduce the tumor growth of the tumor-bearing mice and prolong the survival time, so that the condition of most mice is sustained. Yamaki et al[71] established CRC multifocal liver metastasis model of rats or multifocal lung metastasis model using the expression of mutant (L289A) new town of fusion protein of soluble tumor cystic stomatitis virus carrier (types) [rVSV NDV/F (L289A)][72] for hepatic artery and local drug delivery. The pulmonary metastasis models did not show long-term survival. However, in the mice liver metastasis model, four of the seven rats survived for more than 100 d. This result provided an effective basis for the follow-up clinical trials.
The application of NDV tumor therapy has entered the clinical phase (phases I, II and III)[73], and three methods are generally used in clinical trials: (1) Injection of infectious virus alone; (2) Injection of intact tumor cells infected with NDV; (3) Injection of the protein lysate of NDV-infected tumor cells; and (4) Combined use. As NDV infection of tumor cells can lead to enhanced immunogenicity, an autologous tumor vaccine (ATV-NDV) can be constructed using the tumor cells of a patient[74], and clinically postoperative activity-specific immunotherapy has been performed for patients with CRC (Table 1).
| Ref. | Type of NDV | Aim of the study | Tumor type | Number of subjects | Outcomes |
| Bohle et al[75] | ATV-NDV | Phase I clinical trial | Colon cancer | 16 patients | 2 patients exhibited an enhanced specific anti-tumor response |
| Lehner et al[76] | ATV-NDV | Phase I clinical trial | CRC | 20 patients | 16 patients produced an active, specific immune response |
| Liebrich et al[77] | ATV-NDV | Phase II clinical trial | CRC | 23 patients | The active immune response in the patients was increased |
| Schlag et al[78] | ATV-NDV | Phase II clinical trial | CRC | 23 patients | A 61% tumor recurrence rate was observed in vaccinated patients compared with 87% of patients treated with surgery alone |
| Schulze et al[79] | ATV-NDV | Phase III clinical trial | CRC | 50 patients | Advantages in terms of overall survival in subgroup; ATV-NDV appears to be beneficial prolonging overall survival and metastasis-free survival |
| Liang et al[80] | ATV-NDV | Phase III clinical trial | CRC | 567 patients | Average survival and median survival of the immunotherapy group (310 patients) were higher than those of the control group (257 patients) |
| Schirrmacher et al[82] | ATV-NDV and bsHN-CD28 | Phase I clinical trial | CRC | 14 patients | 4 patients experienced a partial diminishment of metastases |
Some phase I-IV clinical trials based on NDV vectors have yielded encouraging results. For the first time, Bohle et al[75] treated 16 patients with colon cancer after tumor resection with a live, nontoxic NDV-modified tumor cell vaccine. Of the 16 patients, 12 patients had an enhanced specific anti-tumor response. In a phase I clinical trial, 20 patients with CRC were treated with ATV-NDV-specific immunotherapy, with the exception of four patients with mild fever and no serious side effects. Among them, 16 patients produced an active specific immune response to the vaccine, which provided effectiveness for subsequent clinical trials[76].
Liebrich et al[77] isolated tumor cells from human primary CRC and mixed vaccine with NDV nonlytic strain Ulster and used it for vaccination in 23 patients with colorectal liver metastasis. The results showed the active immune response in the patient is increased and can be further used in related tests. In a phase II clinical trial, 23 patients with liver metastases from CRC were completely resected after a consecutive 3 mo of ATV-NDV vaccination. At least 18 mo follow-up showed a 61% tumor recurrence in vaccinated patients compared with 87% of patients who underwent surgery alone[78]. In a prospective randomized phase III trial, 50 patients with CRC hepatic metastases were randomized into an experimental group and a control group. The experimental group received six doses of ATV-NDV. After approximately 10 years of follow-up, no differences in primary and secondary endpoints were detected in the total patient group. However, the experimental subgroup (13 patients with colon cancer) showed significant advantages in terms of overall survival. The vaccination appeared to help prolong overall survival and metastasis-free survival[79].
In a phase III trial, Liang et al[80] used ATV-NDV to treat 335 patients with stage I-IV CRC. The average survival and median survival of the immunotherapy group (310 patients) were higher than those of the control group. The other 25 patients with advanced disease had a 1-year survival rate of 96%. The total effective rate of immunotherapy was 24%. After NDV vaccine immunotherapy, the number of NK cells increased, and the immune function improved significantly. In patients with stage IV colon cancer, tumor memory T cells (MTCs) may induce tumor-associated antigens either spontaneously or upon vaccination with an antitumor vaccine, which may constitute a potential mechanism in those patients with long-term survival[81].
Multiple immunizations of colon cancer patients with ATV-NDV using CD3 or CD28 on HN or F and T cells simultaneously targeting the virus have been shown to be clinically effective and improve long-term survival[82]. Attachment of the NDV-specific single-chain antibody anti-HNxanti-CD28 (bsHN-CD28) to ATV-NDV enhances T cell costimulatory signals. After using ATV-NDV-bsHN-CD28 to treat 14 cases of advanced CRC not surgically treatable in the phase I clinical trial, no tumor-reactive blood circulation MTCs were detected in any of the patients before vaccination; however, all patients exhibited the immune response of tumor-reactive MTCs at least once during five vaccinations. Among them, four patients experienced partial diminishment of metastases[83].
NDV-based treatment causes only mild pus-like symptoms, and these side effects are temporary and well tolerated; even high doses via intravenous administration did not cause significant toxicity. Although rNDV treatment may lead to some side effects, the response is negligible when compared to the therapeutic effect. When a patient is initially desensitized with a lower dose, the subsequent maximum tolerated dose may be increased by a factor of ten[84].
The multiple preclinical data and clinical trials with oncolytic NDV clearly demonstrate its efficacy for CRC. However, there are several questions that need to be resolved .One of the key issues in oncolytic therapy is targeted delivery[85]. Moreover, the extracellular matrix and other barriers in solid tumors may interfere with and slow viral transmission, thereby reducing oncolysis.
In addition, due to the induction of neutralizing antibodies, the oncolysis effect of the virus after repeated viral administration may decrease over time[86]. To overcome the potential neutralizing effect of the antibody on the virus, an aptamer was used to block the antibody, thereby preventing neutralization of the virus. Furthermore, treatment with a paramyxovirus mesenchymal stem cell vector protects the virus against neutralizing antibodies with efficient transfer of the virus to the tumor.
In terms of safety and treatment outcomes, improvement is needed for the application of NDV. For example, NDV 73T damages normal cells while killing tumor cells. In clinical use, NDV may also pose safety problems for medical personnel and the environment because NDV virulent strains and poisonous strains can cause fatal respiratory and neurological diseases in poultry. Furthermore, insertion of an exogenous gene into the NDV genome has pros and cons: On the one hand, exogenous genes may enhance the anti-tumor effect of NDV; on the other hand, insertion of foreign genes may affect replication of the virus.
In 2005, the Chinese government approved the first transgenic oncolytic type 5 adenovirus (Adeno-5; H101) virus for the treatment of tumors; in 2013, the United States Food and Drug Administration affirmed the use of herpes simplex virus type 1 (HSV1; T-Vec). Thus, oncolytic viruses have become a new generation of biosafety agents. Among these agents, NDV meets many conditions for new drugs used in the human body: Selective potent oncolytic activity; a strong type I IFN reaction and a wide range of immunostimulatory effects. NDV directly destroys cancer cells, induces ICD and activates DC1- and Th1-directed antitumor immune responses, resulting in effective destruction of cancer cells and the development of tumor-associated MTCs after onset, which provides long-term protective activity to prolong survival.
Driven by successful animal experiments and clinical trials, the strategy of introducing, integrating and improving NDV anti-CRC therapy has been widely explored. The development of reverse genetics technology allows NDV to be employed as a vector for the integration of therapeutic genes. In the future, we envision multimodal combination therapy as the ideal option for treating complex diseases, such as tumors. Indeed, by combining oncolytic NDV with hyperthermia, therapeutic transgenes, dendritic cells, T cells, bispecific antibodies or NDV-based vaccines (e.g., ATV-NDV), multimodal combination therapy is expected to be a good application prospect. However, more research is needed to determine the preclinical and clinical effects of NDV to verify its safety and efficacy in CRC therapy.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aykan NF, Katuchova J S-Editor: Gong ZM L-Editor: Filipodia E-Editor: Liu JH
| 1. | Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11065] [Cited by in RCA: 12182] [Article Influence: 1522.8] [Reference Citation Analysis (3)] |
| 2. | Wang W, Kandimalla R, Huang H, Zhu L, Li Y, Gao F, Goel A, Wang X. Molecular subtyping of colorectal cancer: Recent progress, new challenges and emerging opportunities. Semin Cancer Biol. 2019;55:37-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 3. | Geevarghese SK, Geller DA, de Haan HA, Hörer M, Knoll AE, Mescheder A, Nemunaitis J, Reid TR, Sze DY, Tanabe KK, Tawfik H. Phase I/II study of oncolytic herpes simplex virus NV1020 in patients with extensively pretreated refractory colorectal cancer metastatic to the liver. Hum Gene Ther. 2010;21:1119-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Inoda S, Morita R, Hirohashi Y, Torigoe T, Asanuma H, Nakazawa E, Nakatsugawa M, Tamura Y, Kamiguchi K, Tsuruma T, Terui T, Ishitani K, Hashino S, Wang Q, Greene MI, Hasegawa T, Hirata K, Asaka M, Sato N. The feasibility of Cep55/c10orf3 derived peptide vaccine therapy for colorectal carcinoma. Exp Mol Pathol. 2011;90:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Hu Z, He J, Gong W, Zhou N, Zhou S, Lai Z, Zheng R, Wang Y, Yang X, Yang W, Zhong L, Lu X, Zhao Y. TLS11a Aptamer/CD3 Antibody Anti-Tumor System for Liver Cancer. J Biomed Nanotechnol. 2018;14:1645-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Huang Y, Mao Q, He J, Su J, Peng Y, Liang W, Hu Z, Zhou S, Lu X, Zhao Y. Fusions of Tumor-derived Endothelial Cells with Dendritic Cells Induces Antitumor Immunity. Sci Rep. 2017;7:46544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Xu-Monette ZY, Zhang M, Li J, Young KH. PD-1/PD-L1 Blockade: Have We Found the Key to Unleash the Antitumor Immune Response? Front Immunol. 2017;8:1597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 8. | Ganar K, Das M, Sinha S, Kumar S. Newcastle disease virus: current status and our understanding. Virus Res. 2014;184:71-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 259] [Article Influence: 23.5] [Reference Citation Analysis (1)] |
| 9. | Afonso CL, Amarasinghe GK, Bányai K, Bào Y, Basler CF, Bavari S, Bejerman N, Blasdell KR, Briand FX, Briese T, Bukreyev A, Calisher CH, Chandran K, Chéng J, Clawson AN, Collins PL, Dietzgen RG, Dolnik O, Domier LL, Dürrwald R, Dye JM, Easton AJ, Ebihara H, Farkas SL, Freitas-Astúa J, Formenty P, Fouchier RA, Fù Y, Ghedin E, Goodin MM, Hewson R, Horie M, Hyndman TH, Jiāng D, Kitajima EW, Kobinger GP, Kondo H, Kurath G, Lamb RA, Lenardon S, Leroy EM, Li CX, Lin XD, Liú L, Longdon B, Marton S, Maisner A, Mühlberger E, Netesov SV, Nowotny N, Patterson JL, Payne SL, Paweska JT, Randall RE, Rima BK, Rota P, Rubbenstroth D, Schwemmle M, Shi M, Smither SJ, Stenglein MD, Stone DM, Takada A, Terregino C, Tesh RB, Tian JH, Tomonaga K, Tordo N, Towner JS, Vasilakis N, Verbeek M, Volchkov VE, Wahl-Jensen V, Walsh JA, Walker PJ, Wang D, Wang LF, Wetzel T, Whitfield AE, Xiè JT, Yuen KY, Zhang YZ, Kuhn JH. Taxonomy of the order Mononegavirales: update 2016. Arch Virol. 2016;161:2351-2360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 369] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 10. | Liu T, Song Y, Yang Y, Bu Y, Cheng J, Zhang G, Xue J. Hemagglutinin-Neuraminidase and fusion genes are determinants of NDV thermostability. Vet Microbiol. 2019;228:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Yaacov B, Eliahoo E, Lazar I, Ben-Shlomo M, Greenbaum I, Panet A, Zakay-Rones Z. Selective oncolytic effect of an attenuated Newcastle disease virus (NDV-HUJ) in lung tumors. Cancer Gene Ther. 2008;15:795-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Lamb RA, Jardetzky TS. Structural basis of viral invasion: lessons from paramyxovirus F. Curr Opin Struct Biol. 2007;17:427-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 208] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 13. | Fournier P, Schirrmacher V. Oncolytic Newcastle Disease Virus as Cutting Edge between Tumor and Host. Biology (Basel). 2013;2:936-975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Molouki A, Peeters B. Rescue of recombinant Newcastle disease virus: current cloning strategies and RNA polymerase provision systems. Arch Virol. 2017;162:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Fountzilas C, Patel S, Mahalingam D. Review: Oncolytic virotherapy, updates and future directions. Oncotarget. 2017;8:102617-102639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | Tayeb S, Zakay-Rones Z, Panet A. Therapeutic potential of oncolytic Newcastle disease virus: a critical review. Oncolytic Virother. 2015;4:49-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Yurchenko KS, Zhou P, Kovner AV, Zavjalov EL, Shestopalova LV, Shestopalov AM. Oncolytic effect of wild-type Newcastle disease virus isolates in cancer cell lines in vitro and in vivo on xenograft model. PLoS One. 2018;13:e0195425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Zhang S, Sun Y, Chen H, Dai Y, Zhan Y, Yu S, Qiu X, Tan L, Song C, Ding C. Activation of the PKR/eIF2α signaling cascade inhibits replication of Newcastle disease virus. Virol J. 2014;11:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Wang X, Dang R, Yang Z. The interferon antagonistic activities of the V proteins of NDV correlated with their virulence. Virus Genes. 2019;55:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Liu L. Fields Virology, 6th Edition. Clin Infect Dis. 2014;59:613. [RCA] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Kumar R, Tiwari AK, Chaturvedi U, Kumar GR, Sahoo AP, Rajmani RS, Saxena L, Saxena S, Tiwari S, Kumar S. Velogenic newcastle disease virus as an oncolytic virotherapeutics: in vitro characterization. Appl Biochem Biotechnol. 2012;167:2005-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Spalatin J, Turner AJ, Hanson RP. Observations on the transmissibility of lentogenic strains of Newcastle disease virus: significance of variables. Avian Dis. 1976;20:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Heiden S, Grund C, Röder A, Granzow H, Kühnel D, Mettenleiter TC, Römer-Oberdörfer A. Different regions of the newcastle disease virus fusion protein modulate pathogenicity. PLoS One. 2014;9:e113344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | An Y, Liu T, He J, Wu H, Chen R, Liu Y, Wu Y, Bai Y, Guo X, Zheng Q, Liu C, Yin J, Li D, Ren G. Recombinant Newcastle disease virus expressing P53 demonstrates promising antitumor efficiency in hepatoma model. J Biomed Sci. 2016;23:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Abdullah JM, Mustafa Z, Ideris A. Newcastle disease virus interaction in targeted therapy against proliferation and invasion pathways of glioblastoma multiforme. Biomed Res Int. 2014;2014:386470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Al-Shammari AM, Rameez H, Al-Taee MF. Newcastle disease virus, rituximab, and doxorubicin combination as anti-hematological malignancy therapy. Oncolytic Virother. 2016;5:27-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Schirrmacher V. Fifty Years of Clinical Application of Newcastle Disease Virus: Time to Celebrate! Biomedicines. 2016;4:pii: E16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Ricca JM, Oseledchyk A, Walther T, Liu C, Mangarin L, Merghoub T, Wolchok JD, Zamarin D. Pre-existing Immunity to Oncolytic Virus Potentiates Its Immunotherapeutic Efficacy. Mol Ther. 2018;26:1008-1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 29. | Chaurasiya S, Chen NG, Fong Y. Oncolytic viruses and immunity. Curr Opin Immunol. 2018;51:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Liu B, Ji Y, Lin Z, Fu Y, Muhammad Dafallah R, Zhu Q. Two single amino acid substitutions in the intervening region of Newcastle disease virus HN protein attenuate viral replication and pathogenicity. Sci Rep. 2015;5:13038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Ghrici M, El Zowalaty M, Omar AR, Ideris A. Induction of apoptosis in MCF-7 cells by the hemagglutinin-neuraminidase glycoprotein of Newcastle disease virus Malaysian strain AF2240. Oncol Rep. 2013;30:1035-1044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Liao Y, Wang HX, Mao X, Fang H, Wang H, Li Y, Sun Y, Meng C, Tan L, Song C, Qiu X, Ding C. RIP1 is a central signaling protein in regulation of TNF-α/TRAIL mediated apoptosis and necroptosis during Newcastle disease virus infection. Oncotarget. 2017;8:43201-43217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | He D, Sun L, Li C, Hu N, Sheng Y, Chen Z, Li X, Chi B, Jin N. Anti-tumor effects of an oncolytic adenovirus expressing hemagglutinin-neuraminidase of Newcastle disease virus in vitro and in vivo. Viruses. 2014;6:856-874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Molouki A, Hsu YT, Jahanshiri F, Abdullah S, Rosli R, Yusoff K. The matrix (M) protein of Newcastle disease virus binds to human bax through its BH3 domain. Virol J. 2011;8:385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | García-Sastre A, Egorov A, Matassov D, Brandt S, Levy DE, Durbin JE, Palese P, Muster T. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology. 1998;252:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 811] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 36. | Cuadrado-Castano S, Ayllon J, Mansour M, de la Iglesia-Vicente J, Jordan S, Tripathi S, García-Sastre A, Villar E. Enhancement of the proapoptotic properties of newcastle disease virus promotes tumor remission in syngeneic murine cancer models. Mol Cancer Ther. 2015;14:1247-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Bai FL, Tian H, Yu YH, Yin JC, Ren GP, Zhou B, Li DS. TNF-related Apoptosis-inducing Ligand Delivered by rNDV is a Novel Agent for Cancer Gene Therapy. Technol Cancer Res Treat. 2015;14:737-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Lazar I, Perlman R, Lotem M, Peretz T, Ben-Yehuda D, Kadouri L. The clinical effect of the inhibitor of apopotosis protein livin in melanoma. Oncology. 2012;82:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Altieri DC. Survivin - The inconvenient IAP. Semin Cell Dev Biol. 2015;39:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 40. | Ye T, Jiang K, Wei L, Barr MP, Xu Q, Zhang G, Ding C, Meng S, Piao H. Oncolytic Newcastle disease virus induces autophagy-dependent immunogenic cell death in lung cancer cells. Am J Cancer Res. 2018;8:1514-1527. [PubMed] |
| 41. | Cheng JH, Sun YJ, Zhang FQ, Zhang XR, Qiu XS, Yu LP, Wu YT, Ding C. Newcastle disease virus NP and P proteins induce autophagy via the endoplasmic reticulum stress-related unfolded protein response. Sci Rep. 2016;6:24721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | Schwaiger T, Knittler MR, Grund C, Roemer-Oberdoerfer A, Kapp JF, Lerch MM, Mettenleiter TC, Mayerle J, Blohm U. Newcastle disease virus mediates pancreatic tumor rejection via NK cell activation and prevents cancer relapse by prompting adaptive immunity. Int J Cancer. 2017;141:2505-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Tan L, Zhang Y, Qiao C, Yuan Y, Sun Y, Qiu X, Meng C, Song C, Liao Y, Munir M, Nair V, Ding Z, Liu X, Ding C. NDV entry into dendritic cells through macropinocytosis and suppression of T lymphocyte proliferation. Virology. 2018;518:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Cassady KA, Haworth KB, Jackson J, Markert JM, Cripe TP. To Infection and Beyond: The Multi-Pronged Anti-Cancer Mechanisms of Oncolytic Viruses. Viruses. 2016;8:pii: E43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Garg AD, Vandenberk L, Fang S, Fasche T, Van Eygen S, Maes J, Van Woensel M, Koks C, Vanthillo N, Graf N, de Witte P, Van Gool S, Salven P, Agostinis P. Pathogen response-like recruitment and activation of neutrophils by sterile immunogenic dying cells drives neutrophil-mediated residual cell killing. Cell Death Differ. 2017;24:832-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 46. | Sun Y, Yu S, Ding N, Meng C, Meng S, Zhang S, Zhan Y, Qiu X, Tan L, Chen H, Song C, Ding C. Autophagy benefits the replication of Newcastle disease virus in chicken cells and tissues. J Virol. 2014;88:525-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 47. | Li Y, Wang LX, Yang G, Hao F, Urba WJ, Hu HM. Efficient cross-presentation depends on autophagy in tumor cells. Cancer Res. 2008;68:6889-6895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 241] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 48. | Kang Y, Yuan R, Xiang B, Zhao X, Gao P, Dai X, Liao M, Ren T. Newcastle disease virus-induced autophagy mediates antiapoptotic signaling responses in vitro and in vivo. Oncotarget. 2017;8:73981-73993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 49. | Hu L, Sun S, Wang T, Li Y, Jiang K, Lin G, Ma Y, Barr MP, Song F, Zhang G, Meng S. Oncolytic newcastle disease virus triggers cell death of lung cancer spheroids and is enhanced by pharmacological inhibition of autophagy. Am J Cancer Res. 2015;5:3612-3623. [PubMed] |
| 50. | Jiang K, Li Y, Zhu Q, Xu J, Wang Y, Deng W, Liu Q, Zhang G, Meng S. Pharmacological modulation of autophagy enhances Newcastle disease virus-mediated oncolysis in drug-resistant lung cancer cells. BMC Cancer. 2014;14:551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Ahmad U, Ahmed I, Keong YY, Abd Manan N, Othman F. Inhibitory and apoptosis-inducing effects of Newcastle disease virus strain AF2240 on mammary carcinoma cell line. Biomed Res Int. 2015;2015:127828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Zhang CX, Ye LW, Liu Y, Xu XY, Li DR, Yang YQ, Sun LL, Yuan J. Antineoplastic activity of Newcastle disease virus strain D90 in oral squamous cell carcinoma. Tumour Biol. 2015;36:7121-7131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Yaacov B, Lazar I, Tayeb S, Frank S, Izhar U, Lotem M, Perlman R, Ben-Yehuda D, Zakay-Rones Z, Panet A. Extracellular matrix constituents interfere with Newcastle disease virus spread in solid tissue and diminish its potential oncolytic activity. J Gen Virol. 2012;93:1664-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Kim SH, Samal SK. Newcastle Disease Virus as a Vaccine Vector for Development of Human and Veterinary Vaccines. Viruses. 2016;8:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 55. | Sharma KK, Kalyani IH, Mohapatra J, Patel SD, Patel DR, Vihol PD, Chatterjee A, Patel DR, Vyas B. Evaluation of the oncolytic potential of R2B Mukteshwar vaccine strain of Newcastle disease virus (NDV) in a colon cancer cell line (SW-620). Arch Virol. 2017;162:2705-2713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Wu Y, Yan S, Lv Z, Chen L, Geng J, He J, Yu Q, Yin J, Ren G, Li D. Recombinant Newcastle disease virus Anhinga strain (NDV/Anh-EGFP) for hepatoma therapy. Technol Cancer Res Treat. 2014;13:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Bommareddy PK, Kaufman HL. Unleashing the therapeutic potential of oncolytic viruses. J Clin Invest. 2018;128:1258-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Lundstrom K. New frontiers in oncolytic viruses: optimizing and selecting for virus strains with improved efficacy. Biologics. 2018;12:43-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 59. | Cheng X, Wang W, Xu Q, Harper J, Carroll D, Galinski MS, Suzich J, Jin H. Genetic Modification of Oncolytic Newcastle Disease Virus for Cancer Therapy. J Virol. 2016;90:5343-5352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 60. | Wang W, Cheng X, Buske PJ, Suzich JA, Jin H. Attenuate Newcastle disease virus by codon modification of the glycoproteins and phosphoprotein genes. Virology. 2019;528:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Wu Y, He J, An Y, Wang X, Liu Y, Yan S, Ye X, Qi J, Zhu S, Yu Q, Yin J, Li D, Wang W. Recombinant Newcastle disease virus (NDV/Anh-IL-2) expressing human IL-2 as a potential candidate for suppresses growth of hepatoma therapy. J Pharmacol Sci. 2016;132:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 62. | Niu Z, Bai F, Sun T, Tian H, Yu D, Yin J, Li S, Li T, Cao H, Yu Q, Wu Y, Ren G, Li D. Recombinant Newcastle Disease virus Expressing IL15 Demonstrates Promising Antitumor Efficiency in Melanoma Model. Technol Cancer Res Treat. 2015;14:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Zhao L, Mei Y, Sun Q, Guo L, Wu Y, Yu X, Hu B, Liu X, Liu H. Autologous tumor vaccine modified with recombinant new castle disease virus expressing IL-7 promotes antitumor immune response. J Immunol. 2014;193:735-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 64. | Xu X, Sun Q, Mei Y, Liu Y, Zhao L. Newcastle disease virus co-expressing interleukin 7 and interleukin 15 modified tumor cells as a vaccine for cancer immunotherapy. Cancer Sci. 2018;109:279-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Fournier P, Aigner M, Schirrmacher V. Transcriptome analysis and cytokine profiling of naive T cells stimulated by a tumor vaccine via CD3 and CD25. Int J Oncol. 2010;37:1439-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 66. | Chia SL, Yusoff K, Shafee N. Viral persistence in colorectal cancer cells infected by Newcastle disease virus. Virol J. 2014;11:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Schirrmacher V, Griesbach A, Ahlert T. Antitumor effects of Newcastle Disease Virus in vivo: local versus systemic effects. Int J Oncol. 2001;18:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 68. | Apostolidis L, Schirrmacher V, Fournier P. Host mediated anti-tumor effect of oncolytic Newcastle disease virus after locoregional application. Int J Oncol. 2007;31:1009-1019. [PubMed] |
| 69. | Ockert D, Schirrmacher V, Beck N, Stoelben E, Ahlert T, Flechtenmacher J, Hagmüller E, Buchcik R, Nagel M, Saeger HD. Newcastle disease virus-infected intact autologous tumor cell vaccine for adjuvant active specific immunotherapy of resected colorectal carcinoma. Clin Cancer Res. 1996;2:21-28. [PubMed] |
| 70. | Vigil A, Park MS, Martinez O, Chua MA, Xiao S, Cros JF, Martínez-Sobrido L, Woo SL, García-Sastre A. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res. 2007;67:8285-8292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 71. | Yamaki M, Shinozaki K, Sakaguchi T, Meseck M, Ebert O, Ohdan H, Woo SL. The potential of recombinant vesicular stomatitis virus-mediated virotherapy against metastatic colon cancer. Int J Mol Med. 2013;31:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Ebert O, Shinozaki K, Kournioti C, Park MS, García-Sastre A, Woo SL. Syncytia induction enhances the oncolytic potential of vesicular stomatitis virus in virotherapy for cancer. Cancer Res. 2004;64:3265-3270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 73. | Taguchi S, Fukuhara H, Homma Y, Todo T. Current status of clinical trials assessing oncolytic virus therapy for urological cancers. Int J Urol. 2017;24:342-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 74. | Takamura-Ishii M, Nakaya T, Hagiwara K. Regulation of Constitutive Interferon-Stimulated Genes (Isgs) in Tumor Cells Contributes to Enhanced Antitumor Response of Newcastle Disease Virus-Infected Tumor Vaccines. Cancers (Basel). 2018;10:pii: E186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Bohle W, Schlag P, Liebrich W, Hohenberger P, Manasterski M, Möller P, Schirrmacher V. Postoperative active specific immunization in colorectal cancer patients with virus-modified autologous tumor-cell vaccine. First clinical results with tumor-cell vaccines modified with live but avirulent Newcastle disease virus. Cancer. 1990;66:1517-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 76. | Lehner B, Schlag P, Liebrich W, Schirrmacher V. Postoperative active specific immunization in curatively resected colorectal cancer patients with a virus-modified autologous tumor cell vaccine. Cancer Immunol Immunother. 1990;32:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Liebrich W, Schlag P, Manasterski M, Lehner B, Stöhr M, Möller P, Schirrmacher V. In vitro and clinical characterisation of a Newcastle disease virus-modified autologous tumour cell vaccine for treatment of colorectal cancer patients. Eur J Cancer. 1991;27:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 78. | Schlag P, Manasterski M, Gerneth T, Hohenberger P, Dueck M, Herfarth C, Liebrich W, Schirrmacher V. Active specific immunotherapy with Newcastle-disease-virus-modified autologous tumor cells following resection of liver metastases in colorectal cancer. First evaluation of clinical response of a phase II-trial. Cancer Immunol Immunother. 1992;35:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Schulze T, Kemmner W, Weitz J, Wernecke KD, Schirrmacher V, Schlag PM. Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: results of a prospective randomized trial. Cancer Immunol Immunother. 2009;58:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 80. | Liang W, Wang H, Sun TM, Yao WQ, Chen LL, Jin Y, Li CL, Meng FJ. Application of autologous tumor cell vaccine and NDV vaccine in treatment of tumors of digestive tract. World J Gastroenterol. 2003;9:495-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 81. | Schirrmacher V, Fournier P, Schlag P. Autologous tumor cell vaccines for post-operative active-specific immunotherapy of colorectal carcinoma: long-term patient survival and mechanism of function. Expert Rev Vaccines. 2014;13:117-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Haas C, Lulei M, Fournier P, Arnold A, Schirrmacher V. A tumor vaccine containing anti-CD3 and anti-CD28 bispecific antibodies triggers strong and durable antitumor activity in human lymphocytes. Int J Cancer. 2006;118:658-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 83. | Schirrmacher V, Schlude C, Weitz J, Beckhove P. Strong T‑cell costimulation can reactivate tumor antigen‑specific T cells in late‑stage metastasized colorectal carcinoma patients: results from a phase I clinical study. Int J Oncol. 2015;46:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 84. | Hotte SJ, Lorence RM, Hirte HW, Polawski SR, Bamat MK, O'Neil JD, Roberts MS, Groene WS, Major PP. An optimized clinical regimen for the oncolytic virus PV701. Clin Cancer Res. 2007;13:977-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 85. | Wang Y, Deng W, Li N, Neri S, Sharma A, Jiang W, Lin SH. Combining Immunotherapy and Radiotherapy for Cancer Treatment: Current Challenges and Future Directions. Front Pharmacol. 2018;9:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 264] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 86. | Chaurasiya S, Warner S. Viroimmunotherapy for Colorectal Cancer: Clinical Studies. Biomedicines. 2017;5:pii: E11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |