Published online Aug 6, 2019. doi: 10.12998/wjcc.v7.i15.2110
Peer-review started: March 4, 2019
First decision: May 31, 2019
Revised: June 23, 2019
Accepted: July 2, 2019
Article in press: July 2, 2019
Published online: August 6, 2019
Processing time: 160 Days and 5.1 Hours
Cystic fibrosis (CF) is rare in Asian populations relative to the Caucasian population. In this paper, we report the cystic fibrosis transmembrane conductance regulator (CFTR) variation in a family of Chinese CF patients, and systematically review the previous literature.
Here we report a 30-month-old Chinese girl who was diagnosed with CF based on her history and symptoms such as recurrent productive cough, wheezing with repeated infection of Pseudomonas aeruginosa, and parasinusitis. Chest computed tomography (CT) scanning revealed obvious exudative lesions and bilateral bronchiectasis. Liver CT scanning revealed a low-density lesion in the left lobe of the liver. A diagnosis of CF was made based upon CFTR gene tests. The CFTR gene was sequenced using the blood samples of her and her parents and showed a heterozygous novel missense mutation of c.753_754delAG in exon 7. In addition, a heterozygous c.1240 C>T mutation was found in exon 10 of the CFTR. The mutation c.753_754delAG was verified to have been inherited from her mother, and the c.1240 C>T mutation was from her father who was diagnosed with congenital absence of vas deferens.
A novel mutation of CFTR, c.753_754delAG, was found in a Chinese CF child. c.2909G>A is the most common mutation among Chinese CF patients.
Core tip: Cystic fibrosis (CF) is an autosomal recessive inherited disease caused by mutations in the CF transmembrane conduction regulator (CFTR) gene. CF is rare in Chinese. ΔF508 is the most common mutation, accounting for greater than two-thirds of CF alleles worldwide, though it is not a predominant mutation in Chinese CF patients. In this paper, we report a novel homozygous complex rearrangement involving CFTR exon 7 deletion (c.753_754delAG chr7-117176607-117176608) in a Chinese child with CF and describe the clinical feature. Moreover, we further review the literature regarding gene mutations in Chinese CF cases from the 1970s to 2017.
- Citation: Wang YQ, Hao CL, Jiang WJ, Lu YH, Sun HQ, Gao CY, Wu M. c.753_754delAG, a novel CFTR mutation found in a Chinese patient with cystic fibrosis: A case report and review of the literature. World J Clin Cases 2019; 7(15): 2110-2119
- URL: https://www.wjgnet.com/2307-8960/full/v7/i15/2110.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i15.2110
Cystic fibrosis (CF) is an autosomal recessive inherited disease caused by mutations in the CF transmembrane conduction regulator (CFTR) gene. CF is most common in the Caucasian population, with a prevalence of 1/2500-3500 among those with Northern European ancestry[1,2]. CF was once considered extremely rare among the Chinese population, and to date, only about 60 cases of CF have been diagnosed in China[3]. CFTR is responsible for regulating the flow of chloride ions across the epithelial membrane. Since CFTR was first identified as the pathogenic gene of CF in 1989, more than 2000 mutations have been found in CF patients, according to the Cystic Fibrosis Mutation Database (http://www.genet.sickkids.on.ca). ΔF508 is the most common mutation, accounting for greater than two-thirds of CF alleles worldwide, though it is not a predominant mutation in Chinese CF patients[4]. The most common gene mutation in Chinese children with CF is c.2909G-A[5]. With increased awareness of this disease and improvements in diagnostic techniques, we have found that CF is not as rare as once believed in the Chinese population. The novel variants c.699 C-A, c.579+1_579+2insACAT, c.1117-1G>C c.3140-454_c.3367+249del931ins13, and p.R1048_G1123del have been reported in CF patients from China in recent years[6-8]. Interestingly, the gene mutation spectrum of CFTR in Chinese patients with CF is significantly different from that in Caucasian patients. Therefore, it is necessary to establish the Chinese CFTR gene mutation database, which will facilitate the genetic diagnosis of CF patients in China. In the present study, we identified a novel homozygous complex rearrangement involving CFTR exon 7 deletion (c.753_754delAG chr7-117176607-117176608) using multiplex ligation-dependent probe amplification analysis in a Chinese child with CF. We further review the literature regarding Chinese CF patients from the 1970s to 2017. The clinical data of all identified CF patients are summarized.
A girl aged 2 years and 10 months was admitted to Children’s Hospital of Soochow University in May 2018 due to recurrent productive cough and wheezing lasting for 1 month.
She had experienced recurrent pneumonia (2-3 times every year) beginning 4 mo after birth, with repeated infection by Pseudomonas aeruginosa and parasinusitis, but without a history of chronic diarrhea or pancreatic involvement.
The child was conceived through in vitro fertilization. Her father had been diagnosed with congenital absence of vas deferens, and her mother was healthy.
She weighed 11 kg, her height was 89 cm, her body mass index was 13.9, and she presented with shortness of breath and dyspnea. Crackles and wheezing rales were present in bilateral lungs. The heart and abdomen were normal. No clubbed digits were found.
Blood routine examination showed a white blood cell count of 15.59 × 109/L, a C reactive protein concentration of 55.4 mg/L, and positivity for Pseudomonas aeruginosa on bronchoalveolar lavage fluid culture. Findings on other tests, including serum electrolyte measurement, fungus culture, Glactomannan test, T-SPOT tuberculosis test, allergic bronchopulmonary aspergillosis and aspergillus fumigatus specific IgE detection were all negative.
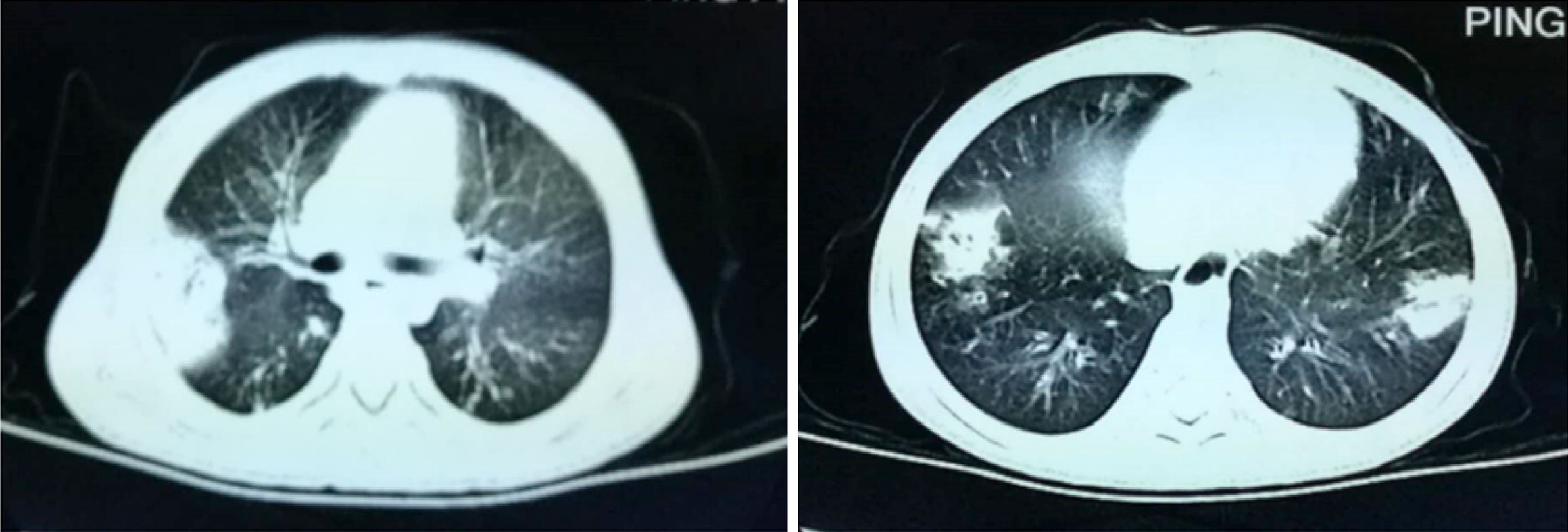
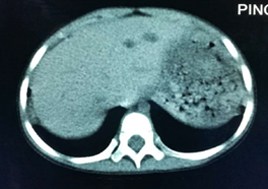
Chest computed tomography (CT) scanning revealed obvious exudative lesions and bilateral bronchiectasis (Figures 1 and 2). Sinus CT scanning revealed bilateral parasinusitis. Liver CT scanning revealed a low-density lesion in the left lobe of the liver. In patients with CF, the liver is also the organ affected by the dense secretion of digestive juice. Bile secreted by the liver can clog bile ducts and damage the liver. Ultrasonography of the pancreas was negative.
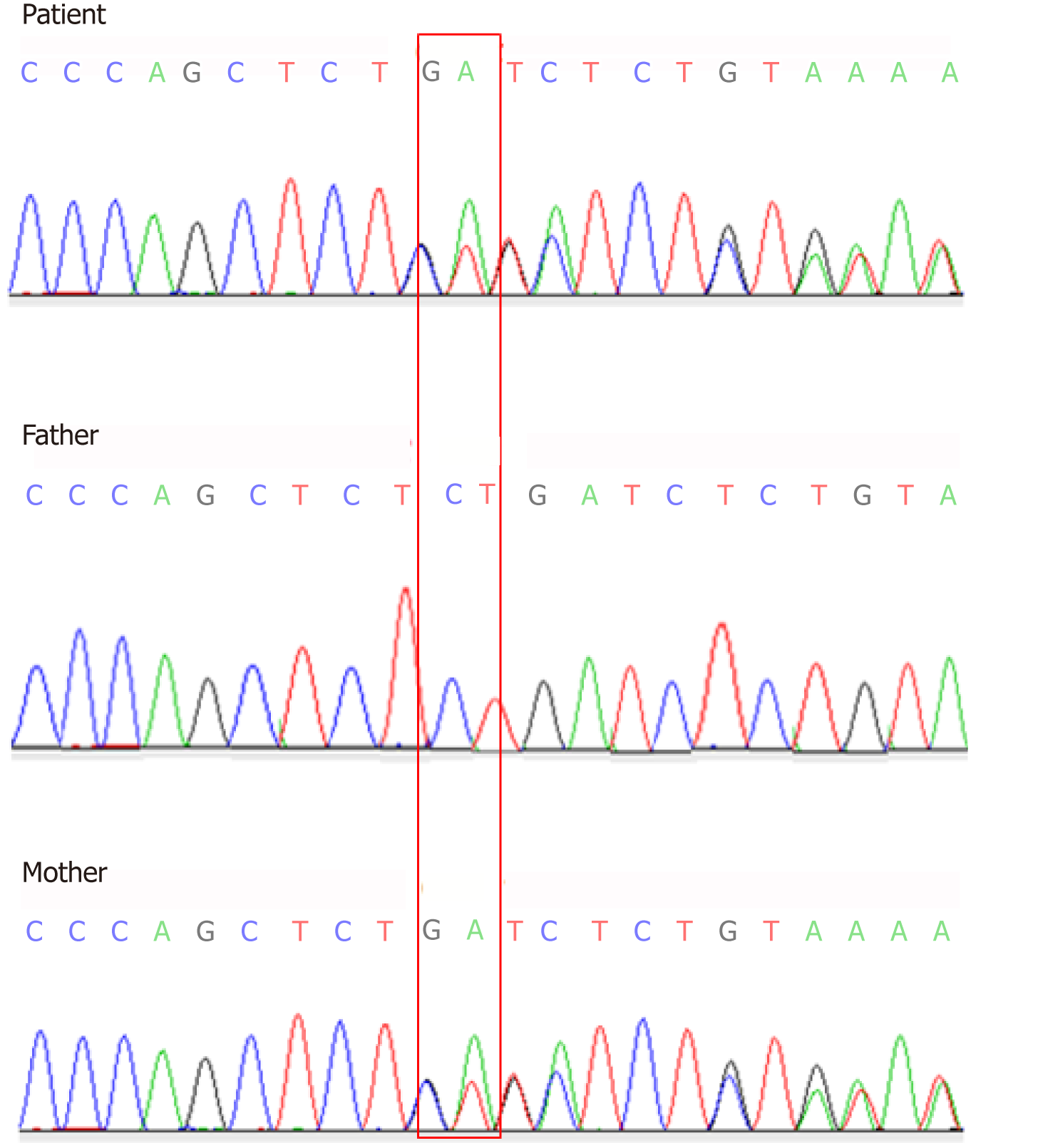
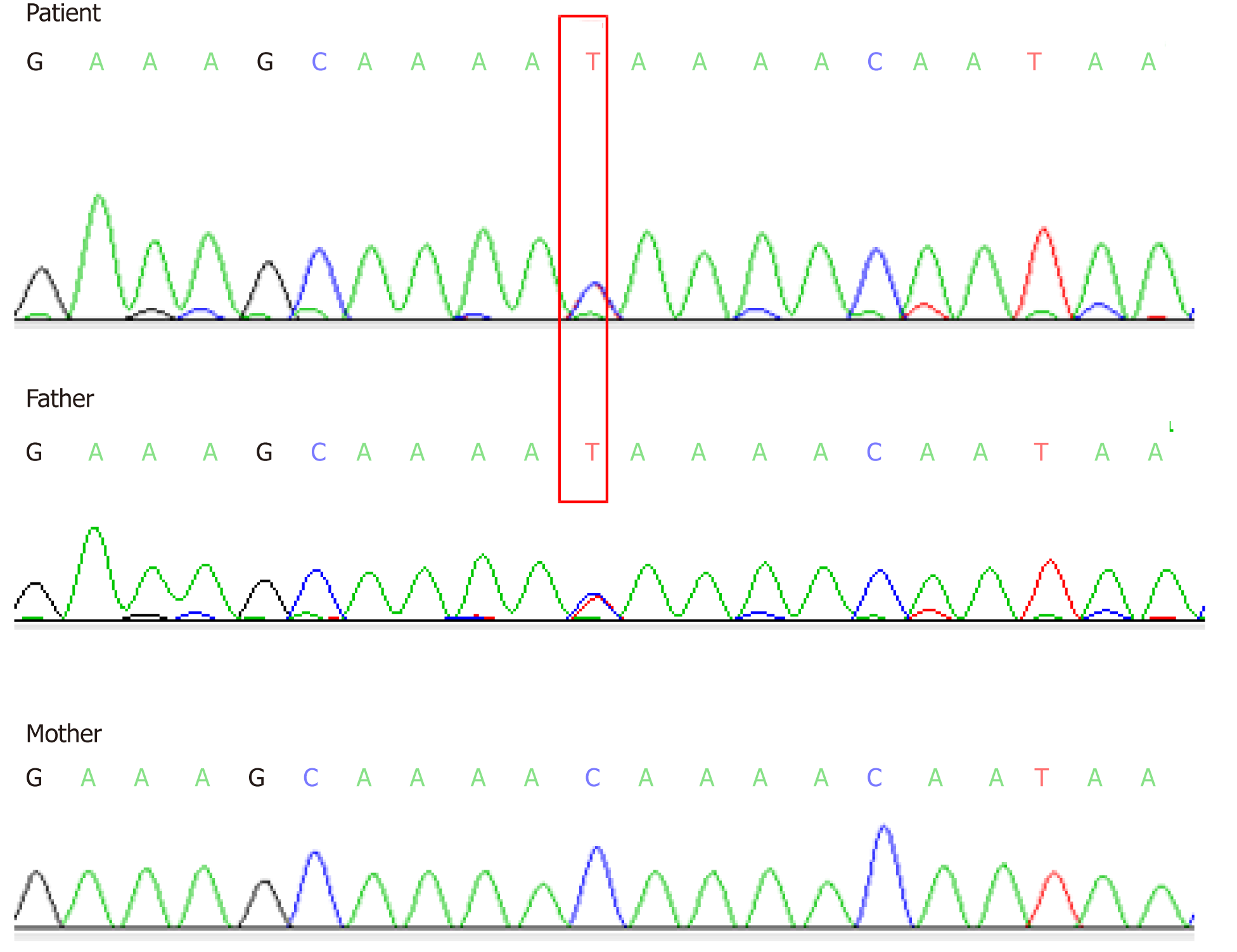
Two heterozygous mutations were found in the CF patient by Sanger sequencing analysis. A heterozygous novel missense mutation of c.753_754delAG chr7-117176607-117176608 was identified in exon 7 (Figure 3), which was inherited from her mother based on its identification in the mother’s sample as well (Figure 3). This novel mutation has not yet been recorded in the CFTR mutation database (http://www.genet.sickkids.on.ca). In addition, a heterozygous c.1240 C>T mutation in exon 10 was observed in CFTR of the CF patient (Figure 4), which was inherited from her father and had already been included in the CFTR mutation database.
CF.
Her symptoms improved after antibiotic treatment with ceftazidime for 3 wk, expectorant, and nutritional support treatment including fat-soluble vitamins and powdered milk with high calorie.
After being discharged from our hospital, the children were followed monthly in the outpatient clinic. We gave low dose azithromycin anti-inflammatory treatment to eradicate P. aeruginosa infection. We did regular examinations of respiratory rate, oxygen saturation, and high-resolution CT of the chest to evaluate the pulmonary disease regression/progression. We introduced regular atomized bronchodilators such as terbutaline and oral secretion expellant including acetylcysteine to help remove respiratory secretions. She had one time of pulmonary infection. The general situation remained well up to date. She weighed 13 kg, her height was 95 cm, and her body mass index was 14.4.
CF is characterized by the abnormal transport of ions and fluid across epithelial cell membranes, resulting from mutations on both alleles in the gene encoding the CFTR[9,10]. CFTR mutations can cause secretions to obstruct the airway, pancreatic tract, and biliary tract and lead to abnormal secretion by the sweat glands. The most important organ to be invaded in CF is the lung, and lung disease is the most lethal factor (85%)[11]. The pancreas is also an important affected organ in CF. Disorders caused by CF include nutritional disorders (fat, protein malabsorption, and fatty diarrhea) and growth retardation. Low body weight caused by pancreatic insufficiency is negatively correlated with lung function and survival rate, and thus, an important factor for poor prognosis[12]. Malnutrition and gastrointestinal symptoms are relatively mild and atypical in Chinese CF patients. Therefore, it is easy for CF diagnosis to be missed or delayed.
For patients with one or more clinical characteristics, such as chronic sinopulmo-nary disease, gastrointestinal and nutritional abnormalities, genital abnormalities in males resulting in obstructive azoospermia, and/or a family history of CF, the measurement of sweat electrolyte concentrations has been the mainstay of CF diagnosis since the standardized procedure was introduced[13]. In the CF case reported here, the patient had chronic sinopulmonary disease, and her father had a CF mutation with obstructive azoospermia. These patients should undergo repeat sweat chloride testing and further evaluation, including detailed clinical assessment and more extensive CFTR gene mutation analysis. CF in Chinese patients is difficult to diagnose, due to insufficient understanding and because sweat examination as well as genetic testing cannot be carried out in most hospitals. It is necessary to educate Chinese pediatricians concerning the clinical manifestations and diagnostic criteria for CF and to promote the implementation of the sweat chloride test.
CFTR mutations are divided into five general classes: mutations affecting biosynthesis, mutations interfering with protein maturation, mutations influencing Cl- channel regulation, mutations intervening Cl- conductance or channel gating, and mutations that reduce CFTR synthesis[14]. Different types of CFFR mutations can cause different clinical phenotypes: I, II, and III mutations are prone to cause pancreatic insufficiency with more serious clinical manifestations. In contrast, because normal Cl- channel function is partially retained, the clinical symptoms of IV and V mutations are relatively mild with pancreatic function remaining normal.
Several studies have demonstrated that p.F508del is the most common mutation in Caucasian CF patients, accounting for approximately 70% of cases[4,5]. The p. F508del mutation is a type II mutation. We review 82 different mutations among 69 Chinese CF patients (40 females and 29 males) reported from the 1970s to 2017. Among them, 53 were from mainland China, 9 from Taiwan, and 4 from Hongkong, with the remaining patients being of Chinese and Vietnamese descent, Chinese and Portuguese descent[7,8,15-40] (Table 1). The age at diagnosis ranged from 0.17 months to 23 years.
| Reference | Location | n | Gender | Age (yr) | Mutation |
| Wang et al[15], 1993 | Taiwan China | 1 | F | 0.5 | 1898+5 G-->T, 2215insG+G2816A |
| Chen et al[16],1995 | Mainland China | 1 | F | — | E2 del about 30 bp |
| Zielenski et al[17], 1995 | Taiwan China | 1 | F | 8 | 1898+5 G-->T, 1898+5 G-->T |
| Crawford et al[18], 1995 | Chinese and Portuguese | 1 | F | 3 | 1898 + 1G>T |
| Wagner et al[19], 1999 | Chinese | 1 | F | 23 | c.319-326delGCTTCCTA, c. 2909G>A |
| Wu et al[20], 2000 | Taiwan China | 2 | F | 14 | 1898+5 G>T, 2215insG+G2816A |
| M | 17 | 1898+5 G>T, 2215insG+G2816A | |||
| Alper et al[21], 2003 | Chinese and Vietnamese | 2 | M | 1.5 | G151T, 989-992insA |
| Taiwan China | F | 0.5 | 1898+5G>T, 2215insG+G2816A | ||
| Chen et al[22], 2005 | Taiwan China | 1 | M | 3 | R553X, R553X |
| Li et al[6], 2006 | Mainland China | 1 | F | 14 | 699C>A, 3821-3823delT |
| Wang et al[23], 2012 | Mainland China | 1 | F | 14 | W679X |
| Liu et al[24], 2012 | Mainland China | 2 | F | 13 | 2909G>A, 362T>G |
| F | 10 | 3196C>T, 3196C>T | |||
| Cheng et al[25], 2013 | Mainland China | 1 | F | 12 | W679X, 1342-11TTT>G, 3120+2T>C |
| Liu et al[26], 2015 | Mainland China | 7 | M | 12 | c.95T>C, c.1657C>T |
| M | 10 | c.293A>G, c.558C>G | |||
| M | 16 | c.2052 dupA, △E18-E20(c.2909-?_3367 + ?del) | |||
| F | 16 | c.2909G>A, △E7-E11†(c.744-?_1584 + ?del) | |||
| F | 10 | c.1679 + 2T>C, c.2658-1G>C | |||
| F | 21 | c.293A>G, c.293A>G | |||
| F | 28 | c.1666A>G | |||
| Shen et al[27], 2016 | Mainland China | 19 | M | 11.58 | c.1699G>T, c.3909C>G |
| F | 10.58 | c.263T>G, c.1766+5G>T, c.110C>G | |||
| M | 13.25 | c.3700A>G, c.960_961insA | |||
| F | 13.67 | c.263T>G, c.2909G>A | |||
| M | 7.17 | c.326A>G, c.1000C>T, c.1666A>G | |||
| F | 10.67 | c.595C>T | |||
| F | 7.75 | c.223C>T, c.326A>G | |||
| F | 7.33 | c.1000C>T | |||
| F | 10.17 | c.263T>G | |||
| F | 11.08 | c.1666A>G | |||
| M | 8.25 | c.293A>G, c.558C>G | |||
| F | 4.17 | c.326A>G, c.2374C>T | |||
| M | 3.67 | c.1666A>G | |||
| F | 12.67 | c.293A>G | |||
| M | 11 | c.648G>A, c.2491-126T>C | |||
| F | 10.33 | c.3196C>T | |||
| M | 11.17 | c.414_415insCTA | |||
| F | 3.42 | c.1075C>T, c.3307delA | |||
| F | 14 | c.2909G>A | |||
| Chu et al[28], 2016 | Mainland China | 1 | M | 9 | C.579+2insACAT, C.F481766+5G>T |
| Xu et al[29], 2016 | Mainland China | 1 | M | 0.67 | c.595C>T, C.2290C>T |
| Li et al[30], 2016 | Mainland China | 1 | M | 0.42 | c.214G>G/A, c.650A>A/G,c.3406G>G/A |
| Tian et al[31], 2016 | Mainland China | 8 | F | 15 | c.2909G>A, c.2374C>T |
| F | 1 | c.2909G>A, c.2125C>T | |||
| M | 13 | c.3700A>G, c.959–960insA | |||
| M | 15 | c.3635delT | |||
| F | 4 | c.2909G>A, c.263T>G | |||
| F | 13 | c.2909G>A, c.2907A>C | |||
| M | 20 | c.2909G>A, c.1521_1523delCTT | |||
| F | 22 | c.2909G>A, c.1997T>G | |||
| Leung et al[32], 2017 | HongKong China | 4 | M | 17 | c.1766+5G>T, c.3068T>G |
| M | 0.5 | c.1766+5G>T, c.3140-26A>G | |||
| M | 0.17 | c.868C>T, c.3068T>G | |||
| F | 0.75 | c.1657C>T, c.3068T>G | |||
| Xie et al[33], 2017 | Mainland China | 2 | M | 12 | c.865A>T,c.3651_3652insAAAT |
| M | 15 | c.865A>T,c.3651_3653insAAAT | |||
| Zheng et al[34], 2017 | Mainland China | 2 | M | 5 | c.3196C>T, c.870-1G>C |
| F | 5 | c.3G>A , c.1572C>A | |||
| Xu et al[7], 2017 | Mainland China | 4 | M | 9 | c.579+1_579+2insACAT, c.1766+5G>T |
| M | 5 | c.595C>T | |||
| F | 6 | c.1117-1G>C, c.2909G>A | |||
| M | 13 | c.4056G>C | |||
| Liu et al[8], 2017 | Mainland China | 1 | M | 11 | c.3140-454_c.3367+249del931ins13 |
| Yao et al[35], 2017 | Mainland China | 1 | F | 0.5 | c.532G>A |
| Sun et al[36], 2017 | Mainland China | 1 | F | 2 | C.1 666A>G |
| Guo et al[37], 2017 | Mainland China | 1 | F | 0.75 | c.1373G>A(p.G458E), c.271G>A(p.G91R) |
| Li et al[38], 2017 | Mainland China | 1 | F | 1.33 | R709X, G970D |
Among the Chinese CF patients, the c.2909 G>A variant was the most common mutation type (11%), followed by 1898+5G>T (7.3%), c.293A>G (6.1%), and 2215insG+G2816A and c.263T>G (both 4.9%). Nevertheless, no p.F508del mutation was found in the Chinese patients (Table 1). In addition, with the exceptions of c.3909 C>G, R553X, and c.1000 C>T, none of the CFTR mutations in the Chinese patients were present in the common Caucasian CFTR mutation-screening panels, indicating that the mutations identified in Chinese CF patients are obviously different from the common gene mutations in Caucasian CF patients. Further, pulmonary lesions were more prominent in Chinese CF patients with or without pancreatic insufficiency[6-8,26,27]. Therefore, it is necessary to establish a Chinese gene mutation database to facilitate genetic diagnosis of CF in China to clarify the relationship between genotype and clinical phenotype.
In the case reported herein, the c.1240C>T mutation resulted in the alteration of amino acid p.Q414* (glutamine > termination). This mutation type has been reported already as a pathogenic mutation in the HGMD pro database[14]. c.753_754A del A.G is a novel mutation (deletion mutation) that results in amino acid changes P.R251Sfs * 6 (frame-shifting mutation - 6 termination). According to the ACMG guidelines, the mutation site c.753_754delAG could be classified as a pathogenic mutation[39]. Both mutations could result in the early termination of CFTR protein translation, which might have a great impact on protein function. The double heterozygous mutation came from the patient’s parents separately. As a compound heterozygous mutation, it is consistent with autosomal recessive inheritance and is a theoretically possible cause of disease. This case expands the mutation spectrum of CFTR in patients of Chinese origin. Several studies have shown that only pancreatic function correlates well with CFTR genotypes[40,41]. According to the pancreatic status of patients, CF mutations can be subdivided into two groups: mild and severe mutations[40]. Patients with pancreatic insufficiency are homozygous or compound heterozygous with two “severe” mutations, whereas patients with pancreatic sufficiency have at least one “mild” allele. As it is not clear from the case if the patient had pancreatic sufficiency or insufficiency, we cannot deduce whether the two mutations were severe mutations or not. Elevated serum lipase, which has not been mentioned before, is not a sign of severe mutation, more of possible pancreatitis which is more commonly seen in heterozygous CF carriers or in those with milder mutations and pancreatic sufficiency.
In conclusion, a novel compound heterozygous c.753_754delAG mutation was found in exon 7 of CFTR in the case reported herein. The common CFTR mutation spectrum in Chinese CF patients is quite different from that in Caucasian patients. Therefore, the Chinese common CFTR mutation spectrum provides valuable data for CF diagnosis in Chinese patients and the development of a commercial Chinese CFTR genetic screening kit. The relevant Chinese gene mutation database is urgently needed.
The authors are grateful to all technicians of the Diagnostic Microbiology Laboratory, the Children’s Hospital of Soochow University, for technical contributions and Beijing Precision Gene Technology Company (Beijing, China).
Checklist (2016).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Brecelj J S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Salvatore D, Buzzetti R, Baldo E, Forneris MP, Lucidi V, Manunza D, Marinelli I, Messore B, Neri AS, Raia V, Furnari ML, Mastella G. An overview of international literature from cystic fibrosis registries. Part 3. Disease incidence, genotype/phenotype correlation, microbiology, pregnancy, clinical complications, lung transplantation, and miscellanea. J Cyst Fibros. 2011;10:71-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Southern KW, Munck A, Pollitt R, Travert G, Zanolla L, Dankert-Roelse J, Castellani C; ECFS CF Neonatal Screening Working Group. A survey of newborn screening for cystic fibrosis in Europe. J Cyst Fibros. 2007;6:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 174] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Singh M, Rebordosa C, Bernholz J, Sharma N. Epidemiology and genetics of cystic fibrosis in Asia: In preparation for the next-generation treatments. Respirology. 2015;20:1172-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Tabaripour R, Niaki HA, Douki MR, Bazzaz JT, Larijani B, Yaghmaei P. Poly thymidine polymorphism and cystic fibrosis in a non-Caucasian population. Dis Markers. 2012;32:241-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Boyle MP, De Boeck K. A new era in the treatment of cystic fibrosis: correction of the underlying CFTR defect. Lancet Respir Med. 2013;1:158-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 6. | Li N, Pei P, Bu DF, He B, Wang GF. A novel CFTR mutation found in a Chinese patient with cystic fibrosis. Chin Med J (Engl). 2006;119:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Xu J, Yin Y, Zhang L, Zhang J, Yuan S, Zhang H. Four case reports of Chinese cystic fibrosis patients and literature review. Pediatr Pulmonol. 2017;52:1020-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Liu K, Liu Y, Li X, Xu KF, Tian X, Zhang X. A novel homozygous complex deletion in CFTR caused cystic fibrosis in a Chinese patient. Mol Genet Genomics. 2017;292:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5632] [Cited by in RCA: 5185] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 10. | Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 423] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 12. | Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H; Clinical Practice Guidelines on Growth and Nutrition Subcommittee; Ad Hoc Working Group. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108:832-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 471] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 13. | Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, Durie PR, Legrys VA, Massie J, Parad RB, Rock MJ, Campbell PW; Cystic Fibrosis Foundation. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4-S14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 811] [Cited by in RCA: 695] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 14. | Dörk T, Fislage R, Neumann T, Wulf B, Tümmler B. Exon 9 of the CFTR gene: splice site haplotypes and cystic fibrosis mutations. Hum Genet. 1994;93:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Wang MC, Shu SG, Chang SM, Ho WL, Chi CS. Cystic fibrosis in two Chinese infants in Taiwan. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1993;34:314-321. [PubMed] |
| 16. | Chen BH, Zhang SZ, Yang Y. The first case of CF in Mainland China identified by DNA analysis. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 1995;12:5-9. |
| 17. | Zielenski J, Markiewicz D, Lin SP, Huang FY, Yang-Feng TL, Tsui LC. Skipping of exon 12 as a consequence of a point mutation (1898 + 5G-->T) in the cystic fibrosis transmembrane conductance regulator gene found in a consanguineous Chinese family. Clin Genet. 1995;47:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Crawford J, Labrinidis A, Carey WF, Nelson PV, Harvey JS, Morris CP. A splicing mutation (1898 + 1G-->T) in the CFTR gene causing cystic fibrosis. Hum Mutat. 1995;5:101-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Wagner JA, Vassilakis A, Yee K, Li M, Hurlock G, Krouse ME, Moss RB, Wine JJ. Two novel mutations in a cystic fibrosis patient of Chinese origin. Hum Genet. 1999;104:511-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Wu CL, Shu SG, Zielenski J, Chiang CD, Tsui LC. Novel cystic fibrosis mutation (2215insG) in two adolescent Taiwanese siblings. J Formos Med Assoc. 2000;99:564-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Alper OM, Shu SG, Lee MH, Wang BT, Lo SY, Lin KL, Chiu YL, Wong LJ. Detection of novel CFTR mutations in Taiwanese cystic fibrosis patients. J Formos Med Assoc. 2003;102:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Chen HJ, Lin SP, Lee HC, Chen CP, Chiu NC, Hung HY, Chern SR, Chuang CK. Cystic fibrosis with homozygous R553X mutation in a Taiwanese child. J Hum Genet. 2005;50:674-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Wang B, Yang L. Cystic Fibrosis Involving Multisystem: A Case Report and Literature Review. Huaxi Yixue. 2012;6:852-854. |
| 24. | Liu JR, Peng Y, Zhao YH, Wang W, Guo Y, He JX, Zhao SY, Jiang ZF. [Clinical manifestations and gene analysis of 2 Chinese children with cystic fibrosis]. Zhonghua Er Ke Za Zhi. 2012;50:829-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 25. | Cheng Y, Ning G, Song B, Guo YK, Li XS. A Chinese girl with cystic fibrosis: a case report identified by sweat and genetic tests. Chin Med J (Engl). 2012;125:719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Liu Y, Wang L, Tian X, Xu KF, Xu W, Li X, Yue C, Zhang P, Xiao Y, Zhang X. Characterization of gene mutations and phenotypes of cystic fibrosis in Chinese patients. Respirology. 2015;20:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Shen Y, Liu J, Zhong L, Mogayzel PJ, Zeitlin PL, Sosnay PR, Zhao S. Clinical Phenotypes and Genotypic Spectrum of Cystic Fibrosis in Chinese Children. J Pediatr. 2016;171:269-76.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Chu JL, Wang Y, Qian J. Cystic fibrosis with severe pneumonia in children. Chin Pediatr Emerg Med. 2016;23:501-504. [DOI] [Full Text] |
| 29. | Xu BP, Wang H, Zhao YH, Liu J, Yao Y, Feng XL, Shen KL. [Molecular diagnosis of two Chinese cystic fibrosis children and literature review]. Zhonghua Er Ke Za Zhi. 2016;54:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Li L, Wang NL, Gong JY, Wang JS. [Infantile cholestasis caused by CFTR mutation: case report and literature review]. Zhonghua Er Ke Za Zhi. 2016;54:851-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 31. | Tian X, Liu Y, Yang J, Wang H, Liu T, Xu W, Li X, Zhu Y, Xu KF, Zhang X. p.G970D is the most frequent CFTR mutation in Chinese patients with cystic fibrosis. Hum Genome Var. 2016;3:15063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Leung GK, Ying D, Mak CC, Chen XY, Xu W, Yeung KS, Wong WL, Chu YW, Mok GT, Chau CS, McLuskey J, Ong WP, Leong HY, Chan KY, Yang W, Chen JH, Li AM, Sham PC, Lau YL, Chung BH, Lee SL. <i>CFTR</i> founder mutation causes protein trafficking defects in Chinese patients with cystic fibrosis. Mol Genet Genomic Med. 2016;5:40-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Xie Y, Huang X, Liang Y, Xu L, Pei Y, Cheng Y, Zhang L, Tang W. A new compound heterozygous CFTR mutation in a Chinese family with cystic fibrosis. Clin Respir J. 2017;11:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Zheng B, Cao L. Differences in gene mutations between Chinese and Caucasian cystic fibrosis patients. Pediatr Pulmonol. 2017;52:E11-E14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Yao Y, Feng XL, Xu BP, Shen KL. Pseudo-Bartter Syndrome in a Chinese Infant with Cystic Fibrosis Caused by c.532G>A Mutation in <i>CFTR</i>. Chin Med J (Engl). 2017;130:2771-2772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 36. | Sun Y, Zhong YM, Zhu M, Wang SY, Wang J, Zhang H, Zhang L, Shao H. Clinical and radiological manifestations of 5 pediatric cases with cystic fibrosis. J Clin Pediatr. 2017;35:837-840. [DOI] [Full Text] |
| 37. | Guo ZY, Shi YY, Qian LL, Wang LB. A case report of infantile cystic fibrosis with pseudo-Bartter syndrome. Zhongguo Xunzheng Erke Zazhi. 2017;12:471-473. [DOI] [Full Text] |
| 38. | Li J, Zhang Y, Wang W, Wan WL, Qiu ZQ. One case of cystic fibrosis in children with pseudoBartter syndrome and literature review. Shandong Yiyao. 2017;57:48-50. [DOI] [Full Text] |
| 39. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19696] [Cited by in RCA: 22474] [Article Influence: 2247.4] [Reference Citation Analysis (0)] |
| 40. | Kristidis P, Bozon D, Corey M, Markiewicz D, Rommens J, Tsui LC, Durie P. Genetic determination of exocrine pancreatic function in cystic fibrosis. Am J Hum Genet. 1992;50:1178-1184. [PubMed] |
| 41. | Ferrari M, Cremonesi L. Genotype-phenotype correlation in cystic fibrosis patients. Ann Biol Clin (Paris). 1996;54:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |