Published online Aug 6, 2019. doi: 10.12998/wjcc.v7.i15.2013
Peer-review started: March 28, 2019
First decision: May 31, 2019
Revised: June 18, 2019
Accepted: July 3, 2019
Article in press: July 3, 2019
Published online: August 6, 2019
Processing time: 134 Days and 9.4 Hours
Acute myocardial infarction (AMI) is a leading cause of mortality. Early reperfusion to restore blood flow is crucial to successful treatment. In the current reperfusion regimen, an increasing number of patients have benefited from direct percutaneous coronary intervention (PCI). In order to understand whether there is a correlation between the components of coronary thrombosis and the absence of reflow or slow blood flow after coronary stent implantation in direct PCI, we collected data on direct PCI cases in our hospital between January 2016 and November 2018.
To investigate the correlation between intracoronary thrombus components and coronary blood flow after stent implantation in direct PCI in AMI.
We enrolled 154 patients (85 male and 69 female, aged 36–81 years) with direct PCI who underwent thrombus catheter aspiration within < 3, 3–6 or 6–12 h of onset of AMI between January 2016 and November 2018. The thrombus was removed for pathological examination under a microscope. The patients of the three groups according to the onset time of AMI were further divided into those with a white or red thrombus. The thrombolysis in myocardial infarction (TIMI) blood flow after stent implantation was recorded based on digital subtraction angiography during PCI. The number of patients with no-reflow and slow blood flow in each group was counted. Statistical analysis was performed based on data such as onset time, TIMI blood flow.
There were significant differences in thrombus components between the patients with acute ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction (P < 0.01). In the group with PCI < 3 h after onset of AMI, there was no significant difference in the incidence of no-reflow and slow-flow between the white and red thrombus groups. In the groups with PCI 3-6 and 6-12 h after onset of AMI, there was a significant difference in the incidence of no-reflow and slow-flow between the white and red thrombus groups (P < 0.01). There was a significant correlation between the onset time of AMI and the occurrences of no-reflow and slow blood flow during PCI (P < 0.01).
In direct PCI, the onset time of AMI and color of coronary thrombus are often used to predict whether there will be no reflow or slow blood flow after stent implantation.
Core tip: We investigated the correlation between intracoronary thrombus components and coronary blood flow after stent implantation in direct percutaneous coronary intervention (PCI) in acute myocardial infarction (AMI). A total of 154 patients with direct PCI who underwent thrombus catheter aspiration within < 3, 3-6 or 6-12 h of onset of AMI were included. There was a significant correlation between the onset time of AMI and the occurrence of no reflow and slow blood flow during PCI. In direct PCI, the onset time of AMI and color of coronary thrombus may predict whether there will be no reflow or slow blood flow after stent implantation.
- Citation: Zhang MJ, Liu X, Liu LH, Li N, Zhang N, Wang YQ, Sun XJ, Huang PH, Yin HM, Liu YH, Zheng H. Correlation between intracoronary thrombus components and coronary blood flow after percutaneous coronary intervention for acute myocardial infarction at different onset time. World J Clin Cases 2019; 7(15): 2013-2021
- URL: https://www.wjgnet.com/2307-8960/full/v7/i15/2013.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i15.2013
Acute myocardial infarction (AMI) is a critical illness with high mortality. Early reperfusion to restore blood flow is the key to successful treatment[1]. In the current reperfusion regimen, an increasing number of patients have benefited from direct percutaneous coronary intervention (PCI). However, as well as some coronary revascularization, there have been some cases of coronary artery no-reflow or slow blood flow after stent implantation in direct PCI[2-6]. In order to understand whether there is a correlation between the components of coronary thrombosis and the absence of reflow or slow blood flow after coronary stent implantation in direct PCI, we collected data on direct PCI cases in our hospital between January 2016 and November 2018.
We enrolled 154 patients with AMI who were admitted to our hospital between January 2016 and November 2018. Patients underwent direct PCI within 12 hours of onset of AMI with aspiration catheterization and stent implantation. There were 85 men and 69 women, aged 36–81 years (mean age 59.8 years). There were 116 patients with acute ST-segment elevation myocardial infarction (STEMI), 38 with acute non-ST-segment elevation myocardial infarction (NSTEMI), 85 with right coronary artery infarction, 47 with left anterior descending artery infarction and 22 with left circumflex artery infarction.
Grouping: All patients were divided into 3 groups according to the time of AMI onset: < 3, 3-6 and 6-12 h. According to electrocardiography and coronary angiography, the patients were further divided into acute STEMI and acute NSTEMI groups.
Coronary angiography: The coronary condition and thrombolysis in myocardial infarction (TIMI) blood flow were recorded using the JUDKINS method. TIMI blood flow was graded as follows: Level 0, complete occlusion of the diseased blood vessels, no contrast agent was passed, and the distal myocardium was completely non-perfused; Level 1, the diseased blood vessels had a small amount of contrast agent and blood flow, but the distal arterial vascular bed was not fully developed; Level 2, the contrast agent slowly passed through the stenosis or was delayed at the distal end of the stenosis, the distal segment was developed, and the distal myocardium was perfused, that is, the distal vascular bed was fully developed after 3 cardiac cycles; Level 3, the contrast agent rapidly filled the blood vessel and was rapidly emptied, and all distal myocardial perfusion was complete, that is, the distal vascular bed was fully developed within 3 cardiac cycles. Patients with TIMI blood flow Level 0–2 had no reflow and slow blood flow[7]. The severity of coronary thrombosis load was assessed according to the following 6 points: (1) A long thrombus > 3 times the inner diameter of the reference vessel; (2) A strip-shaped thrombus with a length of > 5 mm at the proximal end of the occlusion; (3) A floating thrombus at the proximal end of the occlusion; (4) Inner luminal diameter > 4.0 mm; (5) Occlusion of the proximal vessel without abrupt blunt occlusion; and (6) Contrast agent retention in the distal end of the occluded vessel[8].
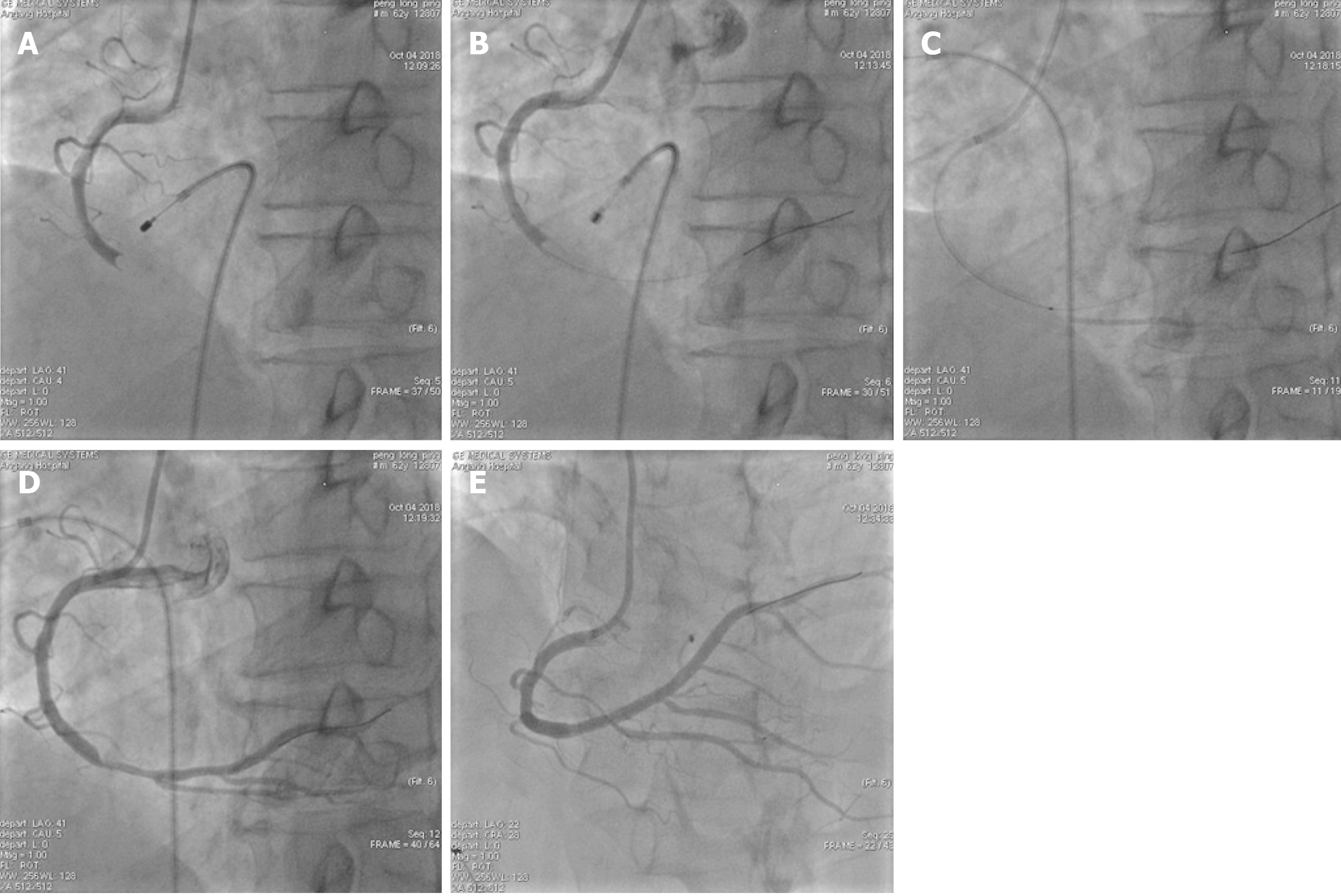
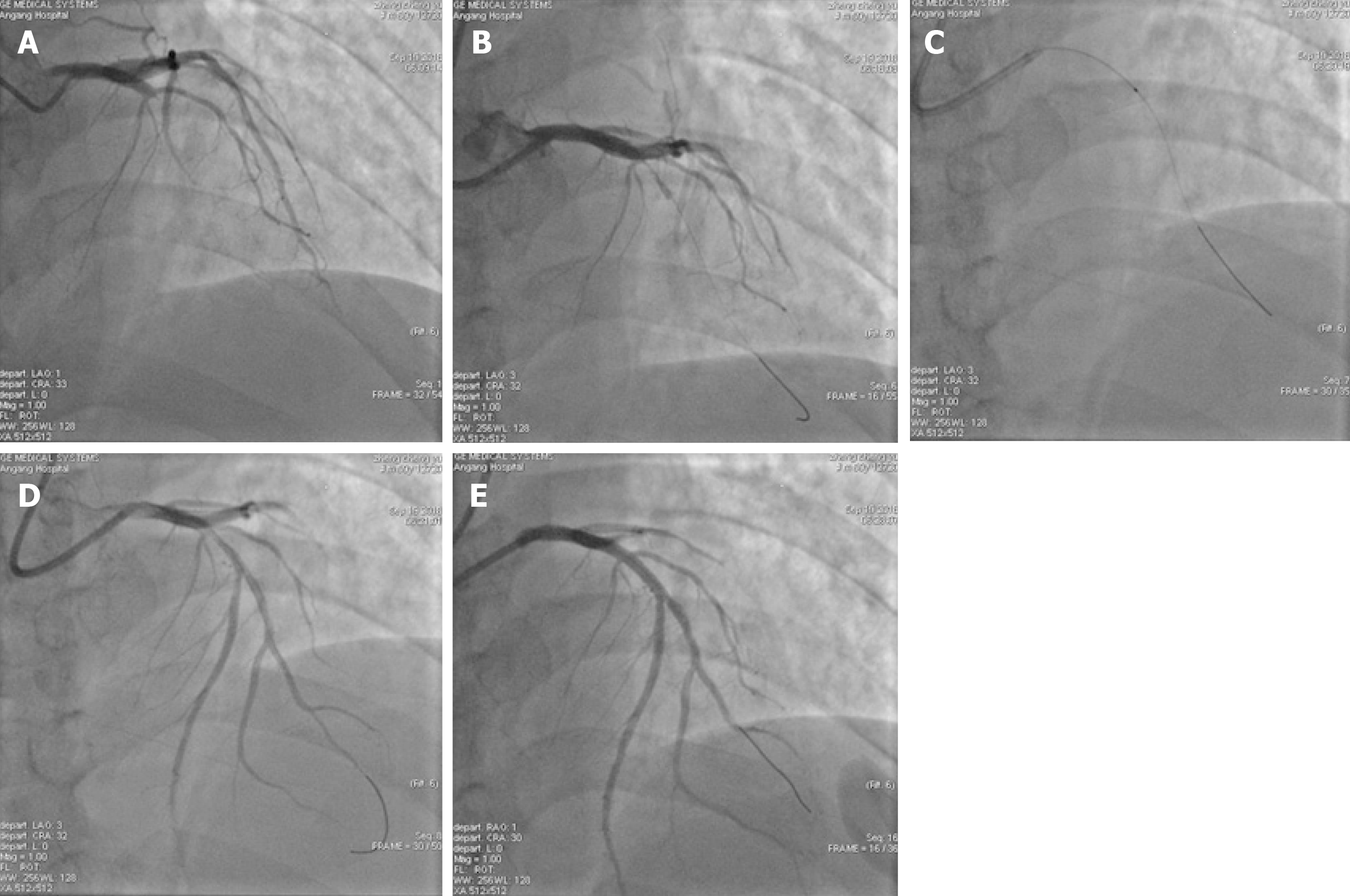
PCI: Signed informed consent was obtained from each patient before surgery, and the double-resistance load was given (aspirin 300 mg and ticagrelor 180 mg orally). For patients older than 75 years, aspirin 300 mg and clopidogrel 300 mg were given orally. The appropriate guiding catheter was inserted into the coronary artery associated with the lesion of the offender and guide the wire to the distal end of the coronary artery through the lesion, and the Export AP thrombus aspiration catheter was passed to the lesion through the guiding wire, and pumping was repeated 3 to 5 times from the proximal end to the distal end of the lesion. According to angiography after aspiration of the thrombus, stent implantation was performed in patients with residual stenosis > 75%. The intraoperative TIMI myocardial perfusion grade was recorded and the number of cases without reflow or slow blood flow was recorded. In patients with no reflow or slow blood flow, tirofiban and sodium nitroprusside were given intracoronally through the aspiration catheter to improve blood flow. Typical cases are shown in Figure 1 and Figure 2.
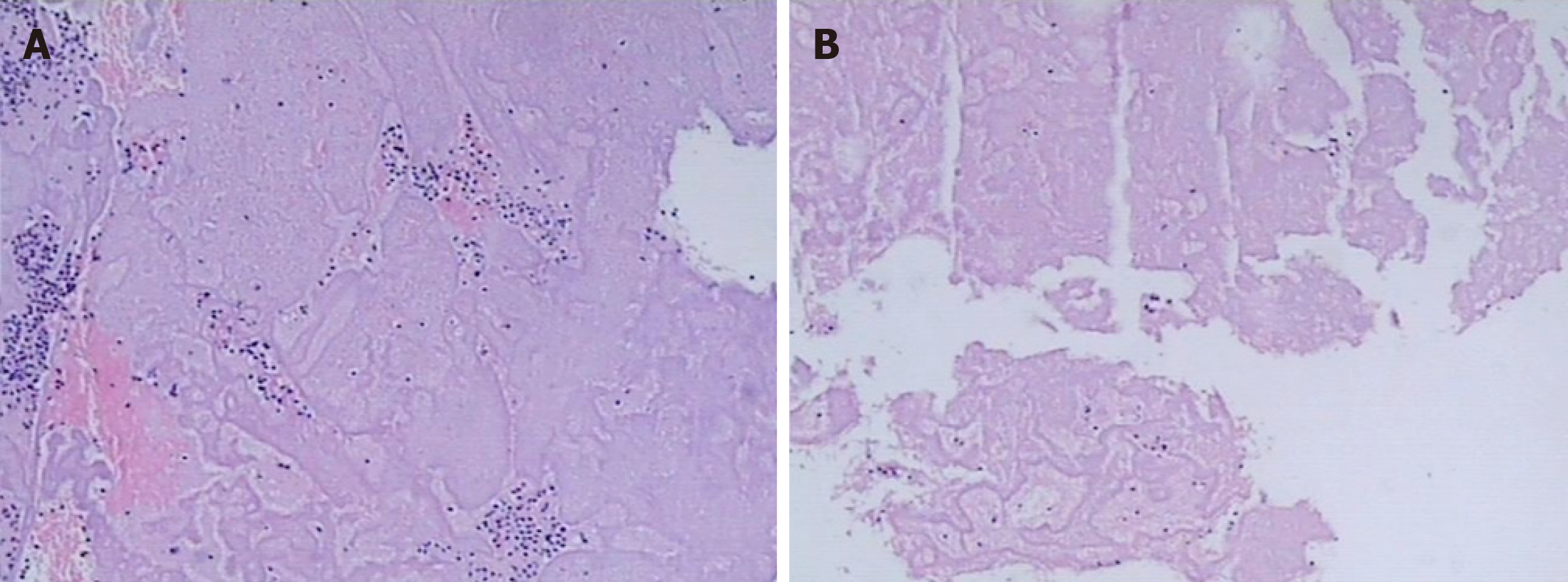
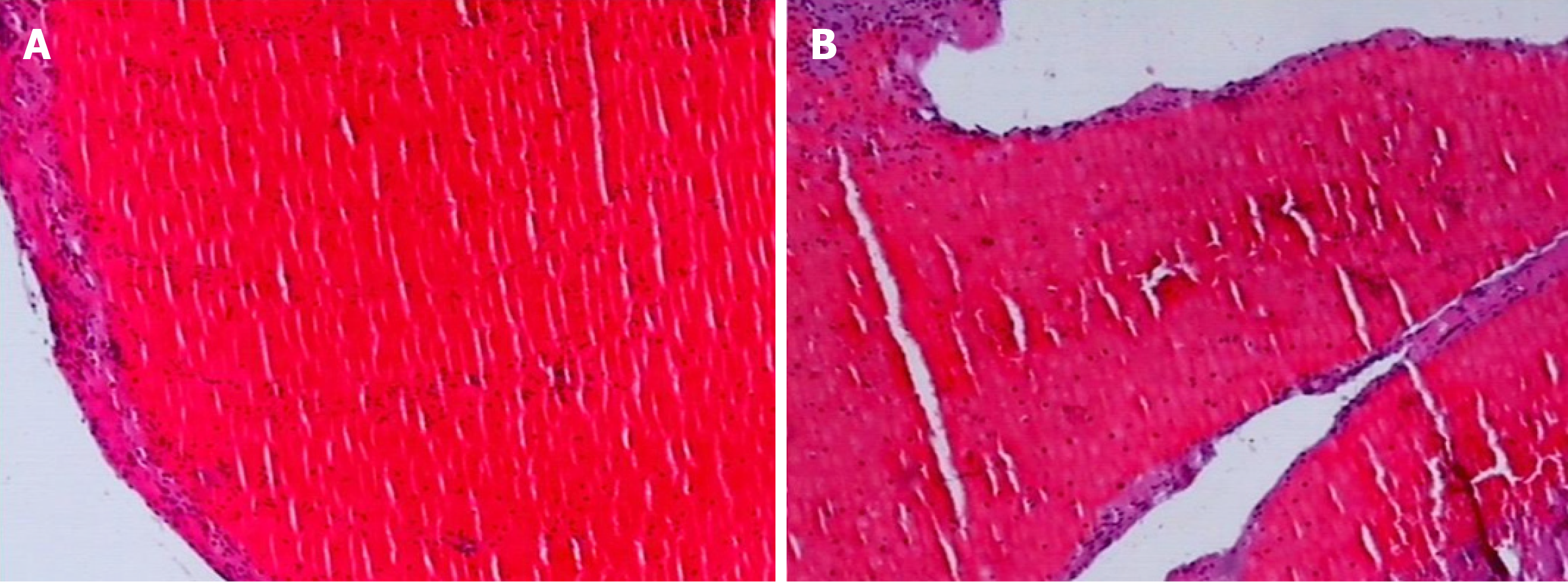
Observation of thrombus components: The thrombus was extracted from the coronary artery and initially observed with the naked eye. The thrombi were classified as white or red, which was confirmed by fixed section microscopy (Figure 3 and Figure 4).
Statistical analysis was performed using SPSS version 19.0 software. Normal distribution measurement data are expressed as mean ± SD. The Chi-square test was used to compare the data between the groups. P < 0.01 was considered statistically significant.
In the NSTEMI group, 28 cases of white thrombus and 10 of red thrombus were extracted. In the STEMI group, 4 cases of white thrombus and 112 of red thrombus were extracted. There was a significant difference in the thrombus components between the two groups (P < 0.01), as shown in Table 1. In the group with PCI at < 3 h after onset of AMI, there were 4 cases of white thrombus (1 had no reflow and slow blood flow), and 27 cases of red thrombus (none had no reflow and slow blood flow). There was no significant difference in the incidence of no reflow and slow blood flow after stent implantation between the two subgroups (P > 0.05). In the PCI at 3-6 and 6-12 h after onset of AMI, there were 28 cases of white thrombus (20 patients had no reflow and slow blood flow), and 95 cases of red thrombus (15 patients had no reflow and slow blood flow). There was a significant difference in the incidence of no reflow and slow blood flow after stent implantation between these two subgroups (P < 0.01), as shown in Table 2. There was a significant correlation between the time of PCI after onset of AMI and the occurrence of no reflow and slow blood flow (P < 0.01), as shown in Table 3.
| Groups | White thrombus | < 3 h | 3–6 h | 6–12 h |
| NSTEMI (38 cases) | Yes | 3 (50.0) | 12 (75.0) | 13 (81.25) |
| No | 3 (50.0) | 4 (25.0) | 3 (18.75) | |
| STEMI (116 cases) | Yes | 1 (4.00) | 1 (1.92) | 2 (5.13) |
| No | 24 (96.0) | 51 (98.08) | 37 (94.87) | |
| Fisher | 37.662 | 29.418 | ||
| P value | 0.016 | < 0.001 | < 0.001 |
| Subgroups | No reflow or slow blood flow | < 3 h | 3-6 h | 6-12 h |
| White thrombus (32 cases) | No | 3 (75.0) | 5 (38.46) | 3 (20.0) |
| Yes | 1 (25.0) | 8 (61.54) | 12 (80.0) | |
| Red thrombus (122 cases) | No | 27 (100) | 50 (90.91) | 30 (75.0) |
| Yes | 0 (0) | 5 (9.09) | 10 (25.0) | |
| Fisher | 15.467 | 13.75 | ||
| P value | 0.143 | < 0.001 | < 0.001 |
| No reflow or slow blood flow | < 3 h | 3-6 h | 6-12 h | Chi-square | P value |
| Yes (36 cases) | 1 (3.23) | 13 (19.12) | 22 (40.0) | 16.201 | < 0.001 |
| No (121 cases) | 30 (96.7)7 | 55 (80.88) | 33 (60.0) |
The main cause of AMI is acute or secondary occlusion of the coronary artery. Early, effective and continuous opening of infarct-related blood vessels and restoration of effective blood perfusion can reduce the area of necrotic myocardium and reduce mortality[9]. Reperfusion of the myocardium by primary PCI is currently the preferred treatment for AMI. However, with the increasing use of primary PCI, some patients have no reflow or slow blood flow in infarct-related blood vessels after emergency stent implantation[10,11]. No reflow or slow blood flow refers to the phenomenon of no blood flow or slow blood flow after infarction-related coronary artery treatment by stent or balloon, resulting in no perfusion or hypoperfusion of myocardial tissue. The exact mechanism of no reflow or slow blood flow is not fully understood at present. It is not the result of simple mechanical microcirculation embolism, but the comprehensive consequences caused by the interaction of various pathophysiological mechanisms. The main mechanisms are currently considered to include: Myocardial ischemic injury, myocardial reperfusion injury, distal coronary artery embolization and microcirculatory injury. Myocardial ischemia can cause damage to vascular endothelial cells and trigger a cascade of cytokines. Ischemia causes damage to the vascular endothelium, adhesion of neutrophils and platelets, and causes stenosis or occlusion of the lumen, further aggravating microcirculatory disorders. During myocardial reperfusion, a series of changes have occurred, such as calcium overload, increased oxygen free radical production, inflammatory cell infiltration, and activation of apoptotic signaling pathways; these changes aggravate ischemia and form a vicious circle. In patients with AMI undergoing direct PCI, due to balloon pre-expansion or stent implantation, unstable intracoronary plaque rupture or microparticles (such as microthrombus of platelets) decrease, leading to vascular obstruction in the distal coronary artery. When the number of particles is < 25 or the diameter of the particles is < 200 μm, it generally does not cause microvascular obstruction. When the number of particles is 25-200 or the particle diameter is > 200 μm, it can cause severe microvascular obstruction. At the same time, thromboxane or angiotensin released by the plaque substance may further lead to microcirculatory disorders[12-15]. Different patients have different mechanisms at different pathological stages. Patients with myocardial no reflow after emergency PCI have individual differences, which may be related to genetic susceptibility, and smoking, hypertension, hyperlipidemia and diabetes may also be unfavorable factors for the no-reflow phenomenon[16,17]. The same patient may have multiple different mechanisms at the same time. TIMI blood flow grading is commonly used in clinical evaluation. Coronary artery angiography TIMI blood flow Level 0 is no reflow, while Levels 1 and 2 are slow blood flow. A large number of clinical studies have found that the incidence of no reflow or slow blood flow in primary PCI is estimated to be 20%-30% with TIMI blood flow grading[18]. The rate of no reflow or slow blood flow estimated by microperfusion such as myocardial contrast echo is as high as 34%-39%[19,20], which can cause an increase in myocardial infarct size, continuous reduction of ventricular function, and further increase mortality[21-23]. The incidence is higher in patients with high thromboembolic lesions[24,25].
In conclusion, we found that there was a significant correlation between the onset time of AMI and no reflow and slow blood flow during surgery. The longer onset time of AMI, the higher the incidence of no reflow or slow blood flow. There was a significant difference in the thrombus components between acute STEMI and acute NSTEMI. Patients with acute STEMI had mainly red thrombus, while those with acute NSTEMI had mainly white thrombus, which was closely related to the mechanism of different types of AMI. In patients with PCI at > 3 h after onset of AMI, those with white thrombus were more likely to have no reflow and slower blood flow after stent implantation than patients with red thrombosis. This can predict whether there is no reflow or slow blood flow after stent implantation. At present, the prevention and treatment of microcirculatory disorders and coronary no reflow are limited[26-32]. Therefore, in patients with hyperthrombotic lesions that achieve complete recanalization of infarcted coronary arteries, pre-coronary administration of drugs, such as glycoprotein IIb/IIIa receptor antagonists or sodium nitroprusside, should be fully evaluated based on the nature of the thrombus extracted from the coronary arteries. Calcium antagonists[33,34] are important to reduce the occurrence of slow blood flow or no reflow.
Acute myocardial infarction (AMI) is a leading cause of mortality. Early reperfusion to restore blood flow is crucial to successful treatment. An increasing number of patients have benefited from direct percutaneous coronary intervention (PCI). However, coronary artery no-reflow or slow blood flow after stent implantation and coronary revascularization occurred in direct PCI in some cases. The exact mechanism of no reflow or slow blood flow remains unclear
Although in the current reperfusion regimen, an increasing number of patients have benefited from direct PCI, there have been some cases of coronary artery no-reflow or slow blood flow after stent implantation in direct PCI as well as coronary revascularization. The exact mechanism of no reflow or slow blood flow is still unclear. In order to understand whether there is a correlation between the components of coronary thrombosis and the absence of reflow or slow blood flow after coronary stent implantation in direct PCI, we collected data on direct PCI cases in our hospital between January 2016 and November 2018.
This study aims to investigate the correlation between intracoronary thrombus components and coronary blood flow after stent implantation in direct PCI in AMI.
A total of 154 patients with direct PCI who underwent thrombus catheter aspiration within < 3, 3–6 or 6–12 h of onset of AMI between January 2016 and November 2018 were included. The thrombus was removed for pathological examination. The patients of three groups according to the onset time of AMI were further divided into those with a white or red thrombus. The thrombolysis in myocardial infarction (TIMI) blood flow after stent implantation was recorded based on digital subtraction angiography during PCI. The number of patients with no-reflow and slow blood flow in each group was counted. Statistical analysis was performed on the onset time, thrombus component, and TIMI blood flow.
There were significant differences in thrombus components between the patients with acute ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction (P < 0.01). In the group with PCI < 3 h after onset of AMI, there was no significant difference in the incidence of no-reflow and slow-flow between the white and red thrombus groups. In the groups with PCI 3–6 and 6–12 h after onset of AMI, there was a significant difference in the incidence of no-reflow and slow-flow between the white and red thrombus groups (P < 0.01). There was a significant correlation between the onset time of AMI and the occurrence of no-reflow and slow blood flow during PCI (P < 0.01).
There was a significant correlation between the onset time of AMI and no reflow and slow blood flow during surgery. There was a significant difference in the thrombus components between acute ST-segment elevation myocardial infarction (STEMI) and acute non-ST-segment elevation myocardial infarction (NSTEMI). Patients with acute STEMI had mainly red thrombus, while those with acute NSTEMI had mainly white thrombus, which was closely related to the mechanism of different types of AMI. In patients with PCI at > 3 h after onset of AMI, those with white thrombus were more likely to have no reflow and slower blood flow after stent implantation than patients with red thrombosis. This can predict whether there is no reflow or slow blood flow after stent implantation. In patients with hyperthrombotic lesions that achieve complete recanalization of infarcted coronary arteries, pre-coronary administration of drugs, such as glycoprotein IIb/IIIa receptor antagonists or sodium nitroprusside, should be fully evaluated based on the nature of the thrombus extracted from the coronary arteries. Calcium antagonists can help reduce the occurrence of slow blood flow or no reflow.
In direct PCI, the onset time of AMI and color of coronary thrombus are often used to predict whether there will be no reflow or slow blood flow after stent implantation. However, the exact mechanism of no reflow or slow blood flow is not fully understood. Multiple pathophysiological mechanisms might be involved, including myocardial ischemia, myocardial reperfusion injury, distal coronary artery embolization and microcirculatory injury. More prospective studies are needed to be carried out in the future in AMI patients with direct PCI after stent implantation.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Campanale M, Sogabe I S-Editor: Wang JL L-Editor: Filipodia E-Editor: Wu YXJ
| 1. | Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Ting HH, O'Gara PT, Kushner FG, Ascheim DD, Brindis RG, Casey DE, Chung MK, de Lemos JA, Diercks DB, Fang JC, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2016;133:1135-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 366] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 2. | Bouleti C, Mewton N, Germain S. The no-reflow phenomenon: State of the art. Arch Cardiovasc Dis. 2015;108:661-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Niccoli G, Scalone G, Lerman A, Crea F. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J. 2016;37:1024-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 313] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 4. | Costa RA, Abizaid A, Lotan C, Dudek D, Silber S, Dizon JM, Maehara A, Dressler O, Brener SJ, Stone GW. Impact of thrombus burden on outcomes after standard versus mesh-covered stents in acute myocardial infarction (from the MGuard for acute ST elevation reperfusion trial). Am J Cardiol. 2015;115:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Barresi L, Tacelli M, Tarantino I, Cipolletta F, Granata A, Traina M. Improving the yield of EUS-guided histology. Endosc Ultrasound. 2018;7:301-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Dietrich CF, Bibby E, Jenssen C, Saftoiu A, Iglesias-Garcia J, Havre RF. EUS elastography: How to do it? Endosc Ultrasound. 2018;7:20-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Mahmoud KS. Left and Right Ventricular Myocardial Performance Index and it,s Relation with TIMI Frame Count in the Coronary Slow Flow Phenomenon. J Cli Exp Cardiol. 2016;7:1-5. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A, Rosengren A, Steg PG, Tubaro M, Verheugt F, Weidinger F, Weis M; ESC Committee for Practice Guidelines (CPG). Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909-2945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1468] [Cited by in RCA: 1425] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 9. | Respaud R, Marchand D, Pelat T, Tchou-Wong KM, Roy CJ, Parent C, Cabrera M, Guillemain J, Mac Loughlin R, Levacher E, Fontayne A, Douziech-Eyrolles L, Junqua-Moullet A, Guilleminault L, Thullier P, Guillot-Combe E, Vecellio L, Heuzé-Vourc'h N. Development of a drug delivery system for efficient alveolar delivery of a neutralizing monoclonal antibody to treat pulmonary intoxication to ricin. J Control Release. 2016;234:21-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Khan AR, Binabdulhak AA, Alastal Y, Khan S, Faricy-Beredo BM, Luni FK, Lee WM, Khuder S, Tinkel J. Cardioprotective role of ischemic postconditioning in acute myocardial infarction: a systematic review and meta-analysis. Am Heart J. 2014;168:512-521.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Eitel I, Stiermaier T, Rommel KP, Fuernau G, Sandri M, Mangner N, Linke A, Erbs S, Lurz P, Boudriot E, Mende M, Desch S, Schuler G, Thiele H. Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST-elevation myocardial infarction: the randomized LIPSIA CONDITIONING trial. Eur Heart J. 2015;36:3049-3057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 187] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 12. | Mazhar J, Mashicharan M, Farshid A. Predictors and outcome of no-reflow post primary percutaneous coronary intervention for ST elevation myocardial infarction. Int J Cardiol Heart Vasc. 2015;10:8-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Toprak C, Tabakci MM, Simsek Z, Arslantas U, Durmus HI, Ocal L, Demirel M, Ozturkeri B, Ozal E, Kargin R. Platelet/lymphocyte ratio was associated with impaired myocardial perfusion and both in-hospital and long-term adverse outcome in patients with ST-segment elevation acute myocardial infarction undergoing primary coronary intervention. Postepy Kardiol Interwencyjnej. 2015;11:288-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Camici PG, d'Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol. 2015;12:48-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 362] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 15. | Task Force Members. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ; ESC Committee for Practice Guidelines, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Document Reviewers, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Rydén L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949-3003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2772] [Cited by in RCA: 2990] [Article Influence: 249.2] [Reference Citation Analysis (0)] |
| 16. | Basso C, Rizzo S, Thiene G. The metamorphosis of myocardial infarction following coronary recanalization. Cardiovasc Pathol. 2010;19:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Yoshino S, Cilluffo R, Best PJ, Atkinson EJ, Aoki T, Cunningham JM, de Andrade M, Choi BJ, Lerman LO, Lerman A. Single nucleotide polymorphisms associated with abnormal coronary microvascular function. Coron Artery Dis. 2014;25:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54:281-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 608] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 19. | Joost A, Stiermaier T, Eitel C, Fuernau G, de Waha S, Desch S, Thiele H, Eitel I. Impact of Initial Culprit Vessel Flow on Infarct Size, Microvascular Obstruction, and Myocardial Salvage in Acute Reperfused ST-Elevation Myocardial Infarction. Am J Cardiol. 2016;118:1316-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Gupta S, Gupta MM. No reflow phenomenon in percutaneous coronary interventions in ST-segment elevation myocardial infarction. Indian Heart J. 2016;68:539-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Ndrepepa G, Tiroch K, Fusaro M, Keta D, Seyfarth M, Byrne RA, Pache J, Alger P, Mehilli J, Schömig A, Kastrati A. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol. 2010;55:2383-2389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 340] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 22. | Carrick D, Oldroyd KG, McEntegart M, Haig C, Petrie MC, Eteiba H, Hood S, Owens C, Watkins S, Layland J, Lindsay M, Peat E, Rae A, Behan M, Sood A, Hillis WS, Mordi I, Mahrous A, Ahmed N, Wilson R, Lasalle L, Généreux P, Ford I, Berry C. A randomized trial of deferred stenting versus immediate stenting to prevent no- or slow-reflow in acute ST-segment elevation myocardial infarction (DEFER-STEMI). J Am Coll Cardiol. 2014;63:2088-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 294] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 23. | De Maria GL, Alkhalil M, Oikonomou EK, Wolfrum M, Choudhury RP, Banning AP. Role of deferred stenting in patients with ST elevation myocardial infarction treated with primary percutaneous coronary intervention: A systematic review and meta-analysis. J Interv Cardiol. 2017;30:264-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Izgi A, Kirma C, Tanalp AC, Dundar C, Oduncu V, Aung SM, Sonmez K, Mutlu B, Mansuroglu D. Predictors and clinical significance of angiographically detected distal embolization after primary percutaneous coronary interventions. Coron Artery Dis. 2007;18:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Kirma C, Izgi A, Dundar C, Tanalp AC, Oduncu V, Aung SM, Sonmez K, Mutlu B, Ozdemir N, Erentug V. Clinical and procedural predictors of no-reflow phenomenon after primary percutaneous coronary interventions: experience at a single center. Circ J. 2008;72:716-721. [PubMed] |
| 26. | Botta I, Devriendt J, Rodriguez JC, Morissens M, Carling A, Gutierrez LB, Preseau T, De Bels D, Honore PM, Redant S. Cardiogenic Shock after Nifedipine Administration in a Pregnant Patient: A Case Report and Review of the Literature. J Transl Int Med. 2018;6:152-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Langabeer SE. Detecting CALR Mutations in Splanchnic Vein Thrombosis: Who and How? J Transl Int Med. 2018;6:55-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Sawada H, Chen JZ, Wright BC, Sheppard MB, Lu HS, Daugherty A. Heterogeneity of Aortic Smooth Muscle Cells: A Determinant for Regional Characteristics of Thoracic Aortic Aneurysms? J Transl Int Med. 2018;6:93-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Rezkalla SH, Stankowski RV, Hanna J, Kloner RA. Management of No-Reflow Phenomenon in the Catheterization Laboratory. JACC Cardiovasc Interv. 2017;10:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 162] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 30. | Elgendy IY, Jneid H. Microvascular obstruction in ST elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: another frontier to conquer? J Thorac Dis. 2018;10:1343-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Ito N, Nanto S, Doi Y, Kurozumi Y, Natsukawa T, Shibata H, Morita M, Kawata A, Tsuruoka A, Sawano H, Okada K, Sakata Y, Kai T, Hayashi T. Beneficial effects of intracoronary nicorandil on microvascular dysfunction after primary percutaneous coronary intervention: demonstration of its superiority to nitroglycerin in a cross-over study. Cardiovasc Drugs Ther. 2013;27:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Zalewski J, Claus P, Bogaert J, Driessche NV, Driesen RB, Galan DT, Sipido KR, Buszman P, Milewski K, Van de Werf F. Cyclosporine A reduces microvascular obstruction and preserves left ventricular function deterioration following myocardial ischemia and reperfusion. Basic Res Cardiol. 2015;110:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Abdelaziz HK, Elkilany W, Khalid S, Sabet S, Saad M. Efficacy and safety of intracoronary verapamil versus sodium nitroprusside for the prevention of microvascular obstruction during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Coron Artery Dis. 2017;28:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Lago IM, Novaes GC, Badran AV, Pavão RB, Barbosa R, Figueiredo GL, Lima MO Filho, Haddad JL, Schmidt A, Marin JA Neto. In-Lab Upfront Use of Tirofiban May Reduce the Occurrence of No-Reflow During Primary Percutaneous Coronary Intervention. A Pilot Randomized Study. Arq Bras Cardiol. 2016;107:403-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |