Published online Aug 6, 2019. doi: 10.12998/wjcc.v7.i15.1954
Peer-review started: March 22, 2019
First decision: May 13, 2019
Revised: June 22, 2019
Accepted: July 3, 2019
Article in press: July 3, 2019
Published online: August 6, 2019
Processing time: 138 Days and 4.9 Hours
Vestigial like family member 3 (VGLL3) is associated with the prognosis of epithelial ovarian cancer and soft tissue sarcoma, but its role in gastric cancer (GC) is unclear.
To explore the expression pattern and clinical significance of VGLL3 in GC.
Integrative analysis was performed on the GC transcriptome profiles and survival information deposited in the ONCOMINE, GEPIA, and ONCOLNC databases. The expression levels of VGLL3 mRNA and protein were analyzed in the freshly resected tumor and normal gastric tissues from GC patients by quantitative RT-PCR and Western blot, respectively. In addition, the in situ expression of VGLL3 in the GC tissues was determined by immunohistochemistry (IHC), and the patients were accordingly classified into the high and low expression groups. The correlation of VGLL3 expression status with patient prognosis was then determined by univariate and multivariate Cox regression analyses.
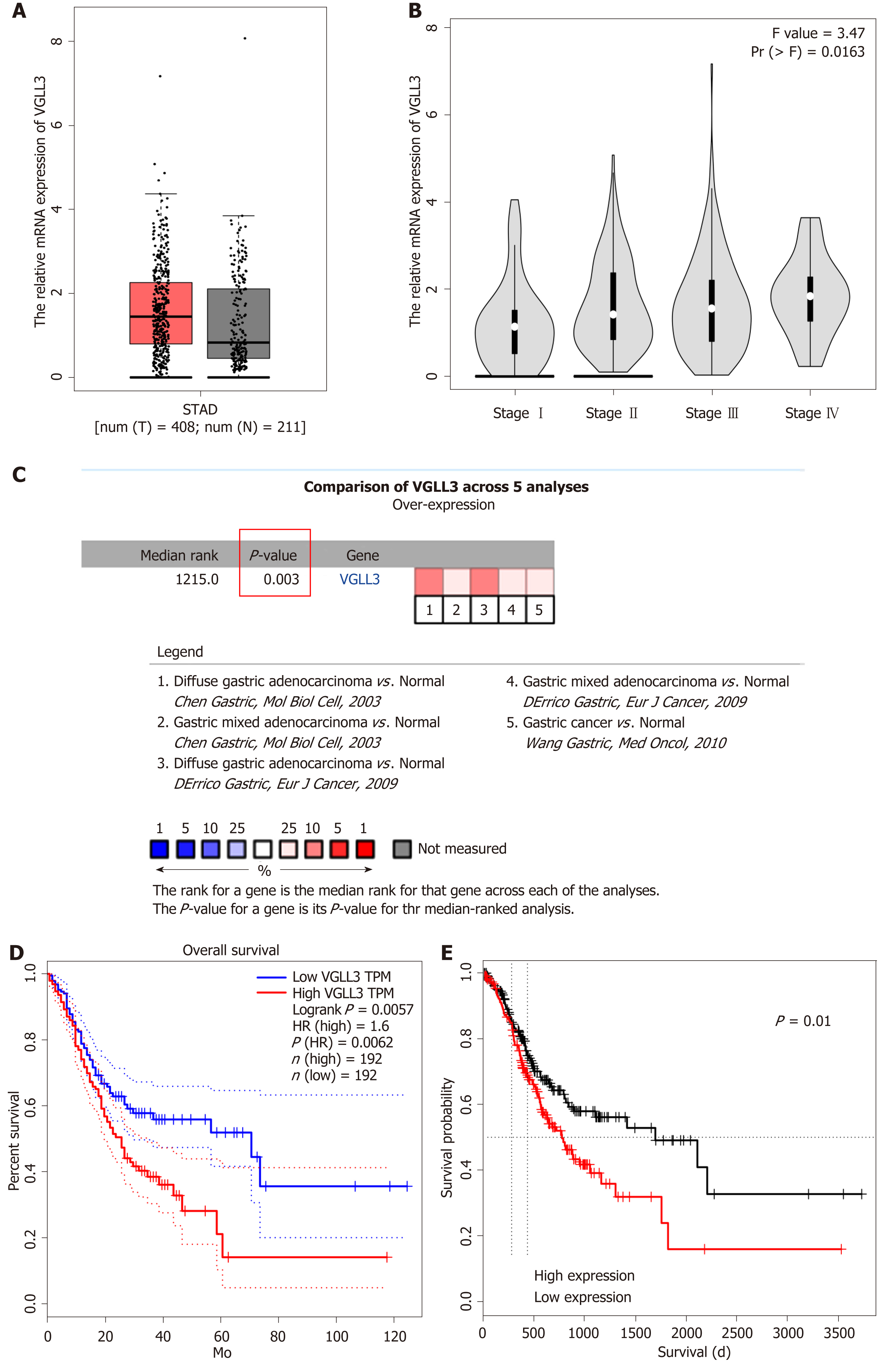
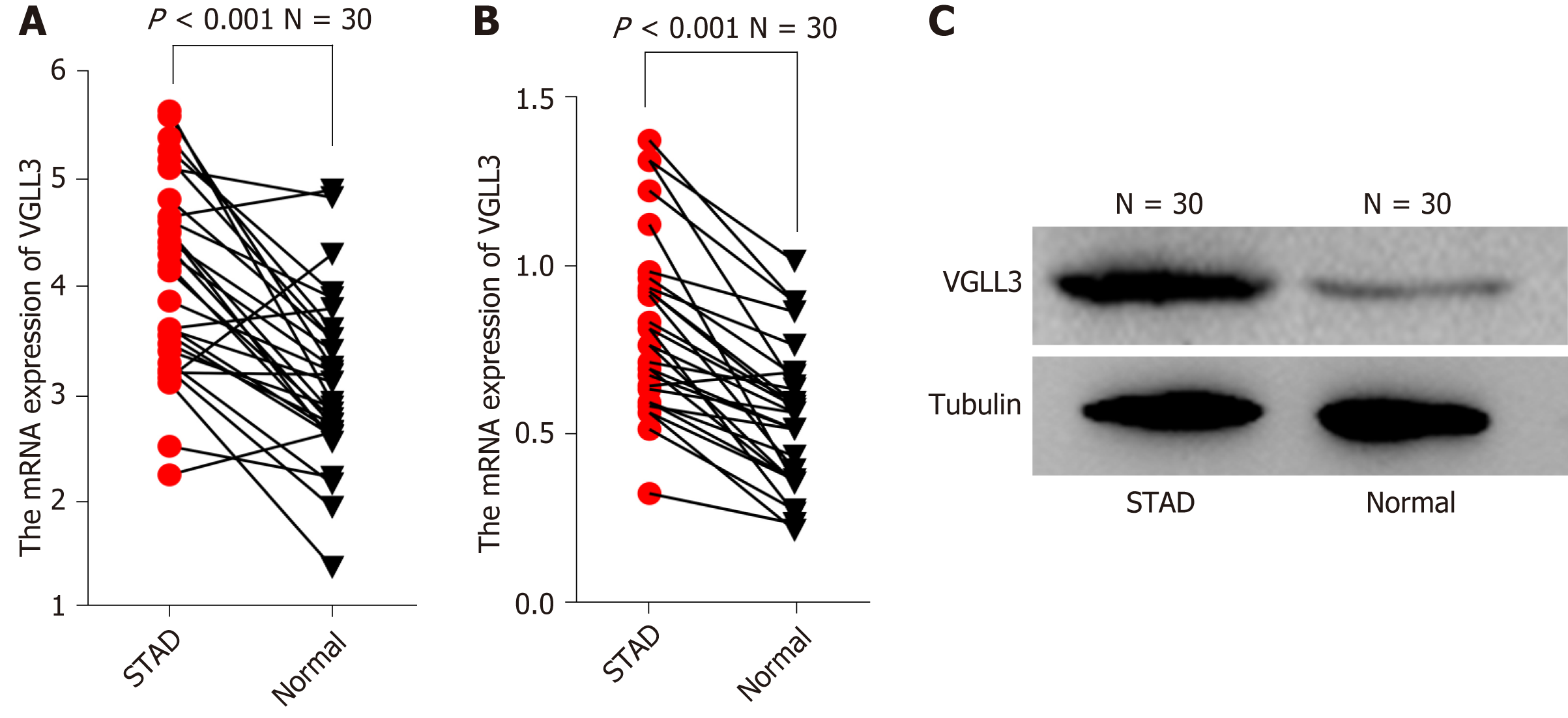
Analysis of the ONCOMINE and GEPIA databases showed that VGLL3 was significantly up-regulated in GC tissues (P = 0.003), and associated with the tumor TNM stage (P = 0.0163). The high VGLL3 expression group had a significantly worse prognosis compared to the low expression group, as per both GEPIA (P = 0.0057) and ONCOLNC (P = 0.01). The bioinformatics results were validated by the significantly higher VGLL3 mRNA and protein levels in the GC tissues compared to the adjacent normal tissues (P < 0.001) in a cohort of 30 GC patients. Furthermore, high in situ expression of VGLL3 protein was associated with more advanced N and TNM stages and HER2 mutation (P < 0.05) in a cohort of 172 patients. Kaplan-Meier analysis showed that the high VGLL3 expression group had a worse prognosis compared to the low expression group (P = 0.019). Multivariate analysis showed that VGLL3 expression status was an independent risk factor for prognosis. In addition, the prognostic risk model nomogram showed that VGLL3 was the most important indicator, with an area under the receiver operating characteristic (ROC) curve (AUC) of 0.613 for 3-year survival and 0.706 for 5-year survival. Finally, the protein interaction network analysis revealed that VGLL3 is likely involved in the Hippo signaling pathway.
VGLL3 is overexpressed in GC tissues and associated with a poor prognosis, indicating its potential as a novel prognosis biomarker and therapeutic target for GC.
Core tip: The present study for the first time revealed the expression of vestigial like family member 3 (VGLL3) in gastric cancer (GC) and its correlation with HER2 mutation. Overall, the findings of the present study suggest that VGLL3 is a novel prognostic biomarker for GC and highlight the significance of VGLL3 as a promising therapeutic target for GC.
- Citation: Zhang LH, Wang Z, Li LH, Liu YK, Jin LF, Qi XW, Zhang C, Wang T, Hua D. Vestigial like family member 3 is a novel prognostic biomarker for gastric cancer. World J Clin Cases 2019; 7(15): 1954-1963
- URL: https://www.wjgnet.com/2307-8960/full/v7/i15/1954.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i15.1954
Gastric cancer (GC) is a frequently occurring malignancy of the digestive tract[1], and it was the fifth most commonly diagnosed and the third most common cause of cancer-related mortality worldwide in 2018[2]. Lack of early diagnosis and ineffective treatment options for the advanced stages are the primary reasons for the dismal 5-year survival rate of GC[3]. Therefore, it is imperative to identify novel biomarkers for early diagnosis and accurate prediction of prognosis. Vestigial like family member 3 (VGLL3) is a member of the vestigial like family of proteins[4], and is associated with epithelial ovarian cancer[5] and soft tissue sarcoma[6]. The aim of this study was to determine the expression status and prognostic utility of VGL33 in GC. To this end, we mined the transcriptomic data of GC from the ONCOMINE and GEPIA databases, and determined its correlation with the survival data from GEPIA and ONCOLINC. VGLL3 levels were upregulated in GC samples and associated with a poor prognosis. The bioinformatics data were successfully validated on the tumor and normal gastric tissues resected from GC patients. Based on the VGLL3 levels, the patients were stratified into the high and low expression groups, and a prognosis prediction model was established on the basis of VGLL3 status and clinical data. Our findings show a strong prognostic role of VGLL3 in GC, which can potentially translate to clinical applications.
A total of 172 tissue samples were obtained from gastric adenocarcinoma patients who underwent major surgery at the Affiliated Hospital of Jiangnan University between 2009 and 2012. The tissues were immediately fixed in formalin and embedded in paraffin for further analysis. In addition, fresh samples were dissected from 30 patients at the Affiliated Hospital of Jiangnan University in November 2018, and immediately stored in liquid nitrogen for molecular analysis. The mean follow-up duration was 43.1 mo and ranged from 0.2 to 86 mo. None of the patients received chemotherapy or radiotherapy before surgery[7]. The tumor stage classification was determined by three pathologists who were blinded to the patient data according to the guidelines of the American Joint Committee on Cancer (AJCC). Freshly resected tissues were obtained from 202 patients, and were fixed in formalin and embedded in paraffin. In addition, tissue specimens from 30 patients were flash frozen in liquid nitrogen. All participating clinicians and patients provided written informed consent.
The VGLL3 mRNA levels in the GC and normal gastric tissues were determined by integrated analysis of the Oncomine database (http://www.oncomine.org)[8] using Coexpedia (http://www.coexpedia.org)[9]. ONCOLINC (http://www.oncolnc.org/cancer/) and Coexpedia were used to analyze the prognostic value of WISP1 in STAD. The STRING database (http://www.string-db.org) was utilized to construct the protein-protein interaction (PPI) network[10].
Total RNA was extracted from frozen tissue samples using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions, and reverse transcribed using the PrimeScript RT-PCR kit (Takara, Japan)[11]. RT-qPCR was performed on the ABI 7500 RealTime PCR System (Applied Biosystems, Inc. USA) using SYBR Green Master Mix (Takara, Japan), and the VGLL3 levels were normalized to β-actin. The following primers were used: Forward primer, 5’-CCAACTACAGTCACC-TCTGCTAC-3’ and reverse primer, 5’-ACCACGGTGATTCCTTACTCTTG-3’ for VGLL3; forward primer, 5’-CCTGTGGCATCCACGAAACT-3’ and reverse primer, 5’-GAAGCATTTGCG GTGGACGAT-3’ for β-actin. All reactions were performed in triplicates, and the 2-∆∆Ct method[12] was used to quantify the relative expression levels of VGLL3.
Total protein was extracted from GC and para-cancerous tissues using the RIPA lysis buffer (Pierce, Thermo Scientifc, Cramlington, United Kingdom)[13], and quantified with the enhanced BCA protein assay kit (KeyGEN BioTECH, Jiangsu, China). Equal amount (40 mg) of proteins per sample were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SD-PAGE), and then transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA, United States). After blocking with 5% non-fat milk for 1 h at room temperature (RT), the membranes were incubated overnight with anti-VGLL3 (ab83555, Abcam) and β-actin (ab8226, Abcam) primary antibodies at 4 °C. After washing thrice with TBST, the membranes were incubated with HRP-conjugated secondary antibody (1:1000) for 1 h at RT. The protein bands were visualized with an ECL chemiluminescence system after short exposure to X-ray films (Kodak, Japan). Densitometric analysis was performed with Image Pro-Plus software, and the relative expression levels of VGLL3 were normalized to tubulin.
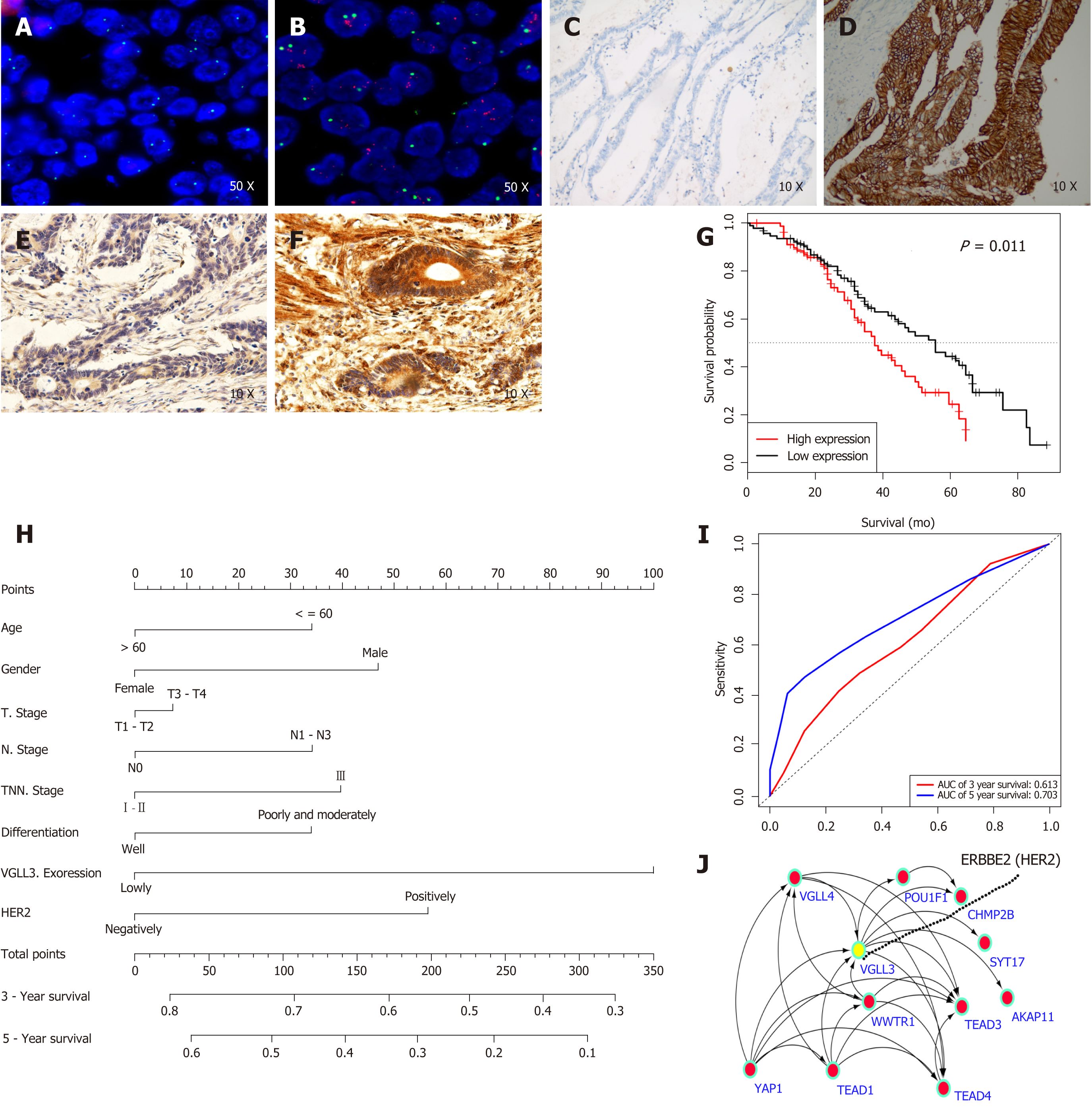
The formalin-fixed tissues were dehydrated, embedded in paraffin, and cut into 5 μm thick sections. IHC was performed according to the manufacturer’s protocol[14]. Briefly, the sections were heated in citrate buffer in a microwave, cooled, and incubated overnight with anti-VGLL3 antibody (1:50, clone PA5-68441, Invitrogen) at 4 °C. After labeling with the secondary antibody for 1 hour at RT, color was developed using liquid DAB substrate. Three pathologists blinded to the patient identity observed and graded VGLL3 positivity as 0, 1 (1%–29% positively-stained cells), 2 (30%–69%), or 3 (70%-100%), and the staining intensity as 0 (negative), 1 (weak staining), 2 (moderate staining), or 3 (strong staining). The immunoreactive score (IRS) was then calculated for each sample by multiplying the staining intensity and positivity scores, and graded as: 0-1, “-”; 2-3, “+”; 4-6, “++”; and 6-9, “+++”[15]. Based on the IRS, the samples were stratified into the high (4-9) and low (0-3) VGLL3 expression groups.
The tissue sections were treated with denaturing solution to denature the DNA into single strands, and hybridized with the PathVysion probes (No.36-161060, PathVysion HER-2DNA Probe Kit). After washing the unbound probe with DNA, the sections were counterstained with the nuclear dye DAPI (4’, 6 diamidino-2-phenylindole). Positive LSI HER-2/neu and CEP 17 signals were counted under a fluorescence microscope, and the ratio of the copy number of HER-2/neu gene to that of chromosome 17 was calculated.
Statistical analyses were performed with R*64 version 3.5.2 software and Graphpad 6.01. HER2 status (positive or negative) was designated on the basis of the combined FISH and IHC results as per the American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guidelines. VGLL3 expression levels in the tumor and normal samples were compared by the Student's t-test, and the association between VGLL3 and clinico-pathological features was assessed by the chi-square test. The overall survival (OS) curve was plotted by the Kaplan-Meier method and analyzed by the log-rank test. Univariate and multivariate analyses of the prognostic factors were performed using the Cox proportional hazard regression model. A nomogram was formulated based on the results of the multivariate analysis with the package of rms in R version 3.5.2 (http://www.r-project.org/)[16]. The receiver operating characteristic (ROC) curve was plotted to determine the sensitivity and specificity of the VGLL3-based prognostic score[17], and the area under the curve (AUC) was calculated. All P-values were two-tailed and considered statistically significant when less than 0.05.
GEPIA database profiling showed that although VGLL3 was significantly overexpressed in GC samples of advanced TNM stages relative to those at the lower stages (P = 0.0163), there was no significant difference between the GC and normal tissues (P > 0.05). Integrated analysis of multiple VGLL3 transcriptome datasets from the ONCOMINE database, however, showed that VGLL3 was significantly overexpressed in GC (P = 0.003), and notably associated with a poor prognosis according to the ONCOLINC database analysis (P = 0.01, n = 378) (Figure 1). In addition, GEPIA online survival analysis further confirmed that VGLL3 was a marker of poor prognosis in GC (P = 0.0062, n = 384). To validate the in silico data, we analyzed the expression levels of VGLL3 in the tumor and normal gastric tissues resected from 30 GC patients, and observed significantly higher levels of VGLL3 mRNA (P < 0.001) and protein (P < 0.001) in the GC tissues compared to normal tissues (Figure 2). Taken together, VGLL3 is upregulated in GC and possibly associated with a worse prognosis.
The relationships between VGLL3 levels and the clinico-pathological characteristics of the patients are summarized in Table 1. High levels of VGLL3 were positively correlated with tumor lymph node metastasis (P = 0.028) and TNM stage (P = 0.041). In addition, the median survival time of the GC patients was significantly lower in the high VGLL3 expression group than in the low expression group (35 mo vs 49 mo, P = 0.019; Figure 3C). Univariate analysis showed that the VGLL3 expression level (P = 0.019) was the only significant prognostic factor of GC, whereas age, gender, differentiation, T stage, lymph node status, TNM stage, and HER2 status did not show any significant results (P > 0.05). After including the significant factors in the Cox proportional hazards regression model for multivariate prognostic analysis, we found that VGLL3 expression (P = 0.019) was an independent risk factor for GC. ROC curves were plotted to determine the power of the VGLL3 prognostic score in predicting the 3- and 5-year survival, and the AUCs were 0.613 and 0.706, respectively (Figure 3I). We constructed a PPI network of VGLL3 using the STRING database, and identified YAP1, TEAD1, WWTR1, and VGLL4 as upstream of VGLL3, and POU1F1, TEAD3, AKAP11, SYT17, CHMP28, TEAD4, and ERBBE2 (HER2) as downstream proteins (Table 2).
| Characteristic | Cases | High expression | Low expression | P-value |
| Age (yr) | ||||
| ≤60 | 97 | 47 | 50 | 0.548 |
| >60 | 75 | 32 | 43 | |
| Gender | ||||
| Female | 101 | 43 | 58 | 0.369 |
| Male | 71 | 36 | 35 | |
| Differentiation | ||||
| Well | 84 | 40 | 44 | 0.779 |
| Poor and moderate | 88 | 39 | 49 | |
| T stage | ||||
| T1/2 | 91 | 36 | 55 | 0.105 |
| T3/4 | 81 | 43 | 38 | |
| N stage | ||||
| N0/1 | 95 | 36 | 59 | 0.028 |
| N2/3 | 77 | 43 | 34 | |
| TNM stage | ||||
| I-II | 119 | 48 | 71 | 0.041 |
| III-IV | 53 | 31 | 22 | |
| HER2 | ||||
| Negative | 89 | 32 | 57 | 0.010 |
| Positive | 83 | 47 | 36 |
| Characteristic | Univariate analysis | Multivariate analysis | ||||
| Hazard ratio | 95%CI | P-value | Hazard ratio | 95%CI | P-value | |
| Age (yr) | 0.83 | 0.55-1.26 | 0.389 | |||
| Gender (Male vs Female) | 1.31 | 0.87-1.96 | 0.194 | |||
| Differentiation (Well vs Poor and moderate) | 0.81 | 0.54-1.22 | 0.304 | |||
| T stage (T3/4 vs T1/2) | 1.16 | 0.77-1.73 | 0.484 | |||
| N stage (N2/3 vs N0/1) | 0.94 | 0.63-1.42 | 0.778 | |||
| TNM stage (III/IV vs I/II) | 1.27 | 0.82-1.97 | 0.283 | |||
| HER2 (Positive vs Negative) | 1.19 | 0.79-1.8 | 0.392 | |||
| VGLL3 (High vs Low) | 1.66 | 1.09-2.55 | 0.019 | 1.66 | 1.09-2.55 | 0.019 |
GC is the third most common cause of cancer-related deaths worldwide, mainly due to ineffective diagnostic and therapeutic options. It is often detected at the advanced stage, and is highly heterogeneous. Therefore, novel molecular biomarkers for the early diagnosis and treatment of GC are urgently needed. VGLL3 is a member of the vestigial-like family proteins that are related to sex and maturation[18-20], and is reportedly associated with epithelial ovarian cancer and soft tissue sarcoma. Through bioinformatics data mining and integrated analysis, we found that VGLL3 mRNA was not only upregulated in the GC tissues relative to normal gastric tissues, but also significantly associated with advanced TNM stage GC and poor survival outcomes. To validate the in silico data, we analyzed the gastric tissues of GC patients, and found abnormally high levels of VGLL3 in the tumor relative to normal tissues. In addition, high in situ expression of VGLL3 was correlated with tumor lymph node metastasis, TNM stage, and HER2 mutation, but not with age, gender, differentiation, T stage, or M stage. The patients were stratified into the high and low VGLL3 expression groups, and the former showed a significantly poor survival. Univariate and multivariate analyses further indicated that VGLL3 expression and TNM stage were independent risk factors for the prognosis of GC. Finally, the respective AUCs of the 3- and 5-year survival of the VGLL3-based prognostic model were 0.613 and 0.706, indicating a somewhat imperfect predictive ability. This could be due to the insufficient number of samples and other confounding factors such as the patient's state of mind, economic status, and family environment. Taken together, we identified VGLL3 as a novel prognostic biomarker for GC.
To further elucidate the underlying molecular mechanisms, we analyzed the PPI network of VGLL3 through the STRING database, and found that the Hippo pathway was significantly enriched. In addition, the identified upstream molecules of VGLL3 are YAP1[6], TEAD1, WWTR1, VGLL4, and the downstream molecules are POU1F1, TEAD3, AKAP11, SYT17, CHMP28, and TEAD4. Interestingly, the expression level of VGLL3 was related to ERBBE2 (HER2) mutation, although the relevant mechanistic connection is unclear.
To conclude, VGLL3 is highly expressed in GC and an independent risk factor, and further studies are needed to determine its underlying mechanism. Nevertheless, VGLL3 is a novel biomarker for GC prognosis and a potential therapeutic target for this malignancy.
Gastric cancer (GC) is the most prevalent gastrointestinal tract malignancy. The prognosis of GC patients remains relatively poor. It is urgent to explore prognostic markers for GC.
There are insufficient reports about the correlation between VGLL3 and GC.
The aim of the present study was to explore the expression pattern and clinical significance of VGLL3 in GC.
It was found that VGLL3 would be a potential prognostic marker by bioinformatics analysis. To validate the in silico data, the authors identified the expression of VGLL3 in GC patient samples by immunohistochemistry and evaluated clinical outcomes.
Analysis of the ONCOMINE and GEPIA databases showed that VGLL3 was significantly up-regulated in GC tissues, and associated with the tumor TNM stage. In addition, the high VGLL3 expression group had a significantly worse prognosis compared to the low expression group, as per both GEPIA and ONCOLNC. The bioinformatics results were validated by the significantly higher VGLL3 mRNA and protein levels in the GC tissues compared to the adjacent normal tissues in a cohort of 30 GC patients. Furthermore, high in situ expression of VGLL3 protein was associated with more advanced N and TNM stages and HER2 mutation in a cohort of 172 patients. Kaplan-Meier analysis showed that the high VGLL3 expression group had a worse prognosis compared to the low VGLL3 expression group. Multivariate analysis showed that VGLL3 expression status was an independent risk factor for prognosis. In addition, the prognostic risk model nomogram showed that VGLL3 was the most important indicator, with an AUC of 0.613 for 3-year survival and 0.706 for 5-year survival. Finally, the protein interaction network analysis revealed that VGLL3 is likely involved in the Hippo signaling pathway.
VGLL3 is overexpressed in GC tissues and associated with a poor prognosis, indicating its potential as a novel prognosis biomarker and therapeutic target for GC.
The present study suggested that VGLL3 is a novel prognostic biomarker for GC, and the significance of VGLL3 as a promising therapeutic target for GC is highlighted.
Manuscript source: Invited Manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bang YJ, Kruszewski WJ S-Editor: Cui LJ L-Editor: Wang TQ E-Editor: Liu JH
| 1. | The Lancet. GLOBOCAN 2018: counting the toll of cancer. Lancet. 2018;392:985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55782] [Article Influence: 7968.9] [Reference Citation Analysis (132)] |
| 3. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15468] [Article Influence: 2578.0] [Reference Citation Analysis (2)] |
| 4. | Gambaro K, Quinn MC, Wojnarowicz PM, Arcand SL, de Ladurantaye M, Barrès V, Ripeau JS, Killary AM, Davis EC, Lavoie J, Provencher DM, Mes-Masson AM, Chevrette M, Tonin PN. VGLL3 expression is associated with a tumor suppressor phenotype in epithelial ovarian cancer. Mol Oncol. 2013;7:513-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Cody NA, Shen Z, Ripeau JS, Provencher DM, Mes-Masson AM, Chevrette M, Tonin PN. Characterization of the 3p12.3-pcen region associated with tumor suppression in a novel ovarian cancer cell line model genetically modified by chromosome 3 fragment transfer. Mol Carcinog. 2009;48:1077-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Hélias-Rodzewicz Z, Pérot G, Chibon F, Ferreira C, Lagarde P, Terrier P, Coindre JM, Aurias A. YAP1 and VGLL3, encoding two cofactors of TEAD transcription factors, are amplified and overexpressed in a subset of soft tissue sarcomas. Genes Chromosomes Cancer. 2010;49:1161-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Sun X, Wang T, Zhang C, Ning K, Guan ZR, Chen SX, Hong TT, Hua D. S100A16 is a prognostic marker for colorectal cancer. J Surg Oncol. 2018;117:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Wang LL, Chen ZS, Zhou WD, Shu J, Wang XH, Jin R, Zhuang LL, Hoda MA, Zhang H, Zhou GP. Down-regulated GATA-1 up-regulates interferon regulatory factor 3 in lung adenocarcinoma. Sci Rep. 2017;7:2551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Yang S, Kim CY, Hwang S, Kim E, Kim H, Shim H, Lee I. COEXPEDIA: exploring biomedical hypotheses via co-expressions associated with medical subject headings (MeSH). Nucleic Acids Res. 2017;45:D389-D396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607-D613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10161] [Cited by in RCA: 11712] [Article Influence: 1952.0] [Reference Citation Analysis (1)] |
| 11. | Petrov A, Beer M, Blome S. Development and validation of a harmonized TaqMan-based triplex real-time RT-PCR protocol for the quantitative detection of normalized gene expression profiles of seven porcine cytokines. PLoS One. 2014;9:e108910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Sun X, Wang T, Guan ZR, Zhang C, Chen Y, Jin J, Hua D. FBXO2, a novel marker for metastasis in human gastric cancer. Biochem Biophys Res Commun. 2018;495:2158-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Cai X, Zheng Y, Speck NA. A Western Blotting Protocol for Small Numbers of Hematopoietic Stem Cells. J Vis Exp. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Wester K, Wahlund E, Sundström C, Ranefall P, Bengtsson E, Russell PJ, Ow KT, Malmström PU, Busch C. Paraffin section storage and immunohistochemistry. Effects of time, temperature, fixation, and retrieval protocol with emphasis on p53 protein and MIB1 antigen. Appl Immunohistochem Mol Morphol. 2000;8:61-70. [PubMed] |
| 15. | Wu J, Wang F, Liu X, Zhang T, Liu F, Ge X, Mao Y, Hua D. Correlation of IDH1 and B7H3 expression with prognosis of CRC patients. Eur J Surg Oncol. 2018;44:1254-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, Wan X, Liu G, Wu D, Shi L, Lau W, Wu M, Shen F. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 831] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 17. | Kuang M, Zheng D, Tao X, Peng Y, Pan Y, Zheng S, Zhang Y, Li H, Yuan C, Zhang Y, Xiang J, Li Y, Chen H, Sun Y. tRNA-based prognostic score in predicting survival outcomes of lung adenocarcinomas. Int J Cancer. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Barson NJ, Aykanat T, Hindar K, Baranski M, Bolstad GH, Fiske P, Jacq C, Jensen AJ, Johnston SE, Karlsson S, Kent M, Moen T, Niemelä E, Nome T, Næsje TF, Orell P, Romakkaniemi A, Sægrov H, Urdal K, Erkinaro J, Lien S, Primmer CR. Sex-dependent dominance at a single locus maintains variation in age at maturity in salmon. Nature. 2015;528:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 353] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 19. | Tu W, Wagner EK, Eckert GJ, Yu Z, Hannon T, Pratt JH, He C. Associations between menarche-related genetic variants and pubertal growth in male and female adolescents. J Adolesc Health. 2015;56:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Liang Y, Tsoi LC, Xing X, Beamer MA, Swindell WR, Sarkar MK, Berthier CC, Stuart PE, Harms PW, Nair RP, Elder JT, Voorhees JJ, Kahlenberg JM, Gudjonsson JE. A gene network regulated by the transcription factor VGLL3 as a promoter of sex-biased autoimmune diseases. Nat Immunol. 2017;18:152-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |