Published online Jul 26, 2019. doi: 10.12998/wjcc.v7.i14.1899
Peer-review started: March 26, 2019
First decision: April 18, 2019
Revised: May 10, 2019
Accepted: May 23, 2019
Article in press: May 23, 2019
Published online: July 26, 2019
Processing time: 125 Days and 19.2 Hours
Squamous cell carcinoma (SCC) is one the most common subtypes of non-small cell lung cancer, yet the treatment options for it remain limited. Here, we report a case of advanced SCC and review the related literature focusing on the multiline therapy method.
We report the case of a 45-year-old man with advanced SCC who was deemed inoperable at the time of advanced SCC diagnosis. The patient had been referred to our hospital in April 2013 with complaints of a stuffy feeling in the chest, dyspnea, and pain in the right shoulder lasting for 1 mo. Physical examination found no obvious abnormalities, except for lower breath sound in the right lower lung. Laboratory data were within normal limits. Immunohistochemistry analysis of the tumor tissue showed CK5/6 (+), p63 (+), CD56 (+), and Ki-67 (+, approximately 30%), and genetic testing detected no EGFR mutation. He received a multiline treatment that included chemotherapy, radiotherapy, targeted therapy, and antiangiogenic therapy. After more than 5-year comprehensive treatment, the patient remains alive.
This typical case highlights the importance of appropriate multiline therapy for those patients with advanced SCC.
Core tip: Patients with advanced squamous cell carcinoma (SCC) have a poor prognosis and their treatment options are limited. Nab-paclitaxel has a superior antitumor effect and plays a significant role in the treatment of advanced non-small cell lung cancer, especially SCC; radiotherapy, anaplastic lymphoma kinase-targeted therapy, and antiangiogenic therapy also make a great contribution to the patients’ survival. We should attach importance to multiline therapy and be flexible in our choice of the most suitable therapy method for those patients with SCC, including advanced cases.
- Citation: Yang X, Peng P, Zhang L. Multiline treatment of advanced squamous cell carcinoma of the lung: A case report and review of the literature. World J Clin Cases 2019; 7(14): 1899-1907
- URL: https://www.wjgnet.com/2307-8960/full/v7/i14/1899.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i14.1899
Lung cancer is the most commonly diagnosed cancer and the leading cause of cancer death worldwide. According to the latest statistics, lung cancer represents 11.6% of the total new cancer cases and 18.4% of the total cancer deaths[1]. Approximately 85% of all lung cancer patients have histological subtypes of non-small cell lung cancer (NSCLC), of which adenocarcinoma and squamous cell carcinoma (SCC) are the most common subtypes, having an incidence of 50% and 30%, respectively[2,3]. Advanced NSCLC has a relatively poor prognosis, especially for those patients with stage IIIB/IV, with the overall 5-year survival being less than 5%[4]. The SCC subtype is associated with even shorter survival than the nonsquamous NSCLC[5].
Herein, we report a case of advanced stage SCC involving a patient who was inope-rable at the time of diagnosis. The patient underwent comprehensive treatment that included chemotherapy, radiotherapy, antiangiogenic therapy, and targeted therapy; today, at more than 5 years later, he remains alive.
The patient, a 45-year-old man, was referred to our hospital in April 2013 with complaints of a stuffy feeling in the chest, dyspnea, and pain in the right shoulder, without nausea, emesis, fever, or chills.
The patient reported the symptoms as having persisted for 1 mo, without exacer-bating or relieving factors.
The patient reported no known systemic illness and no history of smoking or previ-ous surgery.
The patient reported no known relevant personal or family history.
Physical examination showed a body temperature of 36.8°C, breathing frequency of 19/min, pulse rate of 68 beats/min, blood pressure of 128/70 mmHg, performance status of 1, and lower breath sounds in the right lower lung.
Laboratory examinations showed no obvious abnormalities.
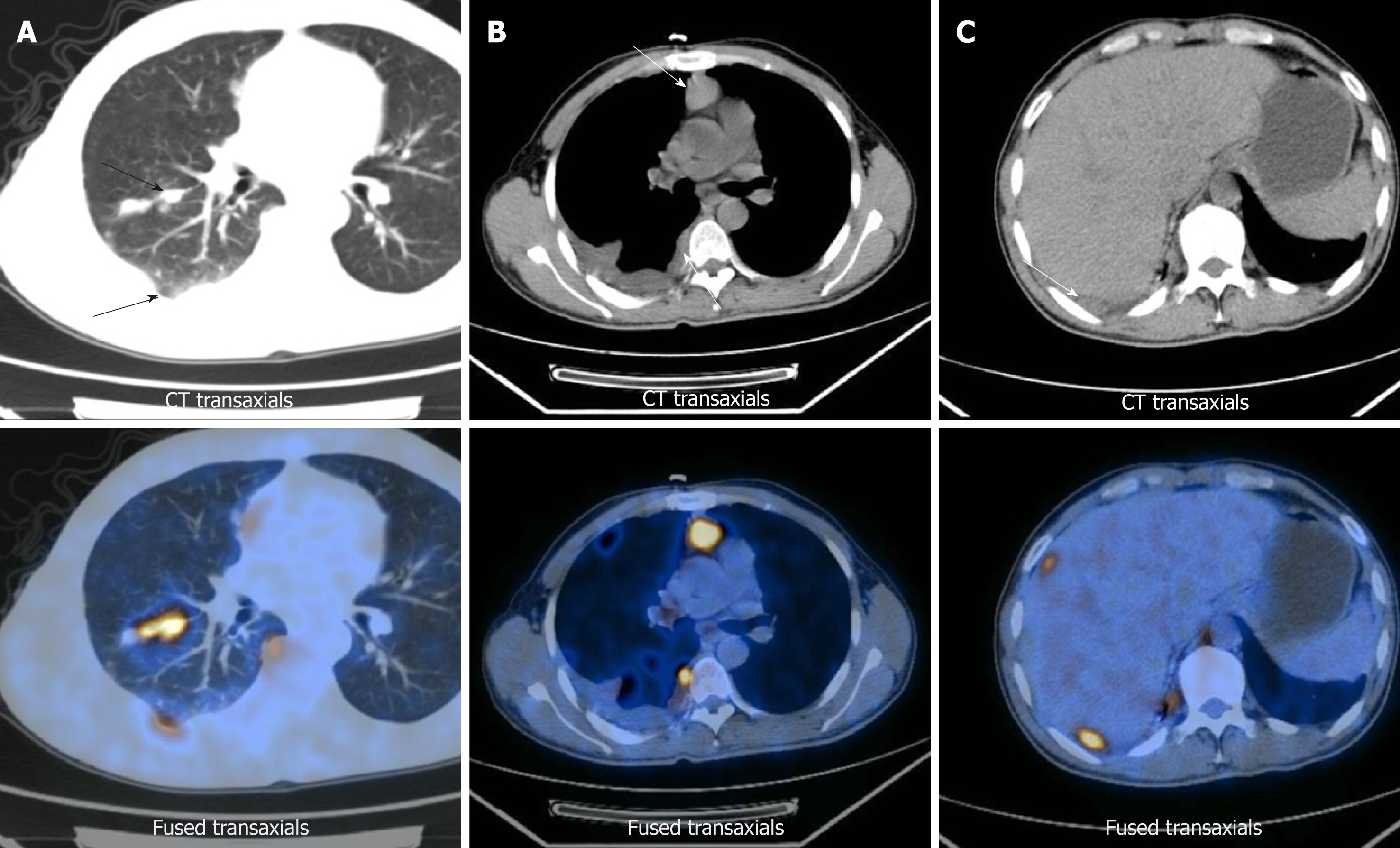
Positron emission tomography/computed tomography imaging was performed on April 12, 2013. Right lung cancer with right pulmonary, right pleural, mediastinal, and right hilar metastases was observed, along with right pleural effusion (Figure 1). Subsequent pleurocytology performed on April 19, 2013 showed the presence of lymphocytes, mesothelial cells, and neutrophils.
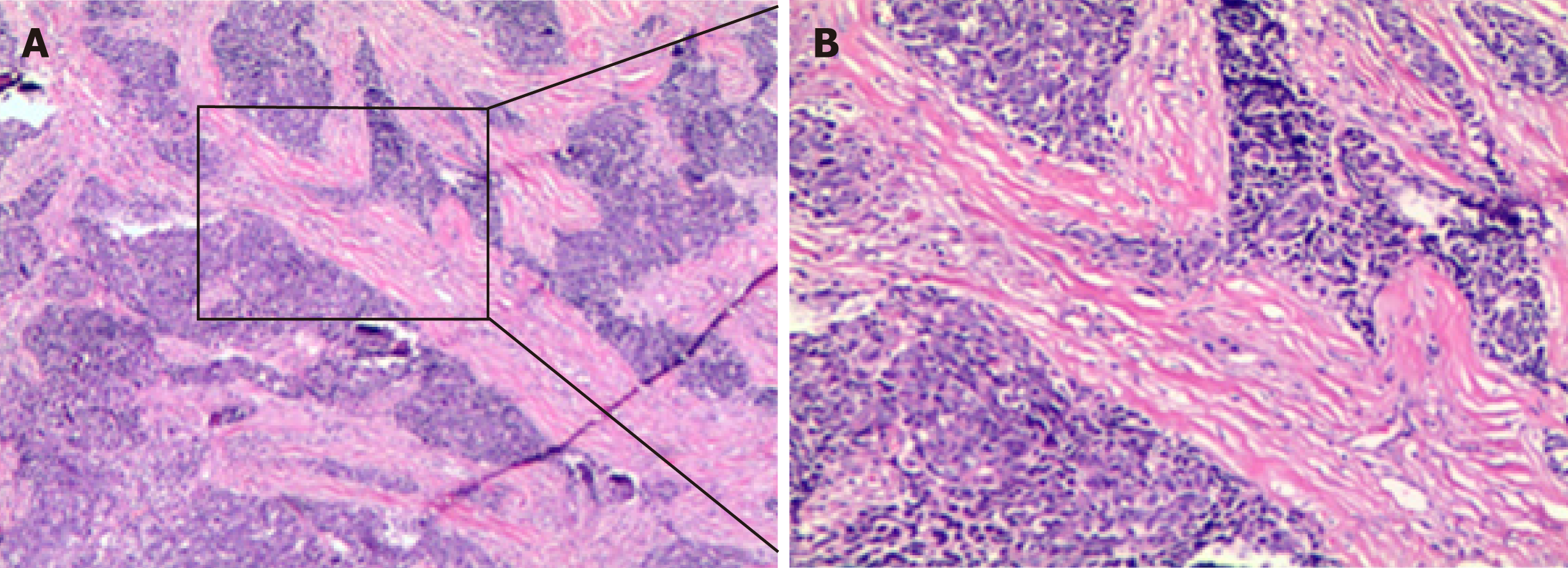
Thoracoscopic biopsy of right ventricular pleural metastases was performed on April 23, 2013. The pathological result showed lowly-differentiated SCC of the chest wall (derived from the right lung) (Figure 2). Immunohistochemistry (Figure 2) analysis characterized the tumor tissue to be PCK (+), CK5/6 (+), p63 (+), and Ki-67 (+, ≈ 30%) (Supplementary Figure 1). Genetic testing detected no EGFR mutation.
According to the collective findings, the patient was diagnosed with right lung SCC stage IV (metastases to the right lung, right pleura, and mediastinum and malignant pleural effusion), EGFR mutation-negative.
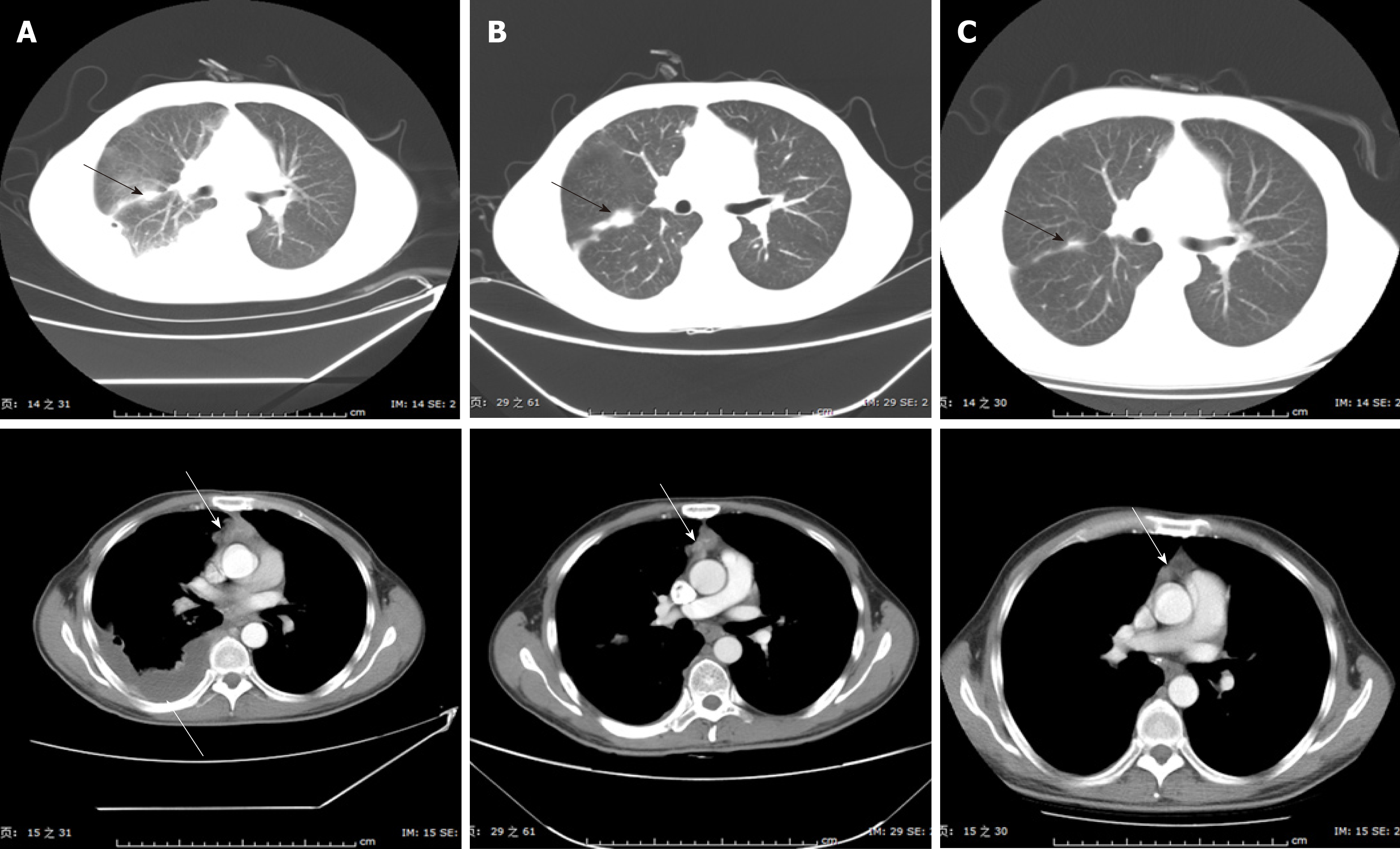
DP chemotherapy, consisting of 75 mg/m2 docetaxel on day 1 and 75 mg/m2 cisplatin on day 1, was administered as first-line therapy, following the NCCN Guidelines V2.2013. After two chemotherapy cycles, the disease was evaluated on June 7, 2013 and characterized as a progressive disease (Figure 3). TP chemotherapy, consisting of 100 mg/m2 nab-paclitaxel (nab-PC) on days 1, 8, and 15, and 75 mg/m2 cisplatin on day 1, was used as second-line therapy starting on June 7, 2013. After six cycles of this therapy, the tumor shrank significantly, as evidenced on November 22, 2013 (Figure 3).
The patient’s condition remained stable over the next 2 years. At follow-up on June 9, 2015, after 23.9 mo of progression-free survival (PFS), evaluation of the state again showed a progressive disease. We looked to the NCCN Guidelines and decided to treat with GP therapy, consisting of 1250 mg/m2 gemcitabine on days 1 and 8, and 75 mg/m2 nedaplatin on day 1, as the third-line therapy method. Unfortunately, the disease progressed after four cycles of GP and three cycles of gemcitabine mo-notherapy, as determined on January 11, 2016, following a total 7.0-mo PFS. Although the fourth-line therapy of NVB + endo, consisting of 25 mg/m2 vinorelbine on days 1 and 8, and 30 mg endostar on days 1-7, had a great effect on the disease, we had to discontinue the therapy after three cycles because of the intolerable gastrointestinal reactions.
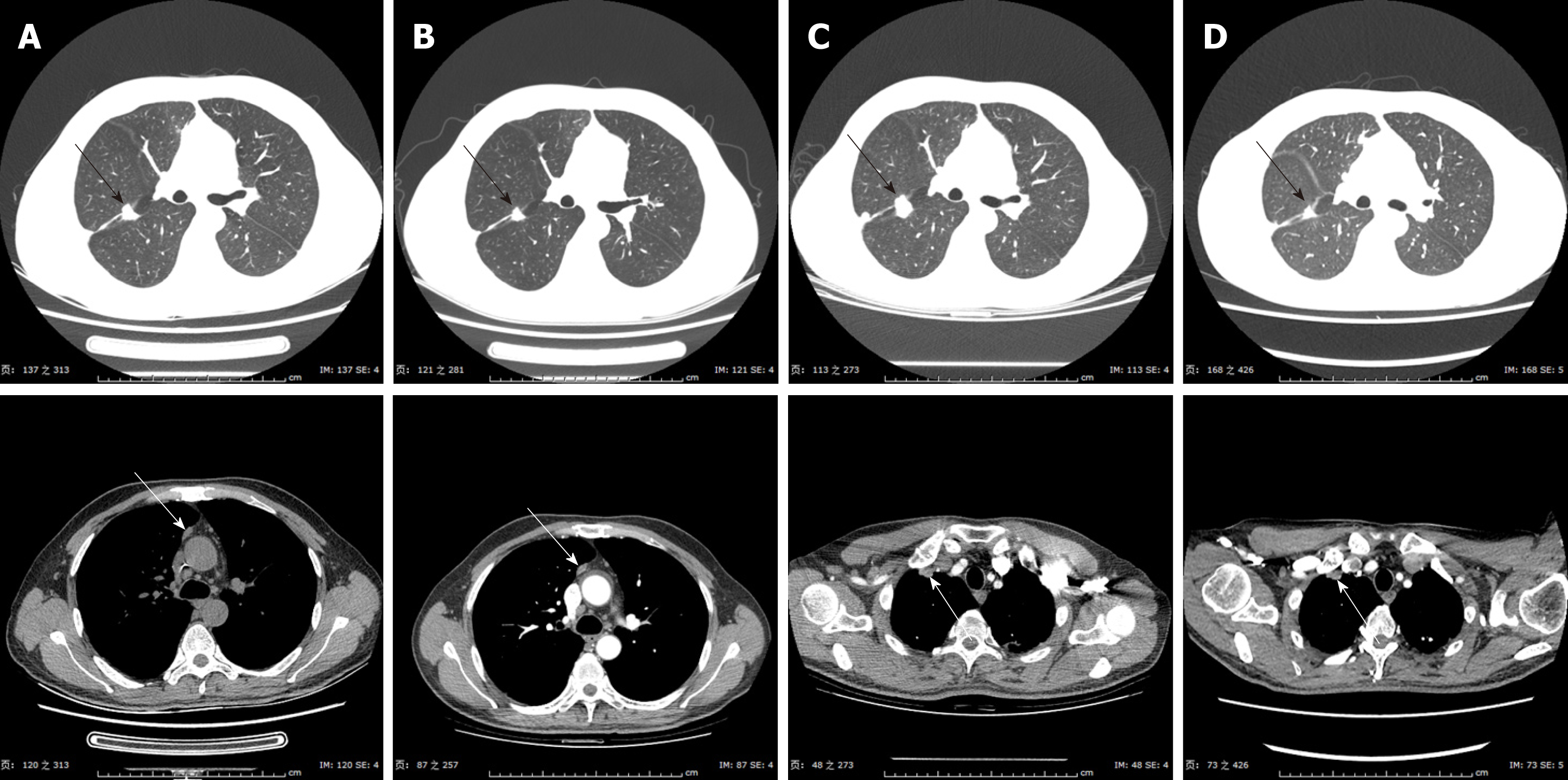
For the next treatment method, we administered nab-PC again, considering the fact that the previous nab-PC had given the patient a total PFS of 23.9-mo. This time, nab-PC monotherapy also showed considerable efficacy after seven cycles of adminis-tration, giving a total PFS of 11.0-mo. Then, after careful and thorough evaluation, the patient started to receive radiotherapy in the right lung (DT = 50 Gy/5F) on April 27, 2017. The tumor shrank, as predicted (Figure 4), but started to grow again after 6 mo. However, radiation-induced lung injury (RILI) was observced after the radiotherapy, accompanied by cough and shortness of breath. Corticosteroid-based therapy was administered to improve RILI. On January 23, 2017, a percutaneous lung biopsy was taken and the pathological result indicated non-keratinized SCC.
Immunohistochemistry analysis showed positivity for anaplastic lymphoma kinase (ALK) D5F3, consistent with the result of blood gene testing, which indicated a rearrangement of ALK [i.e., a gene fusion of SPTBN1-ALK (S27:A20)]. Still, no EGFR mutation was found in the tissue gene testing. Thus, crizotinib was used in the next round of therapy, starting on February 2, 2018. In July of that year, anlotinib was ad-ministered instead.
The entire process of treatment is presented along a timeline in Figure 5.
Up to now, the disease has remained stable in the patient. No obvious enlargement of the tumor was observed, and the RILI also improved, with some small focal fibrosis left. Long-term, regular follow-up is still in progress. The patient has also been advised to receive regular imaging examinations.
Our patient was diagnosed with advanced SCC; multiple biopsies and gene testing offered us a whole and thorough understanding of the disease, so that we could administer an individualized comprehensive treatment that included chemotherapy, radiotherapy, antiangiogenic therapy, and targeted therapy. Multiline therapy made a great contribution to the patient’s survival, although the patient experienced multiline relapse.
Historically, the treatment for SCC has been mostly limited to cytotoxic che-motherapy because of the lack of targetable aberration. Currently, platinum-based chemotherapy is the mainstay of the first-line treatment in those patients without targetable aberration or with high-level expression of PD-L1, and platinum agents are most commonly administered with taxanes, gemcitabine, vinorelbine, or pemetrexed[6]. Solvent-based paclitaxel plus carboplatin is the most frequently used taxane-platinum combination in the United States; reports cite a 15%-32% objective response rate and a median overall survival of 7.9-mo to 10.06-mo for this therapy[7]. Nab-PC is a new type of paclitaxel, which is produced by binding paclitaxel to 130-nm albumin particles, aiming to overcome the solvent-associated limitations. Nab-PC has higher efficiency and fewer toxicities and can be provided to the patient more conveniently due to the fact that it does not require any solvents and eliminates the need for steroid pretreatment.
Some studies have shown that nab-PC elicits superior response rates compared with solvent-based paclitaxel in first-line therapy of patients with advanced NSCLC, especially for those with the SCC subtype[8,9]. In those patients with SCC histology, a 68% improvement in objective response rate was achieved with nab-PC plus carboplatin, as compared to that with solvent-based paclitaxel plus carboplatin (41% vs 24%, respectively). That result was inspiring since the basic treatment options for SCC at the time were limited[9]. Several studies have also demonstrated the efficacy and tolerability of nab-PC as second-line or late-phase chemotherapy in advanced SCC[10-14]. For our patient, nab-PC plus cisplatin was first administered as the second-line therapy, due to the patient’s poor response to the DP therapy; it showed consid-erable efficacy and led to a total PFS of 23.9-mo, without obvious adverse effects. Moreover, when subsequent therapy failed to provide a remarkable benefit, the nab-PC chemotherapy still showed efficacy.
Radiotherapy is not only the main treatment method for early-stage NSCLC patients who are considered inoperable but also plays an important role in those patients with advanced lung cancer. Palliative radiotherapy is effective in improving thoracic symptoms, especially for those patients with advanced lung cancer[15,16]; the positively impacted symptoms include hemoptysis, cough, chest pain, and dyspnea, thus improving the patients’ life quality. Besides controlling symptoms, palliative radiotherapy is also beneficial to patient survival. Several clinical trials have demonstrated the ability of radiotherapy to improve survival in patients administered with palliative intention for locally advanced lung cancer[17-19]. Our patient received radiotherapy in the right lung after becoming resistant to chemotherapy; the tumor shrank, as predicted, offering the patient a PFS of 6-mo.
When radiotherapy was no longer useful, results of pathological analysis and blood gene testing indicated a gene fusion of SPTBN1-ALK (S27:A20), leading to the administration of crizotinib therapy. Our patient then showed a response, although limited, to crizotinib therapy. Lung cancer with ALK-rearrangements are reliant upon ALK signaling and can be inhibited by ALK tyrosine kinase inhibitors (commonly referred to as TKIs). Crizotinib is an oral small-molecule TKI that targets ALK, MET, and ROS1 tyrosine kinases and has demonstrated considerable efficacy in several clinical trials[20,21]. It was approved by the United States Federal Drug Administration in August 2011 for treatment of patients with advanced NSCLC and ALK rear-rangements. However, very little data has been reported on its use in patients with the SCC subtype and ALK rearrangement[22].
The lack of these data in the literature may be due to the fact that ALK rearrange-ment is seen in only 1% of SCC cases , and only about 5% of adenocarcinoma cases[22,23]. Thus, it remains unknown whether or not those patients with ALK rearrangement-positive SCC would benefit from TKIs like crizotinib. Several cases of SCC with ALK rearrangement have been reported as well as cases with considerable responses to crizotinib therapy[24-26], even after failed chemotherapy[25]; the associated PFS times reported are 6.0-mo, 5.9-mo, and 7.0-mo, respectively. We attach importance to ALK gene testing for patients with advanced SCC, as they may benefit from ALK-targeted therapy, let alone the more powerful drugs that are coming out. For example, compared with crizotinib, the new drug alectinib has shown superior efficacy and lower toxicity in the treatment of NSCLC with ALK rearrangement[27,28].
Anlotinib is a new, orally administered multitargeted receptor TKI and has already shown a broad-spectrum antitumor potential. Several clinical trials have revealed the importance of anlotinib as a third-line or late-phase therapy in NSCLC[29-31]. ALTER-0303 was a phase III trial that compared the efficacy and safety of anlotinib with those of placebo in patients with advanced NSCLC who had progressed after at least two lines of prior treatments[29]; the result showed that, compared with placebo, anlotinib provided improvement in the objective response rate (9.18% vs 0.7%, P < 0.0001) and prolonged the median survival rates, both for PFS (5.37 mo vs 1.40 mo) and overall survival (9.63 mo vs 6.30 mo). Subgroup analysis of the anlotinib-related histology revealed that when the drug was given as a subsequent therapy strategy, there was an improvement in PFS for both advanced adenocarcinoma and SCC cases[30]. Anlotinib was approved by the China Food and Drug Administration for third-line treatment or beyond in advanced NSCLC on May 8, 2018. We initiated anlotinib therapy with our patient in July 2018 and that treatment is ongoing to date. Importantly, efficacy has been observed through the latest follow-up appointment (in November 2018), without presentation of any obvious adverse effects.
We present herein a case of advanced SCC treated by administration of multiline treatment. Throughout the entire process of the comprehensive treatment, the patient showed a remarkable response to the nab-PC-based chemotherapy, radiotherapy, ALK-targeted therapy, and antiangiogenic therapy, which also prolongs PFS and overall survival. We attach importance to individualized treatment and hope to focus the attention of clinicians towards the benefits of a flexible application of multiline therapy combination.
Manuscript source: Unsolicited manuscript
Specialty type: Research and Experimental Medicine
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen YK S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Wang J
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55721] [Article Influence: 7960.1] [Reference Citation Analysis (132)] |
| 2. | Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Ganti AK, Govindan R, Grannis FW, Horn L, Jahan TM, Jahanzeb M, Kessinger A, Komaki R, Kong FM, Kris MG, Krug LM, Lennes IT, Loo BW, Martins R, O'Malley J, Osarogiagbon RU, Otterson GA, Patel JD, Pinder-Schenck MC, Pisters KM, Reckamp K, Riely GJ, Rohren E, Swanson SJ, Wood DE, Yang SC, Hughes M, Gregory KM; NCCN (National Comprehensive Cancer Network). Non-small cell lung cancer. J Natl Compr Canc Netw. 2012;10:1236-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 279] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 3. | Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1607] [Cited by in RCA: 3074] [Article Influence: 439.1] [Reference Citation Analysis (0)] |
| 4. | Ko EC, Raben D, Formenti SC. The Integration of Radiotherapy with Immunotherapy for the Treatment of Non-Small Cell Lung Cancer. Clin Cancer Res. 2018;24:5792-5806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 199] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 5. | Cheng TY, Cramb SM, Baade PD, Youlden DR, Nwogu C, Reid ME. The International Epidemiology of Lung Cancer: Latest Trends, Disparities, and Tumor Characteristics. J Thorac Oncol. 2016;11:1653-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 434] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 6. | Socinski MA, Evans T, Gettinger S, Hensing TA, VanDam Sequist L, Ireland B, Stinchcombe TE. Treatment of stage IV non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e341S-e368S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 7. | Villaruz LC, Socinski MA. Is there a role of nab-paclitaxel in the treatment of advanced non-small cell lung cancer? The data suggest yes. Eur J Cancer. 2016;56:162-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, Hon JK, Hirsh V, Bhar P, Zhang H, Iglesias JL, Renschler MF. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30:2055-2062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 596] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 9. | Socinski MA, Langer CJ, Okamoto I, Hon JK, Hirsh V, Dakhil SR, Page RD, Orsini J, Zhang H, Renschler MF. Safety and efficacy of weekly nab®-paclitaxel in combination with carboplatin as first-line therapy in elderly patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Gong W, Sun P, Mu Z, Liu J, Yu C, Liu A. Efficacy and Safety of Nab-Paclitaxel as Second-line Chemotherapy for Locally Advanced and Metastatic Non-small Cell Lung Cancer. Anticancer Res. 2017;37:4687-4691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Higuchi M, Takagi H, Owada Y, Inoue T, Watanabe Y, Yamaura T, Fukuhara M, Muto S, Okabe N, Matsumura Y, Hasegawa T, Yonechi A, Osugi J, Hoshino M, Shio Y, Fujiu K, Kanno R, Ohishi A, Suzuki H, Gotoh M. Efficacy and tolerability of nanoparticle albumin-bound paclitaxel in combination with carboplatin as a late-phase chemotherapy for recurrent and advanced non-small-cell lung cancer: A multi-center study of the Fukushima lung cancer association group of surgeons. Oncol Lett. 2017;13:4315-4321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Hu W, Zhang Z. A phase II clinical study of using nab-paclitaxel as second-line chemotherapy for Chinese patients with advanced non-small cell lung cancer. Med Oncol. 2015;32:498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Jin F, Zhu H, Shi F, Kong L, Yu J. A retrospective analysis of safety and efficacy of weekly nab-paclitaxel as second-line chemotherapy in elderly patients with advanced squamous non-small-cell lung carcinoma. Clin Interv Aging. 2016;11:167-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Wu Y, Feng J, Hu W, Luo Q. A randomized placebo-controlled clinical study of <i>nab</i>-paclitaxel as second-line chemotherapy for patients with advanced non-small cell lung cancer in China. Biosci Rep. 2017;37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Langendijk JA, ten Velde GP, Aaronson NK, de Jong JM, Muller MJ, Wouters EF. Quality of life after palliative radiotherapy in non-small cell lung cancer: a prospective study. Int J Radiat Oncol Biol Phys. 2000;47:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Simpson JR, Francis ME, Perez-Tamayo R, Marks RD, Rao DV. Palliative radiotherapy for inoperable carcinoma of the lung: final report of a RTOG multi-institutional trial. Int J Radiat Oncol Biol Phys. 1985;11:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 130] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Bezjak A, Dixon P, Brundage M, Tu D, Palmer MJ, Blood P, Grafton C, Lochrin C, Leong C, Mulroy L, Smith C, Wright J, Pater JL; Clinical Trials Group of the National Cancer Institute of Canada. Randomized phase III trial of single versus fractionated thoracic radiation in the palliation of patients with lung cancer (NCIC CTG SC.15). Int J Radiat Oncol Biol Phys. 2002;54:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Macbeth FR, Bolger JJ, Hopwood P, Bleehen NM, Cartmell J, Girling DJ, Machin D, Stephens RJ, Bailey AJ. Randomized trial of palliative two-fraction versus more intensive 13-fraction radiotherapy for patients with inoperable non-small cell lung cancer and good performance status. Medical Research Council Lung Cancer Working Party. Clin Oncol (R Coll Radiol). 1996;8:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 154] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Kramer GW, Wanders SL, Noordijk EM, Vonk EJ, van Houwelingen HC, van den Hout WB, Geskus RB, Scholten M, Leer JW. Results of the Dutch National study of the palliative effect of irradiation using two different treatment schemes for non-small-cell lung cancer. J Clin Oncol. 2005;23:2962-2970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Ou SH, Jänne PA, Bartlett CH, Tang Y, Kim DW, Otterson GA, Crinò L, Selaru P, Cohen DP, Clark JW, Riely GJ. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol. 2014;25:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 176] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 21. | Wu YL, Lu S, Lu Y, Zhou J, Shi YK, Sriuranpong V, Ho JCM, Ong CK, Tsai CM, Chung CH, Wilner KD, Tang Y, Masters ET, Selaru P, Mok TS. Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib versus Chemotherapy in East Asian Patients with ALK-Positive Advanced Non-Small Cell Lung Cancer. J Thorac Oncol. 2018;13:1539-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 22. | Caliò A, Nottegar A, Gilioli E, Bria E, Pilotto S, Peretti U, Kinspergher S, Simionato F, Pedron S, Knuutila S, Tortora G, Eccher A, Santo A, Tondulli L, Inghirami G, Tabbò F, Martignoni G, Chilosi M, Scarpa A, Brunelli M. ALK/EML4 fusion gene may be found in pure squamous carcinoma of the lung. J Thorac Oncol. 2014;9:729-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, Jenkins RB, Kwiatkowski DJ, Saldivar JS, Squire J, Thunnissen E, Ladanyi M. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med. 2013;137:828-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 336] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 24. | Zhang Q, Wang J, Zhang S. ALK-rearranged squamous cell lung cancer: a case report. Int J Clin Exp Pathol. 2015;8:2195-2198. [PubMed] |
| 25. | Wang Q, He Y, Yang X, Wang Y, Xiao H. Extraordinary response to crizotinib in a woman with squamous cell lung cancer after two courses of failed chemotherapy. BMC Pulm Med. 2014;14:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Vergne F, Quéré G, Andrieu-Key S, Descourt R, Quintin-Roué I, Talagas M, De Braekeleer M, Marcorelles P, Uguen A. ALK-rearranged squamous cell lung carcinoma responding to crizotinib: A missing link in the field of non-small cell lung cancer? Lung Cancer. 2016;91:67-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T, Takiguchi Y, Nishio M, Yoshioka H, Imamura F, Hotta K, Watanabe S, Goto K, Satouchi M, Kozuki T, Shukuya T, Nakagawa K, Mitsudomi T, Yamamoto N, Asakawa T, Asabe R, Tanaka T, Tamura T. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 672] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 28. | Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, Ou SI, Pérol M, Dziadziuszko R, Rosell R, Zeaiter A, Mitry E, Golding S, Balas B, Noe J, Morcos PN, Mok T; ALEX Trial Investigators. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1385] [Cited by in RCA: 1736] [Article Influence: 217.0] [Reference Citation Analysis (0)] |
| 29. | Han B, Li K, Wang Q, Zhao Y, Zhang L, Shi J, Wang Z, Cheng Y, He J, Shi Y, Chen W, Wang X, Luo Y, Nan K, Jin F, Li B, Chen Y, Zhou J, Wang D. Third-line treatment: A randomized, double-blind, placebo-controlled phase III ALTER-0303 study—Efficacy and safety of anlotinib treatment in patients with refractory advanced NSCLC. J Clin Oncol. 2017;35:9053-9053. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Cheng Y, Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, He J, Shi Y, Chen W, Wang X, Luo Y, Nan K, Jin F, Li B. Subgroup analysis of histology in ALTER0303: Anlotinib hydrochloride as 3rd line and further line treatment in refractory advanced NSCLC patients (pts). J Clin Oncol. 2018;36:9080-9080. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Han B, Li K, Zhao Y, Li B, Cheng Y, Zhou J, Lu Y, Shi Y, Wang Z, Jiang L, Luo Y, Zhang Y, Huang C, Li Q, Wu G. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer. 2018;118:654-661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (0)] |