Published online Jul 6, 2019. doi: 10.12998/wjcc.v7.i13.1711
Peer-review started: February 18, 2019
First decision: March 14, 2019
Revised: March 29, 2019
Accepted: May 2, 2019
Article in press: May 3, 2019
Published online: July 6, 2019
Processing time: 138 Days and 22.5 Hours
Hepatoid adenocarcinoma (HAC) occurs in extrahepatic organs such as the gastrointestinal tract, testes, ovaries, lungs, mediastinum and pancreas, and frequently produces α-fetoprotein (AFP). HAC of the lung (HAL) is rare, characterized by difficult treatment and poor prognosis. There are no reports of HAL in Yunnan-Guizhou Plateau, China.
A 60-year-old male patient was clinically diagnosed with HAL pT3N0M0, stage IIB. Chest computed tomography revealed a 7.5 cm × 7.2 cm soft tissue mass located in the right lung upper lobe and the adjacent superior mediastinum. Right upper lobectomy was performed. The diagnosis of HAL was confirmed by pathological examination, and the patient received paclitaxel and carboplatin as adjuvant chemotherapy after surgery.
Clinical manifestations, pathological features, imaging findings, auxiliary examination, and treatment planning of HAL are presented to help clinicians improve their diagnosis and treatment.
Core tip: We present a patient with primary hepatoid adenocarcinoma of the lung (HAL) in Yungui Plateau, China. HAL is a rare tumor involving difficult treatment and poor prognosis. We discussed the clinical manifestations, pathological features, imaging findings, auxiliary examination performance and treatment planning of HAL in order to help clinicians improve their diagnosis and treatment.
- Citation: Shi YF, Lu JG, Yang QM, Duan J, Lei YM, Zhao W, Liu YQ. Primary hepatoid adenocarcinoma of the lung in Yungui Plateau, China: A case report. World J Clin Cases 2019; 7(13): 1711-1716
- URL: https://www.wjgnet.com/2307-8960/full/v7/i13/1711.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i13.1711
Hepatoid adenocarcinoma (HAC) is a rare neoplasm with aberrant hepatocellular differentiation, and morphological features similar to hepatocellular carcinoma[1]. HAC occurs in extrahepatic organs, and the most common site of origin is the stomach[2-4]. There have been a few reports of HAC in extrahepatic organs such as the ovaries, mediastinum and pancreas[1]. Primary HAC of the lung (HAL) is rare.
HAL was first formally described by Ishikura et al[3] in 1990. Compared with the other more common types of lung tumors, HAL has a poorer prognosis[5-7] and its invasiveness may explain its high mortality rate[3,7]. The clinical symptoms of HAL are uncertain, and its biological significance remains to be elucidated[4]. Computed tomography (CT) images, combined with morphology and immunohistochemistry, may provide some indications for confirmation of this diagnosis. The following two points about diagnostic criteria of HAL were proposed by Ishikura[3]: (1) Typical acinar or papillary adenocarcinoma; and (2) α‑fetoprotein (AFP). AFP expression is positive and/or the level of AFP is elevated in most HAL cases[8]. The present literature review found no relevant cases of HAL in Yunnan-Guizhou Plateau, China. The limited literature regarding HAL is summarized in Table 1. Here, we present a case that was diagnosed as HAL, and the postoperative pathological stage was pT3N0M0, stage IIB.
| Ref. | Year | Region | Age / Sex | Maximum diameter in cm | Ki-67 | VEGF | Immunohistochemical AFP | Serum AFP as μg/L | Hepatocyte | Survival time |
| Bai et al[9] | 2006 | Shanghai | 48/M | 7 | Low-medium level | no data | - | - | + | 9 mo |
| Cao et al[12] | 2008 | Beijing | 64/M | 3 | no data | no data | no data | 2037 | no data | 12 mo |
| Feng et al[13] | 2013 | Shanghai | 46/M | 2.4 | no data | no data | little + | 586 | little + | 6 mo |
| Haninger et al[14] | 2013 | Louisville | 51/M | lowly proliferative | no data | 14 mo | ||||
| Shaib et al[15] | 2014 | Atlanta | 53/F | no data | no data | 4 yr | ||||
| Gavrancic et al [16] | 2014 | New York | 64/M | no data | no data | 2 mo | ||||
| Che et al[17] | 2014 | Beijing | 48/M | no data | no data | |||||
| Liu et al[18] | 2014 | Guangzhou | 64/M | no data | no data | |||||
| Zhong et al[19] | 2015 | Jiangxi | 61/M | 5.7 | 40% + | no data | ++ | 943 | - | 2 wk |
| Motooka et al[20] | 2016 | Kumamoto | 69/M | 4.3 | no data | no data | + | 4497 | + | 51 mo |
| Sun et al[21] | 2016 | Shandong | 59/M | 20% | no data | 23 mo | ||||
| Grossman et al[22] | 2016 | New York | 54/M | no data | no data | 3 mo | ||||
| Hou et al[11] | 2017 | Shandong | 59/M | 4 | 20% | no data | - | - | no data | 12 mo |
| YF Shi | 2018 | Yunnan | 60/M | 7 | 50% + | no data | no data | 1210 | weak+ | 15 mo |
A 60-year-old man was hospitalized with repeated coughing and expectoration for > 4 years, and the symptoms worsened for 1 mo.
The patient had no history of the present illness.
He had undergone esophageal polypectomy, and suffered from gastric ulcers for > 20 years.
No specific personal and family history of disease.
Physical examination showed that thick breathing sounds could be heard in the upper right lung, along with moist rales.
No obvious abnormalities were found in liver and kidney function. Serum AFP (1210.0 ng/mL), cancer antigen (CA) 125 (38.43 U/mL), carcinoembryonic antigen, CA15-3 and CA72-4 were normal. C antibodies, E antibodies and surface antibodies were all positive for six items of hepatitis B, and the rest were negative.
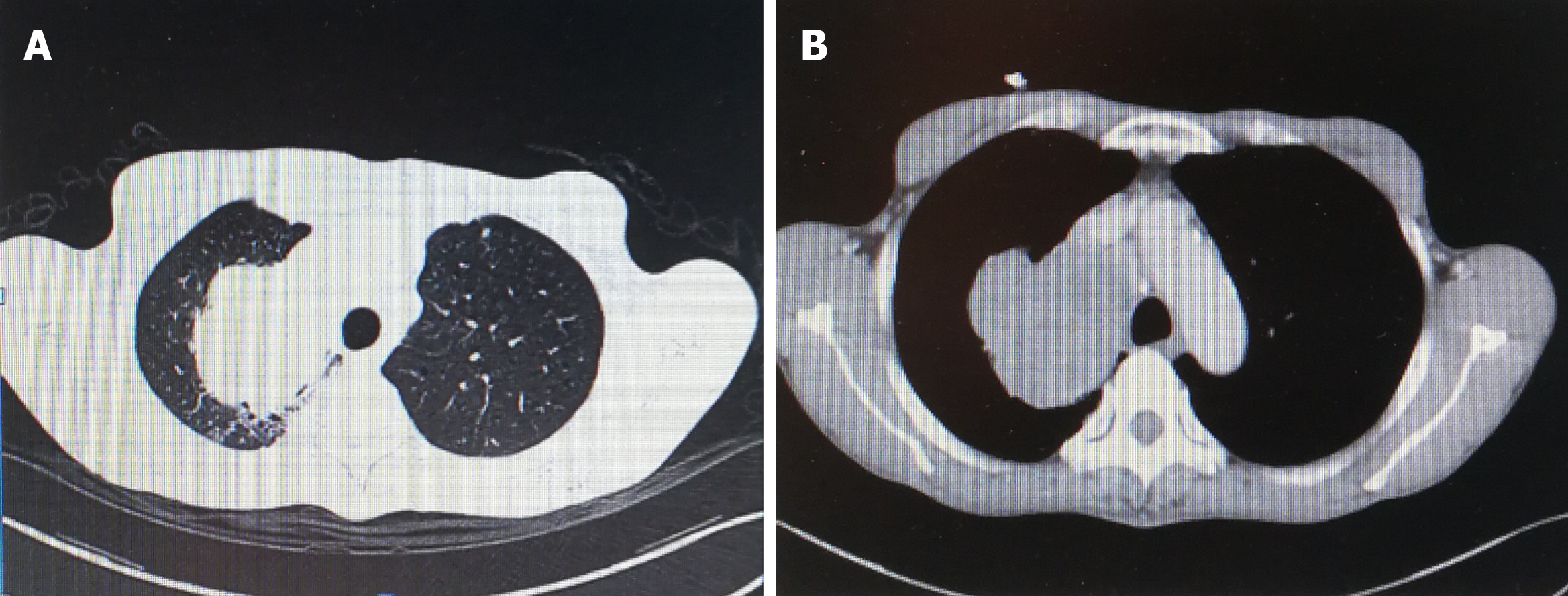
A 7.5 cm × 7.2 cm soft tissue mass in the right lung upper lobe and adjacent superior mediastinum was observed by CT. The boundary of the soft tissue was clear, and there was significant inhomogeneous enhancement after enhanced CT (Figures 1 and 2). Numbers of enlarged lymph nodes were found in the mediastinum. The largest lymph node diameter was 1.4 cm. There were calcified nodules in the right middle lobe, and subpleural nodules in the left upper lobe. Abdominal magnetic resonance imaging, including the liver, gallbladder, pancreas and spleen, and double kidney B ultrasound did not show any occupying lesions.
The specimen was sent for pathological examination after surgery. The diagnosis of HAL was confirmed by pathological examination.
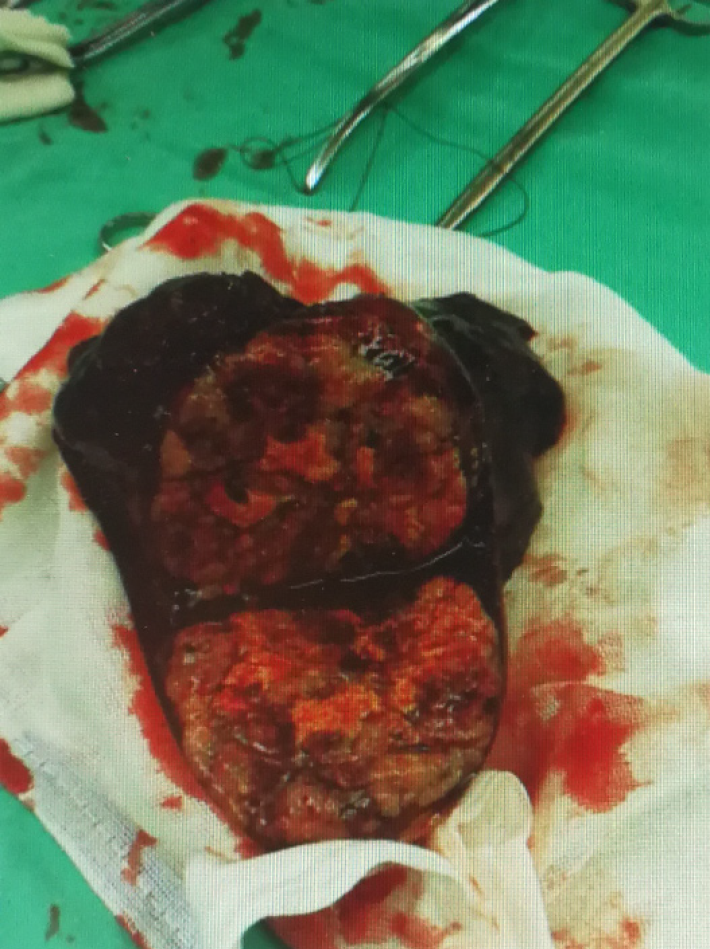
The patient had no surgical contraindications after examination. Resection of the right upper lung was performed. The tumor was located in the upper right lobe of the right lung, and measured about 7 cm × 7 cm × 5 cm. The 1st, 2nd, 3rd, 4th, 7th and 11th groups of mediastinal lymph nodes were resected. The specimens were sent for pathological examination after surgery
Right upper lobectomy was performed. The diagnosis of HAL was confirmed by pathological examination, and the patient received paclitaxel and carboplatin as adjuvant chemotherapy after surgery. For economic reasons, we did not carry out detection of tumor histogenetics and immune-related indicators. Chemotherapy was given once in the first month after surgery. Paclitaxel and carboplatin were added. Because of a serious adverse reaction to chemotherapy, the patient did not continue with the chemotherapy and other treatment. He died 15 mo after surgery.
HAC is a rare tumor that was first recognized as a gastric tumor in 1985 by Ishikura et al[3], and its etiology is still unclear. It has hepatocellular carcinoma-like differentiation and cytological characteristics. It is an aggressive tumor that most commonly arises from the gastrointestinal tract, and there are also a few reports in the ovaries, mediastinum, pancreas and other organs. Primary HAL is very rare. Ishikura et al[3] showed that the pharynx, esophagus, upper gastroduodenal region, liver, gallbladder, pancreas and respiratory system below the throat all develop in the anterior intestine of the embryonic primitive digestive tract. Due to abnormalities in the differentiation process, certain adenocarcinomas of the lungs and other tissues may differentiate into hepatocytes. However, a small number of HACs are also seen in organs such as sweat glands, ovaries, endometrium, frontal sweat glands, and adrenal glands. In terms of embryonic histology, the above organs do not develop from the original digestive tract, or even from the endoderm. Therefore, further research and discussion are needed to explain the origin of extrahepatic HAC in relation to embryonic development.
HAL commonly occurs in older male patients aged > 50 years. It frequently occurs in the upper lobe, and metastasis of the adrenal gland and spine has been reported[9]. The prognosis is closely related to the pathological stage, and the clinical manifestations are nonspecific. According to existing reports[9], there may be symptoms in common with those of other lung tumors, such as cough, expectoration and hemoptysis, and other clinical manifestations of lumbar and back pain. The patient reported in this article only showed symptoms of repeated cough and expectoration. Most of the tumors were located near the hilar area, and the margins were more regular based on clinicopathological and radiographic findings. Tumor marker AFP was often elevated, but cases with normal AFP have also been reported. Postoperative pathological specimens were often grayish white, with a tough, well-defined mass, and internal necrosis. The lung mass was biopsied via bronchoscopy, revealing histopathological findings of carcinoma with hepatoid features. Tumor cells were large and irregular in shape. Circles and polygons were also visible. The cytoplasm was rich, acidophilic or transparent, with obvious heteromorphism. The nuclear mitotic figures were easy to see, and the nucleus was large and irregular. Positive corpuscle was seen in the cytoplasm by periodic acid–Schiff (PAS) staining. Immunohistochemical staining showed that tumor cells were positive for AFP, hepatocytes and cytokeratin (CK) 18. Compared with the reported cases, the clinical manifestations of this case were nonspecific, AFP level was abnormal, the mass was large, but there was no distant metastasis. Bronchoscopy features and immuno-histochemical results were similarly to those reported previously.
The clinical manifestations of HAC are nonspecific, and the imaging features are often larger than those of typical lung cancer, but the boundaries are relatively neat. Bronchoscopy often shows dense, small blood vessels in tumor cells, and PAS-positive homogeneous transparent bodies. Elevated AFP is a useful index for the diagnosis of HAL, but lung metastasis of hepatocellular carcinoma must be excluded[10]. However, there are still reports of AFP-negative HAC. Hou et al[11] showed that preoperative patients have the following conditions that can be considered as HAL: (1) Symptoms in common with those of lung cancer, such as cough, expectoration, hemoptysis and other lung cancers; (2) Elderly men; (3) AFP positivity; and (4) Liver without space-occupying lesions. Preoperative diagnosis of HAL is difficult because there are no obvious clinical manifestations, nor clini-copathological or radiographic features.
Although serum AFP levels are not high (Table 1), HAL can be considered when high levels occur before surgery and there is no space-occupying lesion in the liver. It is generally recognized that the diagnosis of HAL depends on the cytopathological characteristics of hepatocellular carcinoma, and immunohistochemical staining for AFP, hepatocytes and CK18. Simultaneously, we can exclude lung metastasis of hepatocellular carcinoma. However, there are also reports of HAL with negative immunohistochemical staining for AFP and hepatocytes (Table 1). Therefore, HAL is still diagnosed based on comprehensive evaluation, and there are no highly specific indicators. HAL is essentially a special type of lung adenocarcinoma, and guidelines for diagnosis and treatment of lung cancer should be followed. The general principle is that if there is distant metastasis, systemic chemotherapy and local radiotherapy should be performed after a clear diagnosis. If there is no distant metastasis, surgical treatment or local radiotherapy should be performed. Chemotherapy should be considered according to the postoperative stage of HAL. We strongly recommend that patients undergo genetic and immunological tests. However, there are different chemotherapy regimens at present, and the prognosis of patients varies. Therefore, the optimal chemotherapy scheme still requires further research. In the present case, right upper lobectomy was performed. The diagnosis of HAL was confirmed by pathological examination, and the patient received paclitaxel and carboplatin as adjuvant chemotherapy after surgery. For economic reasons, we did not carry out detection of tumor genetics and immune-related indicators. Chemotherapy with paclitaxel and carboplatin was given once in the first month after surgery. Due to a serious adverse reaction to chemotherapy, the patient did not continue with chemotherapy and other treatments, and he died 15 mo after surgery in December 2018. The prognosis of HAL is not clear, and there is no specific retrospective study at present, which may be related to the reported number of cases. Preliminary research has found that vascular endothelial growth factor is highly expressed in HAL, which may be associated with early vascular invasion. The expression of Ki-67 may be directly related to prognosis. It is difficult to review and evaluate the results because of the small number of reported cases and lack of data on the above indicators. It is suggested that Ki-67 is expressed in HAL at levels between 20%-40% (Table 1)[9-22]. We could not perform statistical analysis due to incomplete follow-up data. Early vascular invasion may be the direct cause of early HAL metastasis and high degree of malignancy. However, this patient has a large primary mass, and postoperative pathology confirmed vascular invasion, although distant metastasis was undetected. Therefore, the above speculation needs further confirmation with retrospective studies.
Pathological examination revealed a lump of 7 cm × 7 cm × 7 cm in the upper lobe of the right lung. The patient was considered to have HAC because of the immunohistochemistry results and elevated AFP. Abdominal magnetic resonance imaging, including the liver, gallbladder, pancreas and spleen, and double kidney B ultrasound did not show occupying lesions. Investigation also found that there was no pleural or bronchial invasion, but vascular tumor thrombus was seen. Immu-nohistochemical staining showed that tumor cells were positive for epidermal growth factor receptor, pan-CK, CK18, Napsin A (small focus), CK8 (weak), CD117 (small), Ki-67 (50%), and hepatocytes (weak). In contrast, TTF-1, Syn, CgA, PD-1, PAS, Villin, VIM and CK7 were negative. Finally, no lymph node metastasis was seen.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Turner AM S-Editor: Cui LJ L-Editor: Filipodia E-Editor: Wu YXJ
| 1. | Su JS, Chen YT, Wang RC, Wu CY, Lee SW, Lee TY. Clinicopathological characteristics in the differential diagnosis of hepatoid adenocarcinoma: a literature review. World J Gastroenterol. 2013;19:321-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 118] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Wu Z, Upadhyaya M, Zhu H, Qiao Z, Chen K, Miao F. Hepatoid adenocarcinoma: computed tomographic imaging findings with histopathologic correlation in 6 cases. J Comput Assist Tomogr. 2007;31:846-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Ishikura H, Kanda M, Ito M, Nosaka K, Mizuno K. Hepatoid adenocarcinoma: a distinctive histological subtype of alpha-fetoprotein-producing lung carcinoma. Virchows Arch A Pathol Anat Histopathol. 1990;417:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Nagai E, Ueyama T, Yao T, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach. A clinicopathologic and immunohistochemical analysis. Cancer. 1993;72:1827-1835. [PubMed] |
| 5. | Arnould L, Drouot F, Fargeot P, Bernard A, Foucher P, Collin F, Petrella T. Hepatoid adenocarcinoma of the lung: report of a case of an unusual alpha-fetoprotein-producing lung tumor. Am J Surg Pathol. 1997;21:1113-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Hiroshima K, Iyoda A, Toyozaki T, Haga Y, Baba M, Fujisawa T, Ishikura H, Ohwada H. Alpha-fetoprotein-producing lung carcinoma: report of three cases. Pathol Int. 2002;52:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Terracciano LM, Glatz K, Mhawech P, Vasei M, Lehmann FS, Vecchione R, Tornillo L. Hepatoid adenocarcinoma with liver metastasis mimicking hepatocellular carcinoma: an immunohistochemical and molecular study of eight cases. Am J Surg Pathol. 2003;27:1302-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Kishimoto T, Yano T, Hiroshima K, Inayama Y, Kawachi K, Nakatani Y. A case of *-fetoprotein-producing pulmonary carcinoma with restricted expression of hepatocyte nuclear factor-4* in hepatoid foci: a case report with studies of previous cases. Hum Pathol. 2008;39:1115-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Bai CG, Liu XH, Yu YW, Zhang SM, Ma DL. A case of pulmonary hepatic adenocarcinoma and literature review. J Clin Exper Pathol. 2006;22:246-248. [DOI] [Full Text] |
| 10. | Poulakis V, Witzsch U, de Vries R, Becht E, Altmannsberger HM, Störkel S. Alpha-fetoprotein-producing renal cell carcinoma. Urol Int. 2001;67:181-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Hou Q, Zhang X, Zhang Y, Li W. Primary hepatic adenocarcinoma of the lung: a case report. J Diagn Pathol. 2017;24:473-475. [DOI] [Full Text] |
| 12. | Cao YA, Long NZ, Peng CS, Lu P, Xia Q, Wang W, Xie WX. Pulmonary hepatic adenocarcinoma with elevated alpha-fetoprotein 1 case. Diagn Theor and Prac. 2008;35:420-420. [DOI] [Full Text] |
| 13. | Feng GW, Hu JJ, Li B. 18F-FDGPET/CT detected pulmonary hepatic adenocarcinoma with elevated alpha-fetoprotein 1 case. Diagn Theor and Prac. 2013;231-233. [DOI] [Full Text] |
| 14. | Haninger DM, Kloecker GH, Bousamra Ii M, Nowacki MR, Slone SP. Hepatoid adenocarcinoma of the lung: report of five cases and review of the literature. Mod Pathol. 2014;27:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Shaib W, Sharma R, Mosunjac M, Farris AB, El Rayes B. Hepatoid adenocarcinoma of the lung: a case report and review of the literature. J Gastrointest Cancer. 2014;45 Suppl 1:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Gavrancic T, Park YH. A novel approach using sorafenib in alpha fetoprotein-producing hepatoid adenocarcinoma of the lung. J Natl Compr Canc Netw. 2015;13:387-391; quiz 391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Che YQ, Wang S, Luo Y, Wang JB, Wang LH. Hepatoid adenocarcinoma of the lung: Presenting mediastinal metastasis without transfer to the liver. Oncol Lett. 2014;8:105-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Liu HY, Wang XM, Cheng ZQ, Peng QZ, Sun XF, Hu JT, Jin HT, He LS. Clinicopathological observation of primary hepatic adenocarcinoma of the lung. Doc dissert. 2014;. [DOI] [Full Text] |
| 19. | Zhong MY, Liu B. A case report of pulmonary hepatic adenocarcinoma and literature review. J Cap Med Univ. 2015;36:515-516. [DOI] [Full Text] |
| 20. | Motooka Y, Yoshimoto K, Semba T, Ikeda K, Mori T, Honda Y, Iyama K, Suzuki M. Pulmonary hepatoid adenocarcinoma: report of a case. Surg Case Rep. 2016;2:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Sun JN, Zhang BL, Li LK, Yu HY, Wang B. Hepatoid adenocarcinoma of the lung without production of α-fetoprotein: A case report and review of the literature. Oncol Lett. 2016;12:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Grossman K, Beasley MB, Braman SS. Hepatoid adenocarcinoma of the lung: Review of a rare form of lung cancer. Respir Med. 2016;119:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |