Published online Jul 6, 2019. doi: 10.12998/wjcc.v7.i13.1703
Peer-review started: February 11, 2019
First decision: April 18, 2019
Revised: April 22, 2019
Accepted: May 3, 2019
Article in press: May 3, 2019
Published online: July 6, 2019
Processing time: 154 Days and 1.8 Hours
Synchronous multiple primary cancers (SMPC) mean two or more malignant tumors occurring simultaneously and with different origins no matter what types they are or where they are located. The carcinogenesis of SMPC often involves variations of some specific genes. However, the correlation between CDH1 mutations and synchronous multiple primary gastrointestinal cancers is largely unknown.
A 62-year-old woman had sustained abdominal pain for one week and visited our hospital. Gastrointestinal endoscopy revealed multiple small polypoid lesions in both the stomach and colorectum. Computed tomography and laboratory results were within normal limits. Pathological evaluation confirmed signet ring cell carcinoma without obvious metastatic evidence. Malignant cells showed negativity for E-cadherin and positivity for β-catenin in the cytoplasm and nucleus. DNA sequencing performed on paraffin-embedded tissue revealed two exactly coincident alterations in CDH1, C.57T>G and C.1418A>T.
This case suggests that the combination of CDH1 mutations and WNT/β-catenin signaling activation contributes to the carcinogenesis of gastrointestinal SMPC.
Core tip: A 62-year-old woman was admitted with abdominal pain for one week. Gastrointestinal endoscopy revealed multiple small polypoid lesions in both the stomach and colorectum. A diagnosis of primary signet ring cell carcinoma was established based on the combination of pathological and imageologic evaluations. E-cadherin expression was downregulated in the malignant cells, where β-catenin was aberrantly translocated to the cytoplasm and nucleus. DNA sequencing indicated C.57T>G and C.1418A>T in CDH1, suggesting the important role of CDH1 mutations in the pathogenesis of synchronous multiple primary gastrointestinal cancers.
- Citation: Hu MN, Lv W, Hu RY, Si YF, Lu XW, Deng YJ, Deng H. Synchronous multiple primary gastrointestinal cancers with CDH1 mutations: A case report. World J Clin Cases 2019; 7(13): 1703-1710
- URL: https://www.wjgnet.com/2307-8960/full/v7/i13/1703.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i13.1703
Synchronous multiple primary cancers (SMPC) are defined as two or more primary malignancies with different origins detected in a person within 6 months[1-3]. Although accumulating evidence suggests that genetic alterations are responsible for the tumo-rigenesis of SMPC, the underlying molecular mechanisms are unclear[4]. E-cadherin, a member of the cadherin family, is a calcium-dependent glycoprotein composed of five extracellular cadherin repeats and can serve as one of the most important elements in cell-cell adhesion in epithelial cells. E-cadherin can regulate cell adhesion and polarity by binding p120 and β-catenin[5]. Thus, loss of E-cadherin gene (CDH1) has been linked with hereditary diffuse gastric cancer as well as other cancers with a high propensity for vascular invasion and metastasis[4,6,7]. Genetic variations of CDH1 promote the detachment of malignant cells from primary site[8]. Except for its essential role in carcinogenesis, the crosstalk between E-cadherin and other signaling pathways, including p27, Hippo, WNT/β-catenin, and rho family GTPase, also contributes to the progression of malignant tumors[9-11].
Although CDH1 has been proposed as a tumor suppressor gene, the relationship between CDH1 mutations and the risk of SMPC, especially in the gastrointestinal system, is largely unknown. Here, we report the first case of synchronous multiple primary gastrointestinal cancers, developing in the stomach and colorectum, with CDH1 mutations.
A 62-year-old women presented to the department of gastroenterology with abdomi-nal pain and vomiting for 1 week.
The patient’s symptoms started a week ago.
The patient had a free previous medical history.
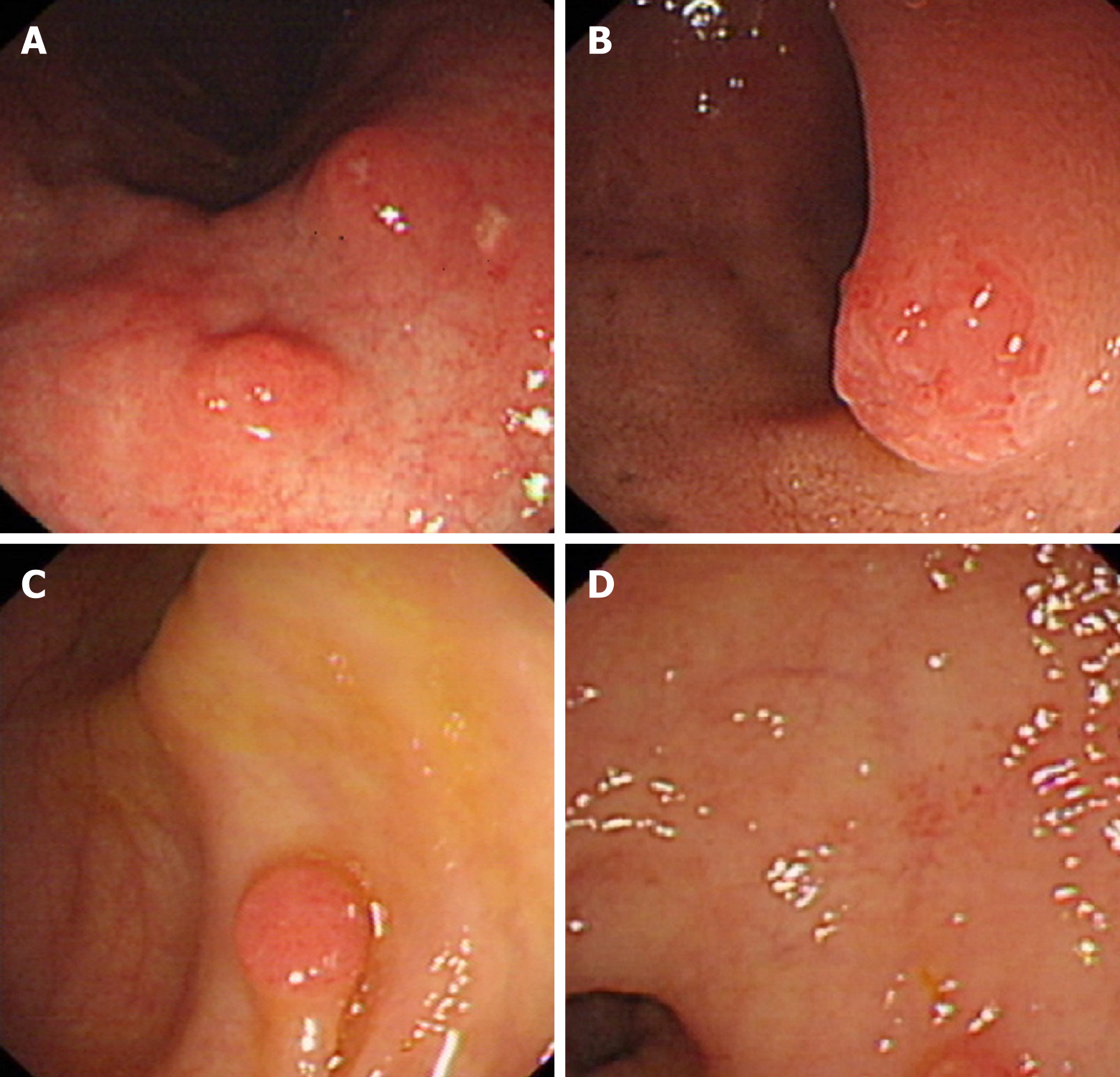
The patient underwent gastroscopy, by which multiple circumferential and polypoid masses, approximately 5 mm in the greatest diameter, in the mucosal surface of the gastric corpus and duodenum bulb were observed. The lesions were characterized by surface congestion and erosion, however, the surrounding mucosa appeared smooth and normal (Figure 1A and 1B). These lesions were clearly distinct from classic gastric adenocarcinoma by its island-like growth pattern. Meanwhile, colonoscopy also found multiple masses, three in the hepatic flexure of the colon, one in the transverse colon, two in the descending colon, and one in the rectum, all of which showed a simi-lar morphology to their counterparts in the stomach (Figure 1C and 1D).
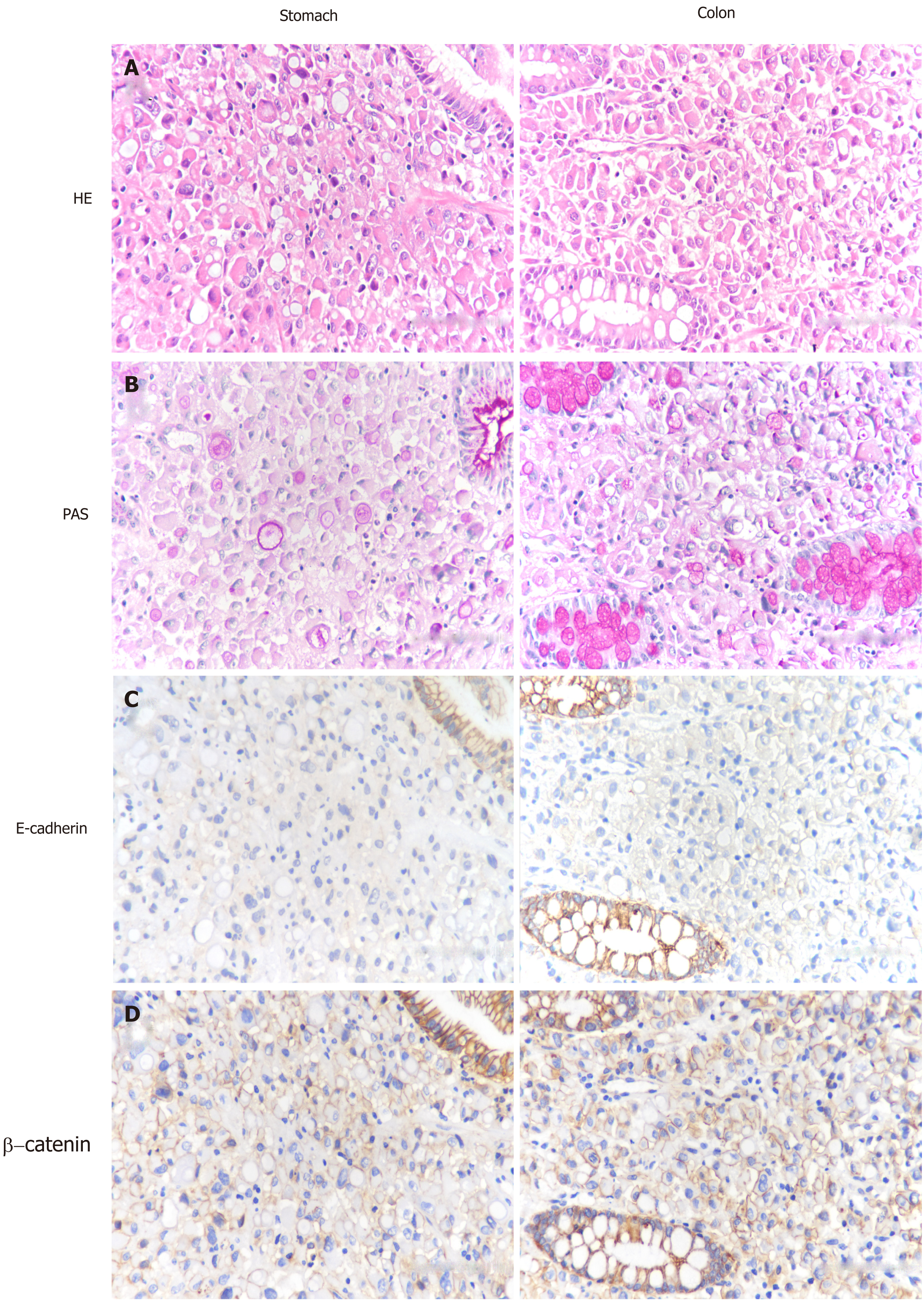
The diagnosis of signet ring cell carcinoma was established based on the pathological evaluation of biopsy specimens (Figure 2A). Periodic acid Schiff (PAS) positivity revealed the presence of mucin in the cytoplasm (Figure 2B). Antibody for E-cadherin decorated normal epithelial cells, rather than surrounding signet ring cells (Figure 2C). Abnormal cytoplasmic and nuclear positivity of β-catenin in cancer cells further encouraged the possibility that CDH1 alterations served as an important intrinsic driver (Figure 2D). Of note is the fact that the poor differentiated cells were restricted to the mucosa.
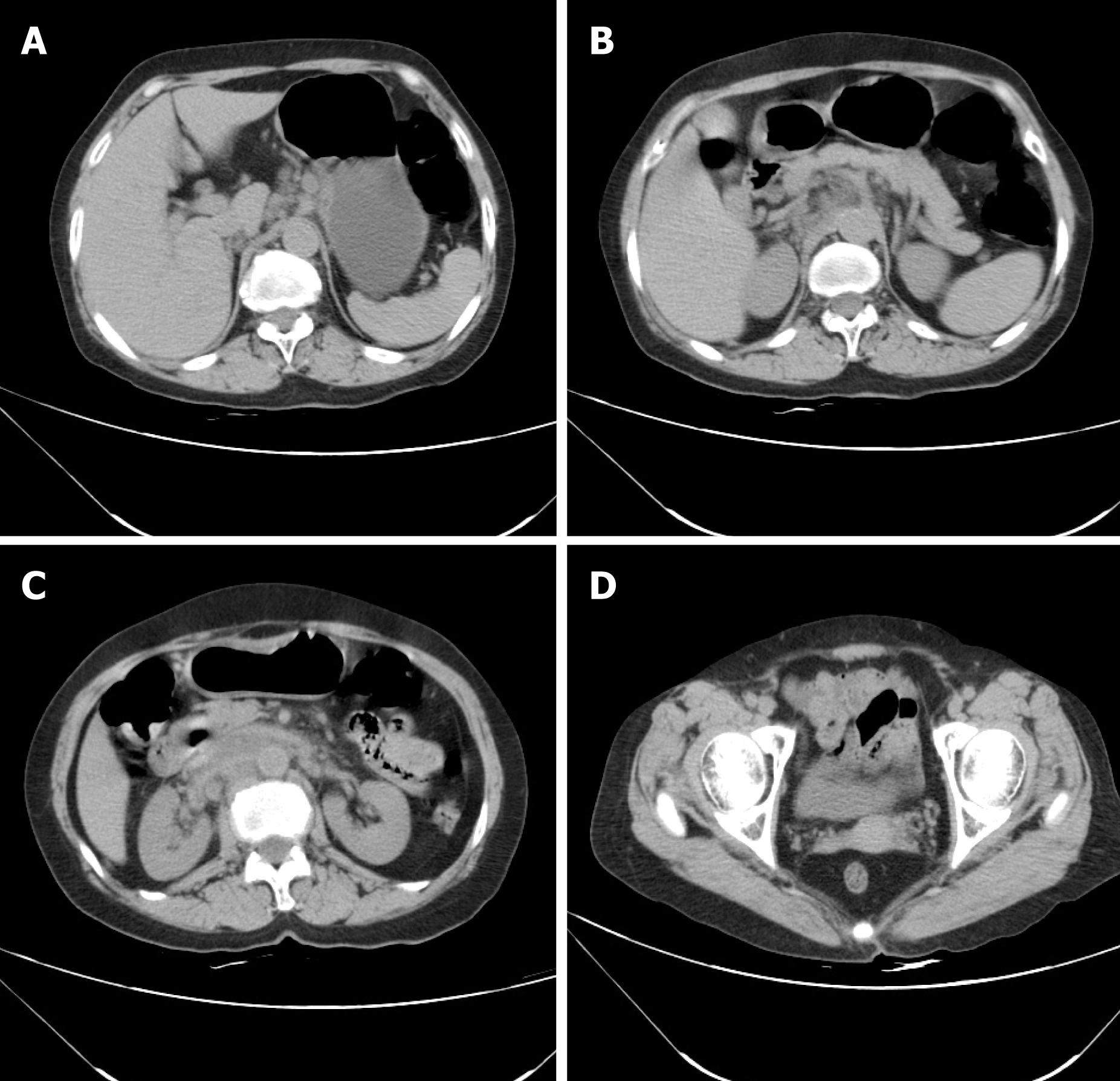
The abdominal computed tomography (CT) scan showed local inconspicuous wall thickening in the stomach, duodenum, colon and rectum, prompting that all may be primary lesions (Figure 3A-D).
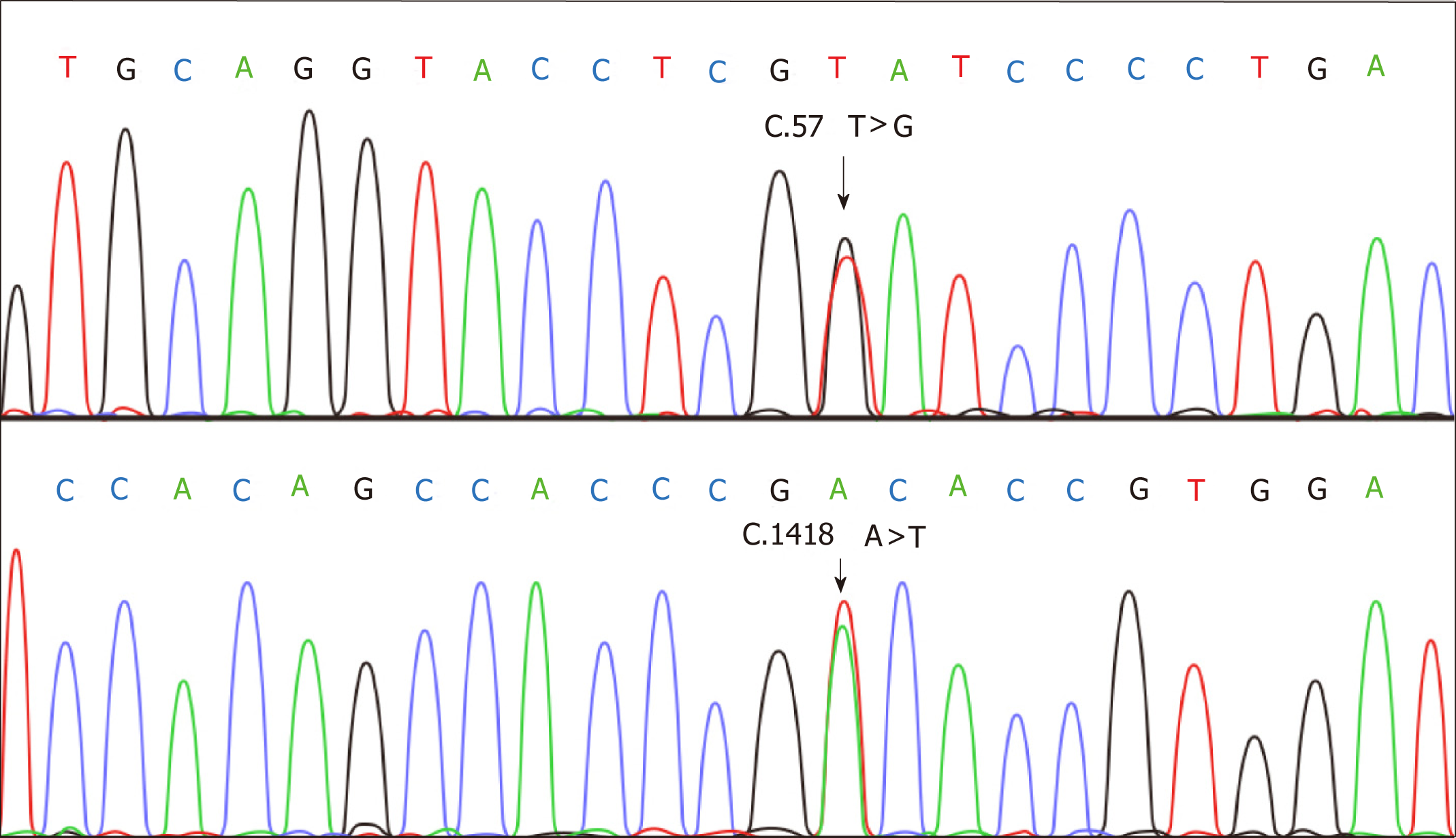
Sequencing was employed to confirm the alterations of CDH1 using DNA extracted from paraffin-embedded tissues. PCR amplification was performed using primers for 16 exons of CDH1 as described by a previous study[12]. DNA sequence was analyzed using the DNA sequencer (ABI Prism, Applied Biosystems) according to the manufa-cturer’s instructions. This experiment was repeated three times to insure the accuracy of results. Specimens exhibited uniform CDH1 alterations C.57T>G and C.1418A>T (Figure 4).
Huan Deng, PhD, MD, Department of Pathology/Molecular Medicine and Genetics Center, the Fourth Affiliated Hospital of Nanchang University; Renmin Institute of Forensic Medicine in Jiangxi
The pathological evaluation of biopsy specimens clearly showed their malignant characteristics. All lesions were diagnosed as poorly signet ring cell carcinomas, which were restricted to the mucosa. Combined with CT findings, these lesions should be derived from different cellular origins. Meanwhile, the immunophenotype of the lesions showed downregulation of E-cadherin and abnormal cytoplasmic and nuclear positivity for β-catenin in cancer cells, supporting the possibility that CDH1 alteration served as an important intrinsic driver during carcinogenesis of gastroin-testinal SMPC.
Multiple circumferential and polypoid masses in the gastrointestinal mucosa were observed by endoscopy. All the lesions have uniform morphological features and were clearly distinct from classic adenocarcinoma because of their island-like growth pattern. The clinical and pathological features support the diagnosis of SMPC. We should distinguish such cases from metastatic cancers based on comprehensive analysis of the results of endoscopy, pathology, positron emission tomography (PET)-CT, and laboratory tests. For patients with SMPC, we need to consider the possibility of heredity, thus relevant genetic testing should be recommended for the patient as well as his/her immediate familial members.
CT results mainly indicated that the lesions were inconspicuous, consistent with the characteristics of early primary gastrointestinal cancers. However, it is hard to give further precise evidences within the limits of inspection items. More substantial data should be obtained to avoid the pitfall of benign diseases or metastatic lesions.
The final diagnosis of the presented case is synchronous multiple primary gastroin-testinal cancers with CDH1 mutations.
The patient was discharged without any treatments.
The patient past away in one year.
The diagnosis of SMPC must meet the following four criteria: (1) Each tumor is malignant; (2) Tumors occur in different organs within 6 months; (3) Each tumor has its own metastatic pathway; and (4) The diagnosis of metastatic or recurrent tumors can be excluded[13-15]. We describe here a patient with synchronous multiple primary gastrointestinal cancers. The small polypoid lesions with distinctive boundary scattered throughout the stomach and colorectum. A combined analysis of restricted distribution of typical signet ring cells in the mucosa and CT findings further supported the diagnosis of primary cancers, rather than invasive or metastatic disea-ses.
Gene alterations contribute to the initiation of tumor genesis[4,16]. Substantial evidence reveals that CDH1 mutations and subsequent E-cadherin dysregulation play a key role in the development of hereditary diffuse gastric cancer (HDGC) as well as lobular breast cancer[17,18]. Patients with HDGC also face a higher risk of primary ma-lignancies in the colonrectum, thyroid, ovary, lung, prostate, salivary gland, and pancreas compared with the normal population[19,20]. A recent study reported a case in which CDH1 mutations are involved in synchronous appendiceal and intramucosal gastric signet ring cell carcinomas[20]. However, the relationship between CDH1 mutations and synchronous multiple primary gastrointestinal cancers has not been introduced before.
In this study, malignant tissues exhibited two CDH1 alterations: C.57T>G and C.1418A>T. Immunohistochemistry results further demonstrated the aberrant expression of CDH1 encoding protein E-cadherin. The downregulation of E-cadherin initiates the carcinogenesis cascade and enhances the motility of tumor cells through crosstalk with other vital signaling pathways such as WNT/β-catenin[21,22]. E-cadherin forms a dimeric complex with β-catenin on the cell membrane. The loss of binding region leads to the aberrant translocation of β-catenin from membrane to the cytoplasm and nucleus, where β-catenin binds TCF/LEF family and serves as a tran-scription factor to promote tumorigenesis[23].
In this study, we attempted to gain further insights into the patient’s family history. Her sisters also died from malignant gastric tumor without definite histopathological type. We lack more substantial evidence to support our hypothesis that it is a hereditary disease because her family members refused to do gene sequencing. Clinical experts are expected to distinguish multiple primary gastrointestinal cancers from metastatic counterparts, which enquires us to perform full diagnostic evalua-tions, involving endoscopy, PET/CT, and pathological biopsy. Meanwhile, genetic analyses can be recommended for patients and family members to predict disease risks.
Considerable publications have revealed that CDH1 mutations can promote the occurrence of malignant comorbidity, especially hereditary diffuse gastric carcinoma. However, the connection between CDH1 mutations and SMPC in the gastrointestinal tract is unclear. We report the first gastrointestinal SMPC case with two CDH1 alterations C.57T>G and C.1418A>T. The WNT/β-catenin signaling pathway was involved in the tumorigenesis. Further work is required to explore the underlying molecular mechanisms.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Karamouzis MV, Xuei X S-Editor: Ji FF L-Editor: Wang TQ E-Editor: Wang J
| 1. | Kim SH, Park BS, Kim HS, Kim JH. Synchronous quintuple primary gastrointestinal tract malignancies: Case report. World J Gastroenterol. 2017;23:173-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Maruyama T, Nakasone T, Maruyama N, Matayoshi A, Arasaki A. Synchronous quadruple multiple primary cancers of the tongue, bilateral breasts, and kidney in a female patient with a disease-free survival time of more than 5 years: a case report. World J Surg Oncol. 2015;13:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Oh SJ, Bae DS, Suh BJ. Synchronous triple primary cancers occurring in the stomach, kidney, and thyroid. Ann Surg Treat Res. 2015;88:345-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Cybulski C, Nazarali S, Narod SA. Multiple primary cancers as a guide to heritability. Int J Cancer. 2014;135:1756-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Chen HN, Yuan K, Xie N, Wang K, Huang Z, Chen Y, Dou Q, Wu M, Nice EC, Zhou ZG, Huang C. PDLIM1 Stabilizes the E-Cadherin/β-Catenin Complex to Prevent Epithelial-Mesenchymal Transition and Metastatic Potential of Colorectal Cancer Cells. Cancer Res. 2016;76:1122-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Hansford S, Kaurah P, Li-Chang H, Woo M, Senz J, Pinheiro H, Schrader KA, Schaeffer DF, Shumansky K, Zogopoulos G, Santos TA, Claro I, Carvalho J, Nielsen C, Padilla S, Lum A, Talhouk A, Baker-Lange K, Richardson S, Lewis I, Lindor NM, Pennell E, MacMillan A, Fernandez B, Keller G, Lynch H, Shah SP, Guilford P, Gallinger S, Corso G, Roviello F, Caldas C, Oliveira C, Pharoah PD, Huntsman DG. Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA Oncol. 2015;1:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 484] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 7. | Mi EZ, Mi EZ, di Pietro M, O'Donovan M, Hardwick RH, Richardson S, Ziauddeen H, Fletcher PC, Caldas C, Tischkowitz M, Ragunath K, Fitzgerald RC. Comparative study of endoscopic surveillance in hereditary diffuse gastric cancer according to CDH1 mutation status. Gastrointest Endosc. 2018;87:408-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Liu X, Chu KM. E-cadherin and gastric cancer: cause, consequence, and applications. Biomed Res Int. 2014;2014:637308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 9. | St Croix B, Sheehan C, Rak JW, Flørenes VA, Slingerland JM, Kerbel RS. E-Cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1). J Cell Biol. 1998;142:557-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 346] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Petrova YI, Schecterson L, Gumbiner BM. Roles for E-cadherin cell surface regulation in cancer. Mol Biol Cell. 2016;27:3233-3244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 187] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 11. | Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci USA. 2011;108:11930-11935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 558] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 12. | Berx G, Cleton-Jansen AM, Nollet F, de Leeuw WJ, van de Vijver M, Cornelisse C, van Roy F. E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. EMBO J. 1995;14:6107-6115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 544] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 13. | Moertel CG, Dockerty MB, Baggenstoss AH. Multiple primary malignant neoplasms. I. Introduction and presentation of data. Cancer. 1961;14:221-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Nyqvist J, Persson F, Parris TZ, Helou K, Kenne Sarenmalm E, Einbeigi Z, Borg Å, Karlsson P, Kovács A. Metachronous and Synchronous Occurrence of 5 Primary Malignancies in a Female Patient between 1997 and 2013: A Case Report with Germline and Somatic Genetic Analysis. Case Rep Oncol. 2017;10:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Xu LL, Gu KS. Clinical retrospective analysis of cases with multiple primary malignant neoplasms. Genet Mol Res. 2014;13:9271-9284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Luo YH, Ho HL, Tsai CM, Shih JF, Chiu CH, Lai SL, Lee YC, Perng RP, Whang-Peng J, Chou TY, Chen YM. The association between tumor epidermal growth factor receptor (EGFR) mutation and multiple primary malignancies in patients with adenocarcinoma of the lungs. Am J Clin Oncol. 2015;38:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Benusiglio PR, Malka D, Rouleau E, De Pauw A, Buecher B, Noguès C, Fourme E, Colas C, Coulet F, Warcoin M, Grandjouan S, Sezeur A, Laurent-Puig P, Molière D, Tlemsani C, Di Maria M, Byrde V, Delaloge S, Blayau M, Caron O. CDH1 germline mutations and the hereditary diffuse gastric and lobular breast cancer syndrome: a multicentre study. J Med Genet. 2013;50:486-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | Pharoah PD, Guilford P, Caldas C; International Gastric Cancer Linkage Consortium. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology. 2001;121:1348-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 425] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 19. | Kim HC, Wheeler JM, Kim JC, Ilyas M, Beck NE, Kim BS, Park KC, Bodmer WF. The E-cadherin gene (CDH1) variants T340A and L599V in gastric and colorectal cancer patients in Korea. Gut. 2000;47:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Hamilton LE, Jones K, Church N, Medlicott S. Synchronous appendiceal and intramucosal gastric signet ring cell carcinomas in an individual with CDH1-associated hereditary diffuse gastric carcinoma: a case report of a novel association and review of the literature. BMC Gastroenterol. 2013;13:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Mohammed MK, Shao C, Wang J, Wei Q, Wang X, Collier Z, Tang S, Liu H, Zhang F, Huang J, Guo D, Lu M, Liu F, Liu J, Ma C, Shi LL, Athiviraham A, He TC, Lee MJ. Wnt/β-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis. 2016;3:11-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 212] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 22. | Wong SHM, Fang CM, Chuah LH, Leong CO, Ngai SC. E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit Rev Oncol Hematol. 2018;121:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 264] [Article Influence: 37.7] [Reference Citation Analysis (1)] |
| 23. | Jang M, Koh I, Lee JE, Lim JY, Cheong JH, Kim P. Increased extracellular matrix density disrupts E-cadherin/β-catenin complex in gastric cancer cells. Biomater Sci. 2018;6:2704-2713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |