Published online Jul 6, 2019. doi: 10.12998/wjcc.v7.i13.1677
Peer-review started: February 15, 2019
First decision: Mach 14, 2019
Revised: April 30, 2019
Accepted: May 10, 2019
Article in press: May 11, 2019
Published online: July 6, 2019
Processing time: 142 Days and 8 Hours
Primary renal synovial sarcoma (PRSS) is an extremely rare tumor with a poor prognosis. Its imaging and immunohistochemical characteristics may overlap with other renal tumors, which renders its early diagnosis in a dilemma. The diagnosis of primary renal synovial sarcoma requires histopathology and the confirmation of SYT-SSX gene fusion using molecular techniques. Cases of primary renal synovial sarcoma have been previously reported in the literature. However, to our knowledge, primary renal allograft synovial sarcoma was never described.
A 43-year-old male patient who underwent kidney transplantation 9 months ago came to our hospital for regular follow-up. Traditional ultrasonography revealed multiple hypo-echo neoplasms in the renal allograft. Contrast-enhanced computed tomography (CECT) showed slightly hyper-density masses with slow homogeneous enhancement. Ultrasound-guided biopsy was conducted for accurate pathological diagnosis. The neoplasms were diagnosed as synovial sarcoma by pathological, immunohistochemical, and genetic analyses. Positron emission tomography/CT showed no evidence of metastasis. At approximately one week post biopsy, contrast-enhanced ultrasound was conducted to eliminate active hemorrhage. One month later, CECT showed that the biggest neoplasm grew from 3.3 cm to 5.7 cm in diameter. Parametric imaging was conducted with SonoLiver CAP to conduct further quantitative analysis, which showed that the enhancement pattern was heterogeneous hyper-vascular enhancement. Radical surgical resection of the whole renal allograft and ureter was conducted without additional adjuvant chemotherapy or external radiotherapy. Anlotinib was chosen for targeted therapy with a good response.
We propose multimodality imaging for accurate diagnosis of renal allograft synovial sarcoma especially when it is formed by spindle-shaped cells.
Core tip: Primary renal synovial sarcoma is an extremely rare tumor with a poor prognosis. We present the multimodality imaging characteristics of primary renal allograft synovial sarcoma in a 43-year-old male patient, which manifested as new hypo-echo solid neoplasms on ultrasonography or cystic-like neoplasms on contrast-enhanced computed tomography. After an accurate diagnosis was made, radical allograft nephrectomy was done without additional adjuvant chemotherapy or external radiotherapy. Anlotinib was chosen for targeted therapy. This case highlights the importance of multimodality imaging for early accurate diagnosis of renal allograft synovial sarcoma, especially when it is formed by spindle-shaped cells.
- Citation: Xu RF, He EH, Yi ZX, Lin J, Zhang YN, Qian LX. Multimodality-imaging manifestations of primary renal-allograft synovial sarcoma: First case report and literature review. World J Clin Cases 2019; 7(13): 1677-1685
- URL: https://www.wjgnet.com/2307-8960/full/v7/i13/1677.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i13.1677
Synovial sarcomas (SS) are generally deep-seated tumors affecting the proximity of large joints among adolescents and young adults and account for 5-10% of all adult soft-tissue sarcomas[1,2]. SS typically affect proximal limbs of young adults, but also involve other sites (the head and neck, duodenum, lung, mediastinum, abdominal wall, kidney, and prostate)[3]. Primary renal SS (PRSS) is a rare tumor with a poor prognosis. PRSS was first described in 1999 and published in 2000, which was previously included in embryonal sarcomas of the kidney[4,5]. The histopathological diagnosis for PRSS is difficult, and always needs further immunohistochemical and cytogenetic analyses. The translocation t (X; 18) (p11.2; q11.2) is pathognomonic for an accurate diagnosis. Moreover, metastatic sarcoma, sarcomatoid renal carcinoma, and hemangiopericytoma should be considered in differential diagnosis.
A 43-year-old man came to ultrasound department for regular follow-up, without any discomfort complaints.
The patient underwent allogeneic kidney transplantation (donation after cardiac death, DCD) because of chronic renal insufficiency and uremia at our hospital 9 months ago.
The patient was diagnosed with renal hypertension 8 years ago, with the highest blood pressure being about 160/100 mmHg. By taking adalat and metoprolol, his blood pressure was stable after dialysis. He was diagnosed with renal anemia 8 years ago and was periodically given hematopoietin for improvement. He was diagnosed with renal osteopathy 8 years ago, and his condition was stable with oral admini-stration of calcium tablets and rocaltrol. He underwent appendectomy 10 years ago because of acute appendicitis. The patient had no medical history of malignancy before transplantation.
The patient’s body temperature was 36.6 °C, heart rate was 78 bpm, respiratory rate was 18 breaths per minute, and blood pressure was 150/80 mmHg. One 5 cm oblique scar could be seen in the right lower abdomen. An arteriovenous fistula could be seen in the left forearm.
The postoperative creatinine was maintained at about 100 μmol/L during regular follow-ups.
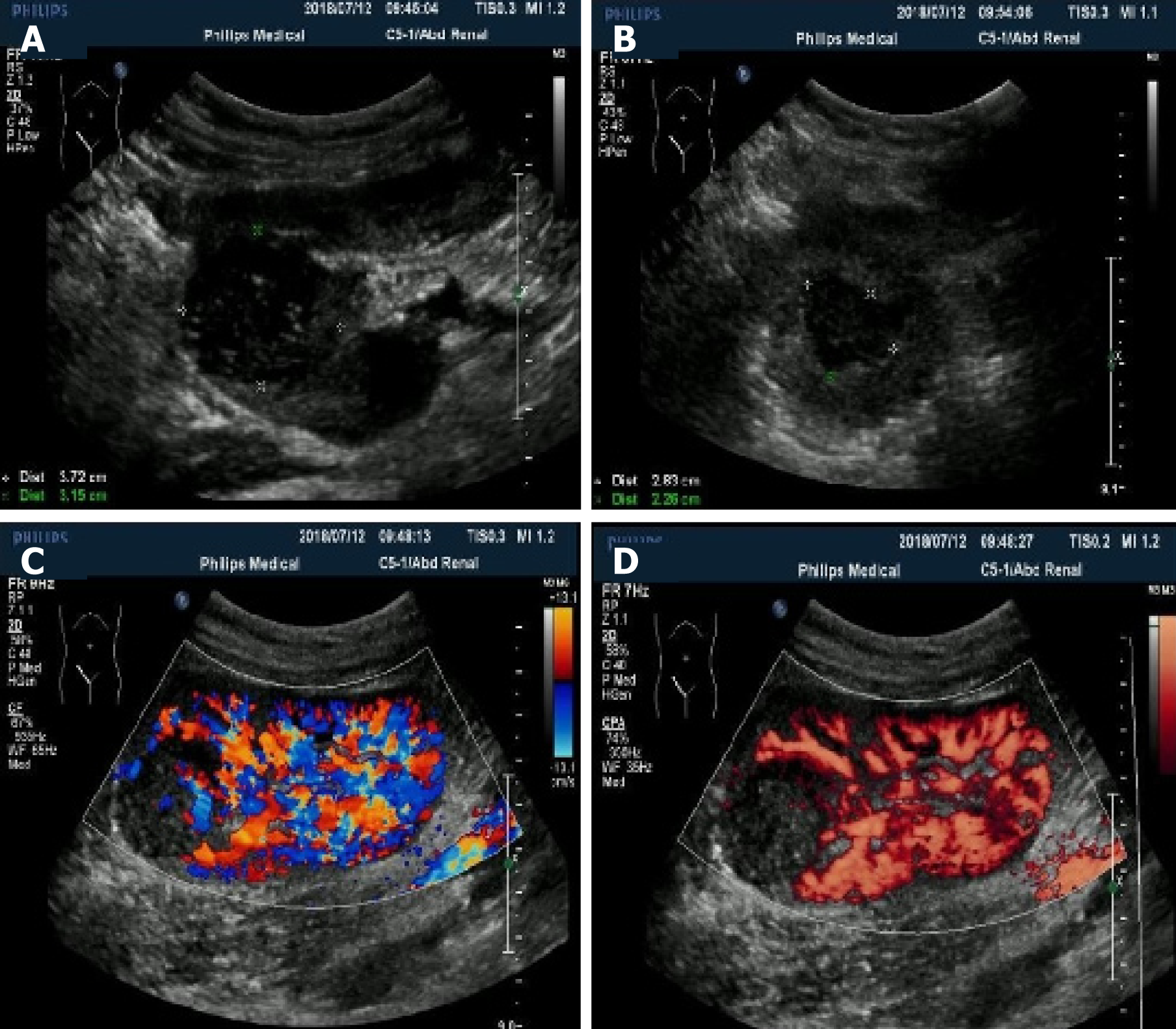
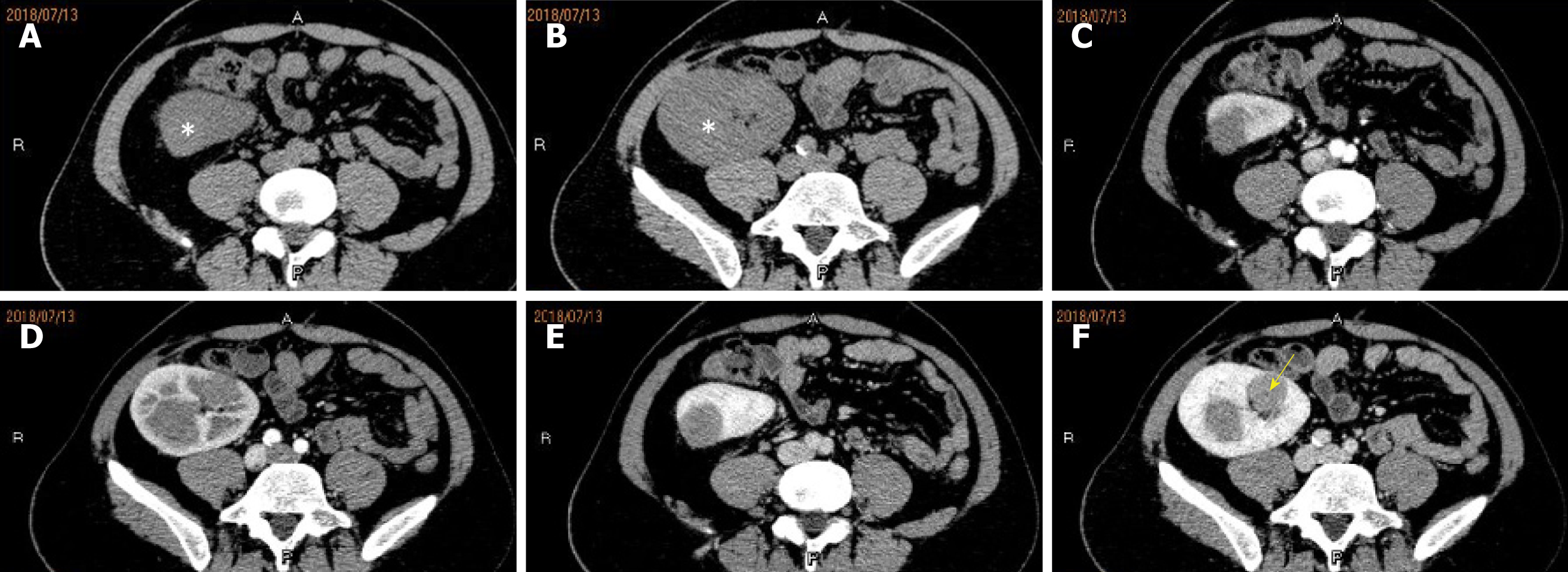
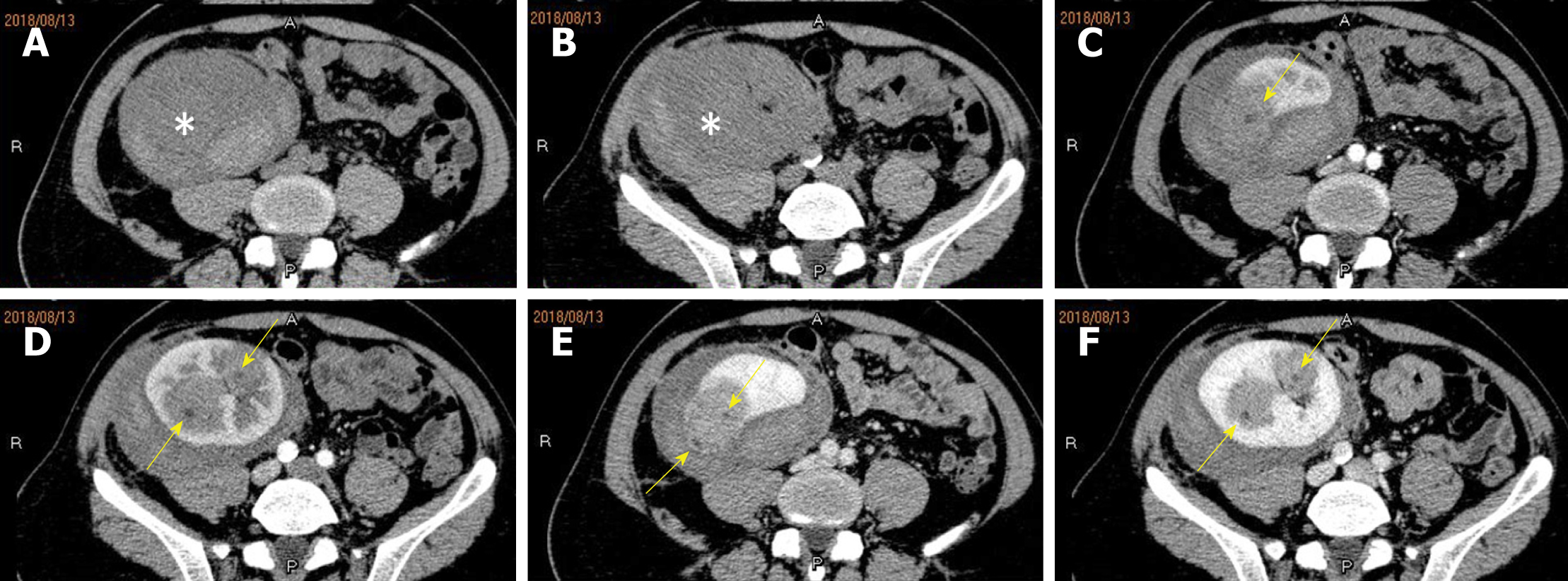
Multiple hypo-echo neoplasms were accidently found in the renal allograft during ultrasonography examination at that time. No discomfort was complained of in the past few months. Using a 1-5 MHz convex transducer (iuElite, Philips, Bothell, WA 98021, USA), ultrasonography revealed multiple well-defined solid hypo-echo neoplasms in the renal allograft (Figure 1A and B ), and the size of the biggest lesion was 3.7 cm × 3.2 cm × 3.5 cm. Color Doppler flow imaging (CDFI) and power Doppler imaging (PDI) showed that the neoplasms were hypo-vascular only with punctiform blood flow signal inside the neoplasms (Figure 1C and D). Contrast-enhanced computed tomography (CECT) (iCT 256, Philips Medical Systems, Best, The Netherlands) showed multiple neoplasms inside the renal allograft, located mainly in the upper pole and middle of the allograft (Figure 2). The renal allograft pelvis and ureter were not involved. The neoplasms were manifested as slightly hyper-density masses with slow homogeneous enhancement. CT values of no-enhancement, arterial-phase, and venous-phase images were 41 HU, 51 HU and 76 HU, respectively. The imaging features on CECT were similar to those of simple cysts. There was no evidence of metastases on CT examination. Post-transplant lymphoproliferative disorder (PTLD) was suspected, and ultrasound-guided biopsy was conducted for accurate pathological diagnosis.
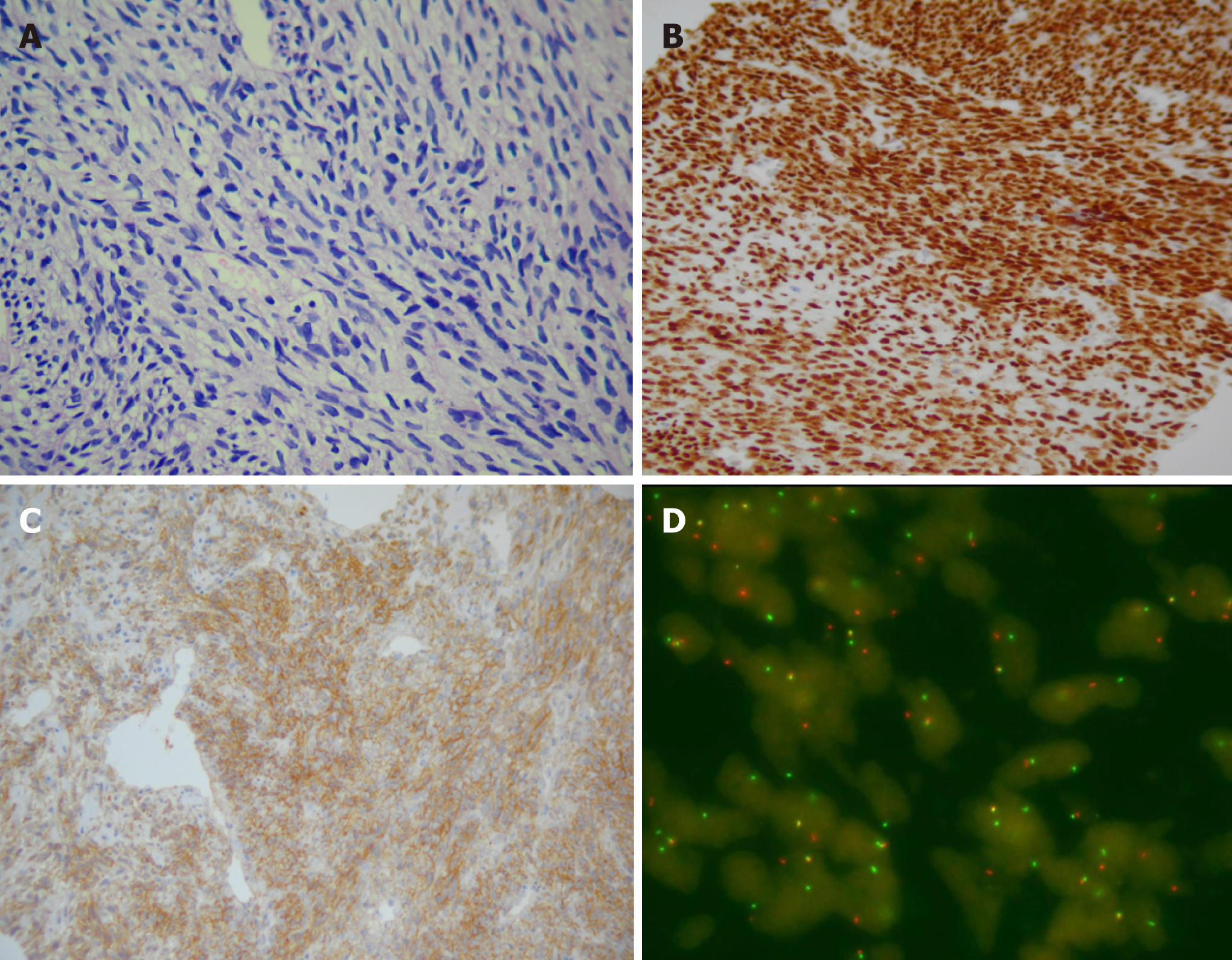
Pathological result showed monotonous neoplasms formed by spindle-shaped cells (Figure 3A), of which the atypia of cells was not obvious and the nuclear division was common with prominent mitotic activity (the cell growth was active). Further immunohistochemical analysis demonstrated positivity for Bcl-2, transducing-like enhancer of split (TLE-1) (Figure 3B), and vimentin CD99 (Figure 3C), focal positivity (30%) for Ki-67, and negativity for INI-1, which was typical for SS. As the neoplasm revealed no epithelial component, it was classified as monophasic SS. Genetic analysis confirmed the presence of SYT-SSX (X, 18) translocation using a SYT dual color break apart probe-based (Z-2097-50; Zytovision, Bremerhaven, Germany) fluorescence in situ hybridization (FISH) test (Figure 3D). Positron emission tomography/computed tomography (PET/CT) showed multiple hyper-density entities in the renal allograft with increased fluorodeoxyglucose (FDG) metabolism, which were clearer during the delayed phase. There was no evidence of metastases.
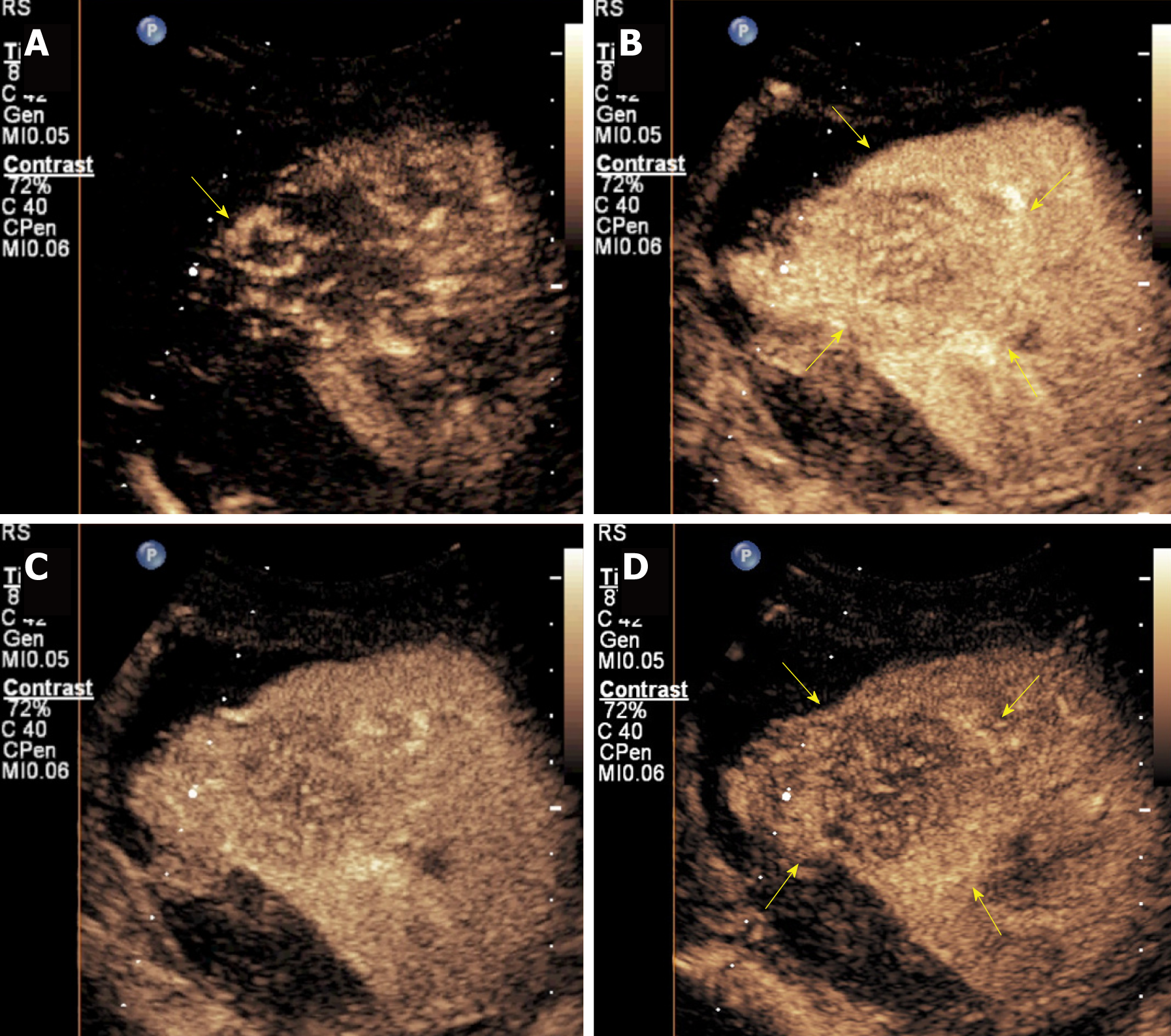
About a week post biopsy, the patient complained of right low abdominal pain around the renal allograft, and traditional ultrasound showed a heterogeneous region around the upper pole of the renal allograft. Thus, bleeding was considered, and contrast-enhance ultrasound (CEUS) was conducted with a SonoVue® (Bracco Int; Milana, Italy) bolus of 1.5 mL injected through a 20-gauge intravenous cannula, followed by a 5 mL saline flush. CEUS excluded active hemorrhage and showed synchronous enhancement of the neoplasm with the renal allograft parenchyma during the cortical phase. Branchlike, twisted, and tortuous vessel perfusion was seen, the neoplasms manifested as heterogeneous slight hyper-enhancement consistently, and no clear earlier wash-out than the peripheral parenchyma could be seen (Figure 4A-D). A pseudocapsule surrounding the neoplasm could be seen as a slightly higher enhancement ring around the mass compared with normal peripheral parenchyma (Figure 4B and D). CECT was conducted again before surgery. The neoplasms grew relatively rapidly during that month (Figure 5). The biggest neoplasm grew from 3.3 cm to 5.7 cm in diameter of the transverse section. Non-enhancing images manifested slightly hyper-density neoplasms (Figure 5A and B). The enhancement of neoplasms was heterogeneous with few spotted and patchy non-enhanced foci inside (Figure 5C-F), and the margins were clearer during the venous phase.
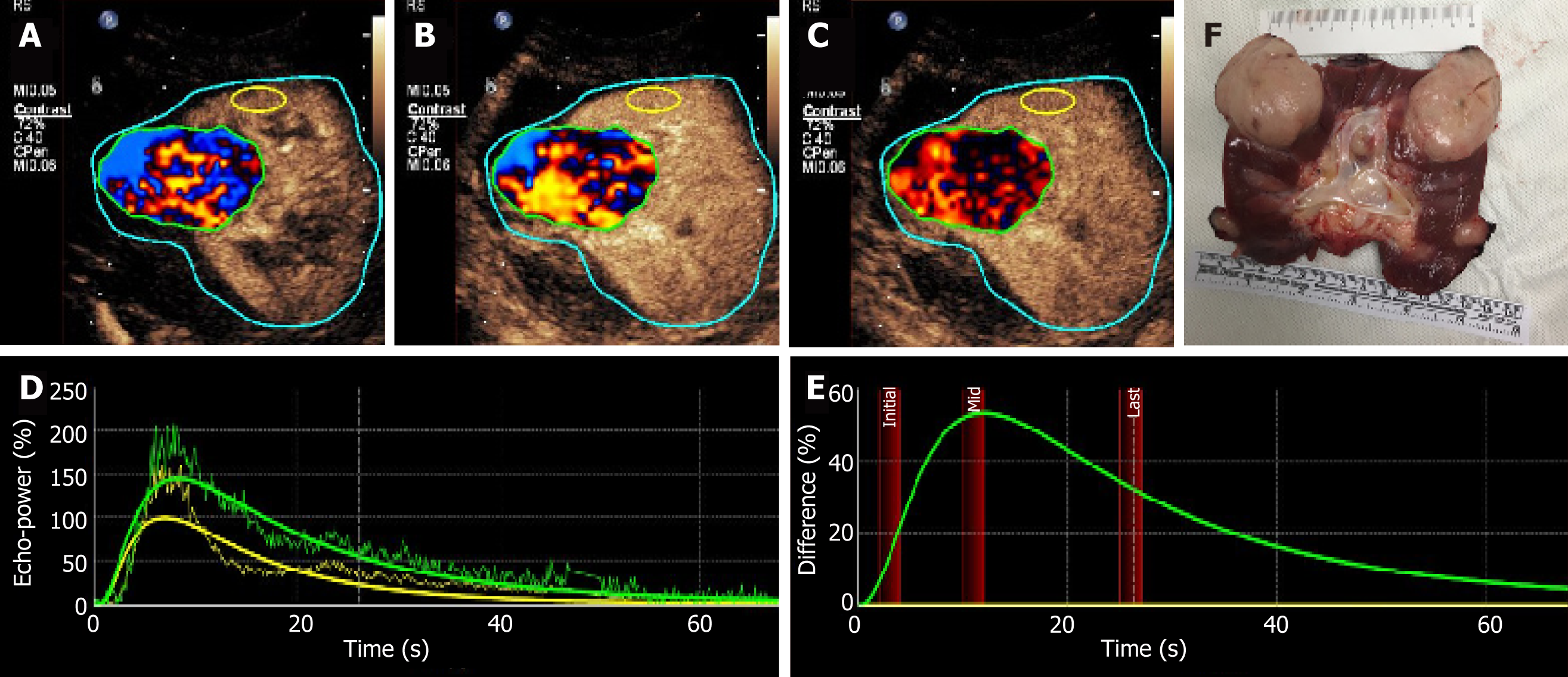
Parametric imaging was conducted with SonoLiver CAP version 1.0 (TomTec Imaging Systems GmbH, Unterschleissheim, Germany) software to conduct further quantitative analysis. The CEUS characteristics were defined by dynamic vascular pattern (DVP) curves and quantitative parameters. The DVP curves reflected the enhancement level change with time between the tumor and the surrounding normal tissue. The regions of interest (ROIs) were painted as follows: (1) Borderline ROI (blue), the imaging acoustic window to be analyzed, including the lesion and the surrounding tissue; (2) Analyzing ROI (green), area including both the central and marginal area of the neoplasm avoiding large vessels or necrotic areas as much as possible, and minor adjustments was performed to correct movement; and (3) Reference ROI (yellow): Peripheral parenchyma avoiding extra vessels, and time gain compensation was selected to correct depth-related attenuation, since it was not possible to select reference ROI of the same depth to the analyzing ROI. The DVP curves were reconstructed based on the difference of enhancing intensity between the entity and the peripheral parenchyma. DVP curves had an automatic correction function by applying motion compensation to reduce or eliminate the artifacts caused by the movement of ROIs. The DVP parametric imaging showed that the enhance-ment pattern of the neoplasm was heterogeneous hyper-vascular enhancement, performing as irregular disorder wash-in (red and blue) and wash-out (red and black) of the analyzing ROI (Figure 6A-C). According to the time-intensity curve of the analyzing ROI and reference ROI (Figure 5D), the DVP curve of this neoplasm was non-washout type, of which the curve was similar to the downwards parabola with the start origin of the whole curves above X-axis (Figure 5E).
The quantitative parameters included maximum intensity (IMAX, the highest percentage ratio of intensity during the whole perfusion process), rise time (RT, the time from 10% IMAX to 90% IMAX), time to peak (TTP, the time from contrast agent arrival in the tumor to IMAX), mean transit time (mTT, the time from contrast agent wash-in to 50% wash-out), and quality of fit (QOF, the fitness between raw data theoretical curve which is required to be higher than 75% when performing quantification analysis ). The IMAX, RT, TTP, mTT, and QOF of the analyzing ROI and reference ROI were 145.1% and 100.0%, 7.2 s and 5.9 s, 8.3 s and 7.0 s, 23.3 s and 17.1 s, and 88.6% and 78.6%, respectively. Principle findings for each image study are summarized in Table 1.
| Examination | Manifestations |
| Ultrasonography | Multiple, well-defined, solid, hypo-echo neoplasms, hypo-vascular with punctiform blood flow signal |
| CECT-1 | Slightly hyper-density neoplasms, slow homogeneous enhancement similar to simple cysts |
| CEUS | Branchlike, twisted, and tortuous vessel perfusion during cortical phase, heterogeneous slightly hyper-enhancement, no clear early wash-out, slightly higher enhanced pseudocapsule could be seen |
| CECT-2 | Neoplasms grew obviously bigger, slightly hyper-density, heterogeneous enhancement with few spotty and patchy non-enhanced foci inside, margins were clearer during the venous phase |
| Parametric imaging for CEUS | DVP parametric imaging showed heterogeneous hyper-vascular enhancement; DVP curve manifested as non-washout type |
The neoplasms were diagnosed as monophasic SS by pathological, immuno-histochemical, and genetic analyses.
Radical surgical resection of the whole renal allograft and ureter was conducted. Macroscopic pathological evaluation revealed a kidney measuring 10.0 cm × 6.5 cm × 5.5 cm (Figure 6F). Five neoplasms were round and well-defined with diameters from 1.0 cm to 5.7 cm infiltrating the renal parenchyma. The biggest neoplasm penetrated the nearby focal renal capsule and grew into the perinephric fat, but the pelvic and sinus fat was not involved. Microscopic vascular invasion could be seen. Resection margins and ureter stump were tumor free. There were no enlarged lymph nodes around the renal hilus. The patients kept on haemato-dialysis three times per week post-surgery without additional adjuvant chemotherapy or external radiotherapy since there was no obvious curative effect reported in the literature.
CECT of the chest, abdomen, and pelvic cavity for postoperative follow-up was done every three months. No occurrence was found around the primary graft area. Unfortunately, lung metastasis was found three months post nephrectomy on chest CECT. Anlotinib was chosen for targeted therapy, which resulted in an obvious decrease in lung metastasis nodules.
To our knowledge, this is the first documented case of renal allograft SS so far. The histological subtypes of SS include monophasic (spindle cells), biphasic (spindle cell component mixed with plump epitheloid cells), and poorly differentiated types. The poorly differentiated SS composes of sheets of undifferentiated round cells with hyperchromatic nuclei and frequent mitoses with the poorest outcome[6,7].
Imaging features can help make a pretreatment diagnosis when renal tumors are suspected. According to previous studies[8-10], PRSS is mostly manifested as a large, well-circumscribed, heterogeneously enhancing, soft-tissue-attenuation neoplasm which may extend into the renal pelvis or the perinephric region. A large number of PRSS present as a predominantly cystic entity with enhancing septa and solid components, with a cyst wall formed by compression of the adjacent renal parenchyma[7,10,11]. In our patient, multiple neoplasms were seen, manifesting as cystic lesions without definite septa or solid components inside on CECT imaging. The feature may be related to the size of the neoplasms, which were found early and in time during regular follow-up. These neoplasms were initially misdiagnosed as benign simple cysts on CT. Fortunately, this is a kidney transplantation patient, who underwent ultrasonographic follow-up regularly. Ultrasonography showed solid neoplasms and PTLD was suspected. Ultrasonography showed new solid neoplasms comparing with past ultrasound examination instead of simple cysts, and biopsy was conducted to make an accurate pathological diagnosis. Actually, when we reviewed CECT imaging of the first time, the enhancement of the lesion was not completely homogenous, and one small spotty non-enhanced focus could be seen (Figure 2F).
The tumors were growing fast, of which the biggest one grew from 3.3 cm to 5.3 cm in diameter in only one month. Lv et al[12] published the CT imaging findings of PRSS, which appeared as a solid-cystic mass with well-defined borders, a cystic wall or pseudo-capsule, heterogeneous or septate enhancement, a “rapid wash-in and slow wash-out” pattern of enhancement in the solid component, and no sign of lymphade-nopathy. The donor of this kidney was 14 years old without medical history of malignant tumors, consistent with literature results that PRSS typically affects younger patients. We, for the first time, describe the characteristics of PRSS of the renal allograft. The neoplasms manifested obvious heterogeneous, irregular wash-in and wash-out, and the whole enhancement was slightly higher than normal parenchyma tissue with a non-washout type of the DVP curve. The different diagnoses made by CEUS and CECT are associated with the principle of different methods. CEUS can outperform the CECT in visualizing tumor blood supply and in qualitative diagnosis. CEUS can detect minute, low-speed blood flows and has a higher sensitivity for small vessels than CECT.
Kidney transplantation is the superior form of renal replacement therapy for end-stage renal disease (ESRD) compared to dialysis, with significantly decreased mortality risk and improved quality of life[13]. Renal transplant recipients (RTR) have a risk three to five times higher than the general population of developing cancer, with lymphoma and skin cancer being the most predominant malignancies, followed by genitourinary tumors[14]. Cancer in RTR is more aggressive, has a higher growth rate, and tends to be multiple and disseminate early, both locally and systemically, in contrast to the general population[15]. No PRSS of five lesions was reported in the literature before, and its multiple feature may be related to the allograft property.
Since it was only a case report, the imaging manifestation of primary renal graft SS is limited and needs analysis of a larger sample.
PRSS is a rare renal neoplasm which can be diagnosed by immunohistochemistry and genetic analysis. The prognosis is bleak despite complete radical nephrectomy, which makes early accurate diagnosis and treatment more important. Although rare, PRSSs should be included in the differential diagnosis when multiple hypo-echo solid neoplasms are seen on ultrasonography or cystic-like well-defined masses on CECT imaging, especially when the donor is young. Combination of ultrasonography and CECT is important for early diagnosis of PRSS.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Choi MR, Ekpenyong CE, Nechifor G S-Editor: Gong ZM L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Thway K, Fisher C. Synovial sarcoma: defining features and diagnostic evolution. Ann Diagn Pathol. 2014;18:369-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 213] [Article Influence: 19.4] [Reference Citation Analysis (1)] |
| 2. | Kransdorf MJ. Malignant soft-tissue tumors in a large referral population: distribution of diagnoses by age, sex, and location. AJR Am J Roentgenol. 1995;164:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 407] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 3. | Wang ZH, Wang XC, Xue M. Clinicopathologic analysis of 4 cases of primary renal synovial sarcoma. Chin J Cancer. 2010;29:212-216. [PubMed] |
| 4. | Bakhshi GD, Khan AS, Shaikh AS, Khan MA, Khan MA, Jamadar NM. Primary renal synovial sarcoma. Clin Pract. 2012;2:e44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Argani P, Faria PA, Epstein JI, Reuter VE, Perlman EJ, Beckwith JB, Ladanyi M. Primary renal synovial sarcoma: molecular and morphologic delineation of an entity previously included among embryonal sarcomas of the kidney. Am J Surg Pathol. 2000;24:1087-1096. [PubMed] |
| 6. | Kawahara T, Sekiguchi Z, Makiyama K, Nakayama T, Nagashima Y, Kita K, Namura K, Itou H, Sano F, Hayashi N, Nakaigawa N, Ogawa T, Uemura H, Yao M, Kubota Y. Primary Synovial Sarcoma of the Kidney. Case Rep Oncol. 2009;2:189-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Perlmutter AE, Saunders SE, Zaslau S, Chang WW, Farivar-Mohseni H. Primary synovial sarcoma of the kidney. Int J Urol. 2005;12:760-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Lalwani N, Prasad SR, Vikram R, Katabathina V, Shanbhogue A, Restrepo C. Pediatric and adult primary sarcomas of the kidney: a cross-sectional imaging review. Acta Radiol. 2011;52:448-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Katabathina VS, Garg D, Prasad SR, Vikram R. Cystic renal neoplasms and renal neoplasms associated with cystic renal diseases in adults: cross-sectional imaging findings. J Comput Assist Tomogr. 2012;36:659-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Gong J, Kang W, Li S, Yang Z, Xu J. CT findings of synovial sarcomas of the kidney with pathological correlation. Clin Imaging. 2013;37:1033-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Chung SD, Huang KH, Chueh SC, Lai MK, Lin WC. Primary synovial sarcoma of the kidney. J Formos Med Assoc. 2008;107:344-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Lv XF, Qiu YW, Han LJ, Cao J, Zhang C, Liu ZY, Zhang XL, Cai PQ, Li L. Primary renal synovial sarcoma: computed tomography imaging findings. Acta Radiol. 2015;56:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Maglakelidze N, Pantsulaia T, Tchokhonelidze I, Managadze L, Chkhotua A. Assessment of health-related quality of life in renal transplant recipients and dialysis patients. Transplant Proc. 2011;43:376-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Lizakowski S, Kolonko A, Imko-Walczuk B, Komorowska-Jagielska K, Rutkowski B, Więcek A, Dębska-Ślizień A. Solid Organ Cancer and Melanoma in Kidney Transplant Recipients: TumorTx Base Preliminary Results. Transplant Proc. 2018;50:1881-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Miao Y, Everly JJ, Gross TG, Tevar AD, First MR, Alloway RR, Woodle ES. De novo cancers arising in organ transplant recipients are associated with adverse outcomes compared with the general population. Transplantation. 2009;87:1347-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |