Published online Jul 6, 2019. doi: 10.12998/wjcc.v7.i13.1591
Peer-review started: January 22, 2019
First decision: April 18, 2019
Revised: April 29, 2019
Accepted: May 1, 2019
Article in press: May 1, 2019
Published online: July 6, 2019
Processing time: 167 Days and 6.7 Hours
Nonfunctional pituitary adenoma is a common type of pituitary adenoma, which can lead to headache, visual field disturbance, and cranial nerve damage due to increased tumor volume. Neuroendoscopic and microscopic transsphenoidal approaches have been widely used in the resection of nonfunctional pituitary adenomas. However, the clinical efficacy in neuroendoscopic and microscopic surgery is still controversial.
To explore the clinical efficacy of neuroendoscopic and microscopic transsphenoidal approach for resection of nonfunctional pituitary adenomas.
We retrospectively analyzed 251 patients with nonfunctional pituitary adenomas; 138 underwent neuroendoscopic surgery via transsphenoidal approach, and 113 underwent microscopic surgery via transsphenoidal approach between July 2010 and September 2015. All patients were followed up for > 6 mo. Gender, age, course of disease, tumor diameter, tumor location, and percentage of patients with headache, visual impairment, sexual dysfunction, and menstrual disorders were contrasted between the two groups to compare the difference of preoperative data. Cure rate, symptom improvement rate, recurrence rate, the postoperative hospital stay, operating time, intraoperative blood loss, and the incidence of postoperative complications were compared in order to evaluate the advantages and disadvantages of neuroendoscopic and microscopic surgery.
There was no significant difference in cure rate, symptom improvement rate, and recurrence rate between neuroendoscopy group and microscopy group (82.6% vs 85.8%, P > 0.05; 90.6% vs 93.8%, P > 0.05; 5.1% vs 9.7%, P > 0.05). In the neuroendoscopy group, the postoperative hospital stay was 8.4 ± 0.6 d; operating time was 167.2 ± 9.6 min; intraoperative blood loss was 83.4 ± 9.3 mL, and the rates of diabetes insipidus and electrolyte imbalance were 4.3% and 8.0%, respectively. The corresponding results in the microscopic group were 11.2 ± 0.6 d, 199.7 ± 9.3 min, 138.8 ± 13.6 mL, and 32.7% and 20.4%, respectively. There were significant differences in postoperative hospital stay, operating time, intraoperative blood loss, and the rates of diabetes insipidus and electrolyte imbalance between the two groups (P < 0.05).
Neuroendoscopic and microscopic transsphenoidal approaches have similar clinical efficacy for the resection of nonfunctional pituitary adenomas. Neuroendoscopic surgery reduces operating time, intraoperative bleeding, postoperative recovery, and complications.
Core tip: Nonfunctional pituitary adenomas often require surgical treatment using a neuroendoscopic and microscopic transsphenoidal approach for resection. However, the clinical efficacy of neuroendoscopic and microscopic surgery may be different. The aim of this study was to explore the clinical efficacy of neuroendoscopic and microscopic transsphenoidal approach for resection of nonfunctional pituitary adenomas. The clinical efficacy of neuroendoscopic and microscopic surgery was similar. Compared with microscopic surgery, neuroendoscopic surgery reduced operating time, intraoperative bleeding, postoperative recovery time, and complications.
- Citation: Ding ZQ, Zhang SF, Wang QH. Neuroendoscopic and microscopic transsphenoidal approach for resection of nonfunctional pituitary adenomas. World J Clin Cases 2019; 7(13): 1591-1598
- URL: https://www.wjgnet.com/2307-8960/full/v7/i13/1591.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i13.1591
Pituitary adenoma is the most common benign tumor in the sellar region. Its incid-ence is 8.2-14.7/100000, accounting for 10%-15% of all intracranial tumors[1]. Pituitary adenoma is next only to gliocytoma and meningioma in terms of prevalence[2]. According to functional classification, it can be divided into functional and nonfunc-tional pituitary adenomas, and nonfunctional pituitary adenomas are more common[3]. Those > 1 cm in diameter are defined as large pituitary adenomas. The main clinical manifestations of pituitary adenomas are hormonal disorder and increasing tumor volume, which leads to headache, visual field disturbance, cranial nerve damage, and reduced quality of life[4]. Except for lactating pituitary adenomas, surgical resection is the first choice of treatment, and most pituitary adenomas can be removed by transsphenoidal surgery. At present, resection of pituitary adenomas by transsphenoidal approach under microscopy and neuroendoscopy is a clinically mature minimally invasive surgery[5].
This study reviewed the clinical data of patients with nonfunctional large pituitary adenomas treated by neuroendoscopic and microscopic surgery in our department and explored the clinical effect of neuroendoscopic and microscopic transsphenoidal approach for resection of nonfunctional large pituitary adenomas.
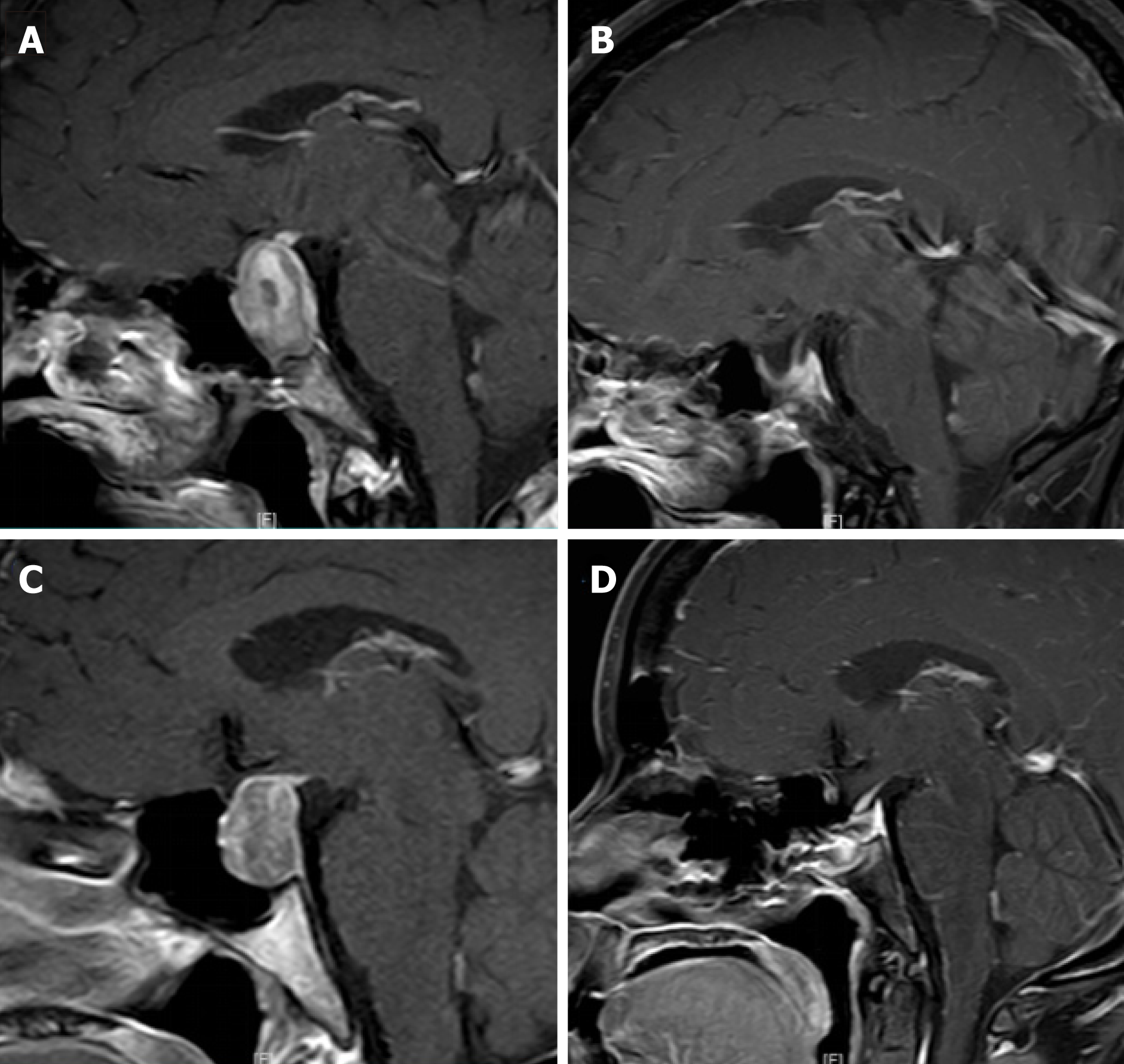
All of the patients received computed tomography and magnetic resonance imaging of the sellar region and underwent transsphenoidal surgery. All tumors were located in the sellar region, and they were confirmed histopathologically as nonfunctional pituitary adenomas. All patients were followed up for > 6 mo.
Patients who were diagnosed with nonfunctional pituitary adenomas and underwent other types of surgery were excluded. If the tumors were confirmed histopatholo-gically as functional pituitary adenomas, meningioma, craniopharyngioma or other tumors but not nonfunctional pituitary adenomas, then they were excluded. Patients with severe cardiopulmonary dysfunction and absence of case data were also exclu-ded.
A total of 326 cases were reviewed between July 2010 and September 2015, and 75 cases were excluded according to the exclusion criteria. Finally, 251 cases of nonfunctional pituitary adenomas were included. Among these, 138 cases (73 female and 65 male) underwent neuroendoscopic surgery, and 113 cases (59 female and 54 male) underwent microscopic surgery. There were no significant differences in gender, age, course of disease, tumor diameter, tumor location, and percentage of patients with headache, visual impairment, sexual dysfunction, and menstrual disorders between the two groups (P > 0.05) (Table 1).
| Neuroendoscopy group, 138 cases | Microscopy group, 113 cases | P-value | |
| Gender, male/female | 65/73 | 54/59 | 0.914 |
| Age in yr | 44.5 ± 1.5 | 42.9 ± 1.3 | 0.413 |
| Course of disease in mo | 25.9 ± 4.2 | 26.7 ± 3.5 | 0.887 |
| Clinical manifestations, n (%) | |||
| Headache | 57 (41.3%) | 47 (41.6%) | 0.963 |
| Visual impairment | 67 (48.6%) | 60 (53.1%) | 0.474 |
| Sexual dysfunction | 4 (2.9%) | 3 (2.7%) | 1.000 |
| Menstrual disorder | 20 (14.5%) | 26 (23.0%) | 0.083 |
| Tumor diameter in mm | 27.7 ± 6.2 | 26.3 ± 7.8 | 0.782 |
| Tumor location, n (%) | |||
| Confined to intrasellar region | 43 (31.2%) | 42 (37.2%) | 0.317 |
| Grow into suprasellar region | 55 (39.9%) | 38 (33.6%) | 0.310 |
| Invades cavernous sinus | 21 (15.2%) | 11 (9.7%) | 0.195 |
| Invades sphenoid sinus | 10 (7.2%) | 12 (10.6%) | 0.347 |
| Invades cavernous and sphenoid sinuses | 9 (6.5%) | 10 (8.9%) | 0.488 |
All 251 cases received computed tomography and magnetic resonance imaging of the sellar region. All tumors were located in the sellar region and were > 1 cm in diameter.
All patients were treated with antibiotic nasal drops for 3 d before surgery, and nasal hair was removed to clean the nasal cavity 1 d before surgery. Each patient was operated upon by a neurosurgeon with a chief surgeon qualification for the resection of pituitary adenomas by transsphenoidal approach under neuroendoscopy or microscopy.
SPSS version 20.0 software was used for statistical analysis. The measurement data were analyzed by t test, and the numerical data were analyzed by χ2 test. P < 0.05 was considered to be statistically significant.
Of 138 cases in the neuroendoscopy group, 114 were cured, 125 were improved, and 7 relapsed. Of 113 cases in the microscopy group, 97 were cured, 106 were improved, and 11 relapsed. There was no significant difference in cure rate, symptom impro-vement rate, and recurrence rate between the two groups (P > 0.05). However, there were significant differences in the duration of postoperative hospital stay, duration of operation, and intraoperative blood loss (P < 0.05) (Table 2 and Figure 1).
| Neuroendoscopy group, 138 cases | Microscopy group, 113 cases | P-value | |
| Cure, n (%) | 114 (82.6%) | 97 (85.8%) | 0.486 |
| Improvement, n (%) | 125 (90.6%) | 106 (93.8%) | 0.348 |
| Relapse, n (%) | 7 (5.1%) | 11 (9.7%) | 0.154 |
| Duration of postoperative hospital stay in d | 8.4 ± 0.6 | 11.2 ± 0.6 | 0.001 |
| Duration of operation in min | 167.2 ± 9.6 | 199.7 ± 9.3 | 0.016 |
| Intraoperative blood loss in mL | 83.4 ± 9.3 | 138.8 ± 13.6 | 0.001 |
In the neuroendoscopy group, 6 cases had transient profuse urination after surgery, 10 had cerebrospinal fluid leakage, 11 had electrolyte disturbance, 32 had hypopi-tuitarism, and 9 had intracranial infection. In the microscopy group, 37 cases had postoperative urine collapse, 12 had cerebrospinal fluid leakage, 23 had electrolyte disturbance, 35 had hypopituitarism, and 11 had intracranial infection. There was a significant difference in the incidence of diabetes insipidus and electrolyte disturbance between the two groups (P < 0.05) (Table 3).
| Neuroendoscopy group, 138 cases | Microscopy group, 113 cases | P-value | |
| Diabetes insipidus | 6 (4.3%) | 37 (32.7%) | 0.000 |
| Cerebrospinal fluid leakage | 10 (7.2%) | 12 (10.6%) | 0.347 |
| Electrolyte disturbance | 11 (8.0%) | 23 (20.4%) | 0.004 |
| Hypopituitarism | 32 (23.2%) | 35 (30.9%) | 0.165 |
| Intracranial infection | 9 (6.5%) | 11 (9.7%) | 0.350 |
Recent improvement in socioeconomic level and health consciousness has resulted in an annual increase in the incidence of pituitary adenomas. Pituitary adenomas not only have various characteristics of tumors, but also can cause abnormalities in endocrine function, affecting physical and mental health in terms of growth, development, and fertility and causing adverse outcomes for patients, families, and society.
Treatment for pituitary adenomas includes medication, surgery, and radioth-erapy[6]. Prolactinoma is the most common type of hormone-secreting pituitary tumor for which dopamine receptor agonists such as bromocriptine are preferred. Cabergo-line is used for patients who are resistant to bromocriptine. Dopamine receptor agonists can reduce the hormone level and tumor size. However, patients whose symptoms cannot be alleviated or who relapse can choose surgery. Surgical resection should be preferred for the other types of pituitary adenomas for which medication has poor efficacy. Radiotherapy is an adjuvant therapy for pituitary adenomas to reduce tumor size and lower surgical difficulty. It can also help with treatment of residual and relapsed pituitary adenomas[7]. Therefore, surgery is the main treatment for nonfunctional pituitary adenomas. The resection of pituitary adenomas by transsphenoidal approach under microscopy and neuroendoscopy is a clinically mature minimally invasive surgery.
In the last 20 years, neuroendoscopic transsphenoidal approach for resection of pituitary adenomas has been rapidly developed with good curative effect[8]. We can observe all aspects of the diseased tissue and its surrounding structures by neuroen-doscopy. The operative vision is so clear that the anatomical structure of the saddle bottom and its surroundings can be clearly identified during the operation. Tumor resection can be observed clearly and the dead angle of view can be reduced[9]. At the same time, we can better avoid injuring the internal carotid artery, cavernous sinus, optic nerve, and oculomotor nerve. Patients incur less damage, which eases their postoperative recovery[10]. However, the surgical difficulty is increased because of the narrow natural channels without a nasal speculum and the small operating space that necessitate single-handed operation. Furthermore, the neuroendoscopic image is only 2D, which lacks any stereoscopic sense[11]. Therefore, it is difficult to stop bleeding, and the surgeon needs a high level of technical expertise[12].
The advantages of the microscope relative to the neuroendoscope are that the former has 3D vision and good spatial stereopsis, and surgeons can operate with both hands, which can stop bleeding easier and keep the surgical field clear. However, the microscope has a limited, tubular field of view, which the anatomical condition of the saddle area cannot be well exposed. In addition, it is difficult to observe the tumor and deep important structures in the blind area of the field, which may easily cause tumor residue and damage surrounding important nerves and tissues resulting in complications[13]. Transsphenoidal surgery under the microscope needs the surgeon to push the nasal septum to the opposite side to cause artificial nasal bone fracture and can cut off part of the bony nasal septum, which may cause more nasal mucosal ble-eding.
In our study, the neuroendoscopy group had a cure rate of 82.6% (114/138), symptom improvement rate of 90.6% (125/138) and recurrence rate of 5.1% (7/138). The microscopy group had a cure rate of 85.8% (97/113), symptom improvement rate of 93.8% (106/113) and recurrence rate of 9.7% (11/113). There was no significant difference in these rates between the two groups. However, in the neuroendoscopy group, the postoperative hospital stay was 8.4 ± 0.6 d, operating time was 167.2 ± 9.6 min, and intraoperative blood loss was 83.4 ± 9.3 mL compared with 11.2 ± 0.6 d, 199.7 ± 9.3 min and 138.8 ± 13.6 mL, respectively, in the microscopy group. These differences between the two groups were significant. We believe that the neuroendo-scopic and microscopic transsphenoidal approaches for resection of nonfunctional pituitary adenomas have equivalent efficacy. However, neuroendoscopic surgery has shorter operating time, less intraoperative bleeding, and shorter recovery time. We consider that we can identify the tumor and its surrounding structures better under neuroendoscopy without breaking the nasal septum, which saves operating time and causes less surgical trauma and quicker recovery.
With regard to complications, in the neuroendoscopy group, the incidence of diabetes insipidus was 4.3% (6/138), cerebrospinal fluid leakage was 7.2% (10/138), electrolyte disturbance was 8.0% (11/138), hypopituitarism was 23.2% (32/138), and intracranial infection was 6.5% (9/138). In the microscopy group, the incidence of diabetes insipidus was 32.7% (37/113), cerebrospinal fluid leakage was 10.6% (12/113), electrolyte disturbance was 20.4% (23/113), hypopituitarism was 30.9% (35/113), and intracranial infection was 9.7% (11/113). There was no difference in the incidence of cerebrospinal fluid leakage, hypopituitarism, and intracranial infection in the two groups. However, the incidence of diabetes insipidus and electrolyte imbalance was significantly different. Neuroendoscopic surgery can reduce the incidence of diabetes insipidus and electrolyte imbalance. We hypothesize that the main reason for cerebrospinal fluid leakage is rupture of the saddle septum, and intracranial infection is often secondary to cerebrospinal fluid leakage. The saddle septum can be broken because it becomes thinner due to the tumor pressure and the tumor falls unevenly during the tumor eradication process, resulting in intraoperative cerebrospinal fluid leakage. However, correct repair of the saddle septum was achieved by neuroendoscopy or microscopy reducing the incidence of cerebrospinal fluid leakage and intracranial infection. Diabetes insipidus and electrolyte imbalance are related to the traction and injury of the pituitary stalk during surgery. Under the neuroendoscope, we can identify the pituitary stalk more clearly, which is helpful for avoiding injury to the pituitary stalk and reducing the occurrence of diabetes insipidus and electrolyte imbalance.
This study had some limitations, such as the limited sample size, lack of randomi-zation of groups, and bias caused by single-center analysis. In the future, multicenter samples and randomization will be used to reduce this bias, and a multicenter prospective study of nonfunctional pituitary adenomas should be conducted to make the research more convincing.
In conclusion, neuroendoscopic surgery does not damage normal structure of the nasal cavity and has the advantages of clearer operative vision, shorter operating time, less intraoperative bleeding, shorter recovery time, and fewer complications. With the development of 3D endoscopic techniques, neuroendoscopic techniques will be one of the main developmental directions of microsurgery.
Nonfunctional pituitary adenoma is a common type of pituitary adenomas, which can lead to headache, visual field disturbance, and cranial nerve damage due to increased tumor volume. Neuroendoscopic and microscopic transsphenoidal approaches have been widely used in the resection of nonfunctional pituitary adenomas. However, the clinical efficacy in neuroendoscopic and microscopic surgery is still controversial.
The purpose of this study was to collect the clinical data of patients with nonfunctional pituitary adenomas treated by neuroendoscopic surgery via transsphenoidal approach or microscopic surgery via transsphenoidal approach at our hospital from 2010 to 2015. The clinical data was compared to explore the clinical efficacy of neuroendoscopic surgery and microscopic surgery, and to provide a direction for the choice of surgical methods for nonfunctional pituitary adeno-mas.
The main objective of this study was to explore the clinical efficacy of neuroendoscopic and microscopic transsphenoidal approach for resection of nonfunctional pituitary adenomas.
From 2010 to 2015, the clinical data of patients with nonfunctional pituitary adenomas treated by neuroendoscopic surgery via transsphenoidal approach or microscopic surgery via trans-sphenoidal approach were analyzed. All patients were followed up for > 6 mo. In this study, the t test and chi-square test were used to test the statistical differences between groups, which clearly confirmed the differences between the two groups.
In the neuroendoscopy group, the cure rate was 82.6%; symptom improvement rate was 90.6%; recurrence rate was 5.1%; the postoperative hospital stay was 8.4 ± 0.6 d; operating time was 167.2 ± 9.6 min; intraoperative blood loss was 83.4 ± 9.3 mL, and the rates of diabetes insipidus and electrolyte imbalance were 4.3% and 8.0%, respectively. The corresponding results in the microscopic group were 85.8%, 93.8%, 9.7%, 11.2 ± 0.6 d, 199.7 ± 9.3 min, 138.8 ± 13.6 mL, and 32.7% and 20.4%, respectively. We found that neuroendoscopic surgery reduces operating time, intraoperative bleeding, postope-rative recovery, and complications. This study will provide a direction for the choice of treatment methods for patients with nonfunctional pituitary adenomas in clinical work.
The clinical efficacy of neuroendoscopic and microscopic transsphenoidal approach for the resection of nonfunctional pituitary adenomas was similar. However, compared to microscopic surgery, neuroendoscopic surgery reduced operating time, intraoperative bleeding, postope-rative recovery, and complications. This study will provide a direction for the choice of treatment methods for patients with nonfunctional pituitary adenomas in clinical work.
This study was a retrospective single-center study, and the sample size was limited. In the future, a multicenter prospective study of nonfunctional pituitary adenomas could be attempted to further explore the long-term safety and efficacy of neuroendoscopic treatment for nonfun-ctional pituitary adenomas.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hicks PB S-Editor: Dou Y L-Editor: Filipodia E-Editor: Wang J
| 1. | Inagawa H, Ishizawa K, Mitsuhashi T, Shimizu M, Adachi J, Nishikawa R, Matsutani M, Hirose T. Giant invasive pituitary adenoma extending into the sphenoid sinus and nasopharynx: report of a case with intraoperative cytologic diagnosis. Acta Cytol. 2005;49:452-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, Nelson M, Weber R, Bernstein DI, Blessing-Moore J, Khan DA, Lang DM, Nicklas RA, Oppenheimer J, Portnoy JM, Randolph C, Schuller DE, Spector SL, Tilles S, Wallace D. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127:S1-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 772] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 3. | Jaffe CA. Clinically non-functioning pituitary adenoma. Pituitary. 2006;9:317-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Karppinen A, Kivipelto L, Vehkavaara S, Ritvonen E, Tikkanen E, Kivisaari R, Hernesniemi J, Setälä K, Schalin-Jäntti C, Niemelä M. Transition From Microscopic to Endoscopic Transsphenoidal Surgery for Nonfunctional Pituitary Adenomas. World Neurosurg. 2015;84:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Akbari H, Malek M, Ghorbani M, Ramak Hashemi SM, Khamseh ME, Zare Mehrjardi A, Emami Z, Ebrahim Valojerdi A. Clinical outcomes of endoscopic versus microscopic trans-sphenoidal surgery for large pituitary adenoma. Br J Neurosurg. 2018;32:206-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Ding D, Starke RM, Sheehan JP. Treatment paradigms for pituitary adenomas: defining the roles of radiosurgery and radiation therapy. J Neurooncol. 2014;117:445-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Cortet-Rudelli C, Bonneville JF, Borson-Chazot F, Clavier L, Coche Dequéant B, Desailloud R, Maiter D, Rohmer V, Sadoul JL, Sonnet E, Toussaint P, Chanson P. Post-surgical management of non-functioning pituitary adenoma. Ann Endocrinol (Paris). 2015;76:228-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Dorward NL. Endocrine outcomes in endoscopic pituitary surgery: a literature review. Acta Neurochir (Wien). 2010;152:1275-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | McLaughlin N, Eisenberg AA, Cohan P, Chaloner CB, Kelly DF. Value of endoscopy for maximizing tumor removal in endonasal transsphenoidal pituitary adenoma surgery. J Neurosurg. 2013;118:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Plunkett C, Barkan AL. The care continuum in acromegaly: how patients, nurses, and physicians can collaborate for successful treatment experiences. Patient Prefer Adherence. 2015;9:1093-1099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Koutourousiou M, Gardner PA, Fernandez-Miranda JC, Paluzzi A, Wang EW, Snyderman CH. Endoscopic endonasal surgery for giant pituitary adenomas: advantages and limitations. J Neurosurg. 2013;118:621-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 12. | Roelfsema F, Biermasz NR, Pereira AM. Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: a structured review and meta-analysis. Pituitary. 2012;15:71-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 13. | Mahvash M, Igressa A, Pechlivanis I, Weber F, Charalampaki P. Endoscopic endonasal transsphenoidal approach for resection of a coexistent pituitary macroadenoma and a tuberculum sellae meningioma. Asian J Neurosurg. 2014;9:236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |