Published online Jun 26, 2019. doi: 10.12998/wjcc.v7.i12.1515
Peer-review started: January 29, 2019
First decision: March 9, 2019
Revised: March 24, 2019
Accepted: April 18, 2019
Article in press: April 19, 2019
Published online: June 26, 2019
Processing time: 148 Days and 22.1 Hours
The female genital tract is an uncommon site of involvement for extra-genital malignancies. Ovarian metastases have been described as disseminations of lung adenocarcinoma; rare cases of secondary localizations in the cervix, adnexa, and vagina have also been reported in the literature. Here, we report two cases of advanced lung adenocarcinoma with female genital tract metastasis.
The first case was a 41-year-old woman with stage IV lung adenocarcinoma metastasizing to the cervix. Immunohistochemistry of the cervical biopsy specimen revealed thyroid transcription factor (TTF)-1(+), cytokeratin (CK)-7(+), and (CK)-20(-). Gene mutational analysis showed epidermal growth factor receptor (EGFR) L858R mutation in exon 21. She had a positive response to gefitinib, for both the pulmonary mass and cervical neoplasm. The second case was a 29-year-old woman who was diagnosed with stage IV lung adenocarcinoma with EGFR mutation. After 12 mo of treatment with icotinib, ovarian biopsy showed adenocarcinoma with CDX2(-), TTF-1(+++), PAX8(-), CK-7(+++), CK-20(++), and Ki67(15%+), accompanied with EGFR 19-del mutation and T790M mutation.
Immunohistochemistry and gene mutational testing have greatly helped in locating the initial tumor site when both pulmonary and female genital tract neoplasms exist.
Core tip: The female genital tract is an uncommon site of involvement for extra-genital malignancies. Ovarian metastases have been described as disseminations of lung adenocarcinoma; rare cases of secondary localizations in the cervix, adnexa, and vagina have also been reported in the literature. Here, we report two cases of advanced lung adenocarcinoma with female genital tract metastasis. The initial tumor site should be considered when both pulmonary and female genital tract neoplasms exist. Immunohistochemistry and gene mutational testing have greatly helped in locating the initial tumor site.
- Citation: Yan RL, Wang J, Zhou JY, Chen Z, Zhou JY. Female genital tract metastasis of lung adenocarcinoma with EGFR mutations: Report of two cases. World J Clin Cases 2019; 7(12): 1515-1521
- URL: https://www.wjgnet.com/2307-8960/full/v7/i12/1515.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i12.1515
Although metastases of lung adenocarcinoma may appear in any organ, they are more commonly observed in the bone, liver, adrenal gland, brain, and skin and seldom seen in the female genital tract, as metastasis in the female genital tract usually corresponds to small cell lung carcinomas[1]. Ovarian metastases have been described as disseminations of lung adenocarcinoma; rare cases of secondary localizations in the cervix, adnexa, and vagina have also been reported in the literature[2]. In these cases, immunohistochemistry and gene mutational analysis play an important role in determining the initial origin of tumor. In this article, we present two cases of advanced lung adenocarcinoma – the first case had cervical metastasis, and the other had metastasis to the ovary. The first case seemed to have cervical metastasis before targeted treatment; the other case developed ovarian metastasis accompanied by the newly presented T790M mutation in exon 20 during treatment with tyrosine kinase inhibitors (TKIs).
Case 1: A 41-year-old never-smoking woman came to our practice because of recurrent cough for 4 mo.
Case 2: A 29-year-old never-smoking woman was accepted because of complaints of cough and chest tightness.
There was no history of fever, weight loss, or sweating.
Case 1: History of past illness The patient’s past medical history was a right lung nodule, which was observed 4 years ago.
Case 1: She was a non-smoker and there was no history of drug abuse or recent travel. The family history was unremarkable.
Case 2: She was a non-smoker and there was no history of drug abuse or recent travel. The family history was unremarkable.
Case 1: The patient had no palpable lymph node.
Case 2: The patient had a low auscultation of breath and had no palpable lymph node.
Case 1: The initial laboratory investigations including complete blood count and blood chemistry tests were normal. Carcinoembryonic antigen level was 1.5 ng/mL (0-5 ng/mL), which was negative.
Case 2: The initial laboratory investigations including complete blood count and blood chemistry tests were normal. Carcinoembryonic antigen level was 4.4 ng/mL (0-5 ng/mL) and tumor-associated carbohydrate antigen 125 level was 42.9 U/mL (0-35 U/mL).
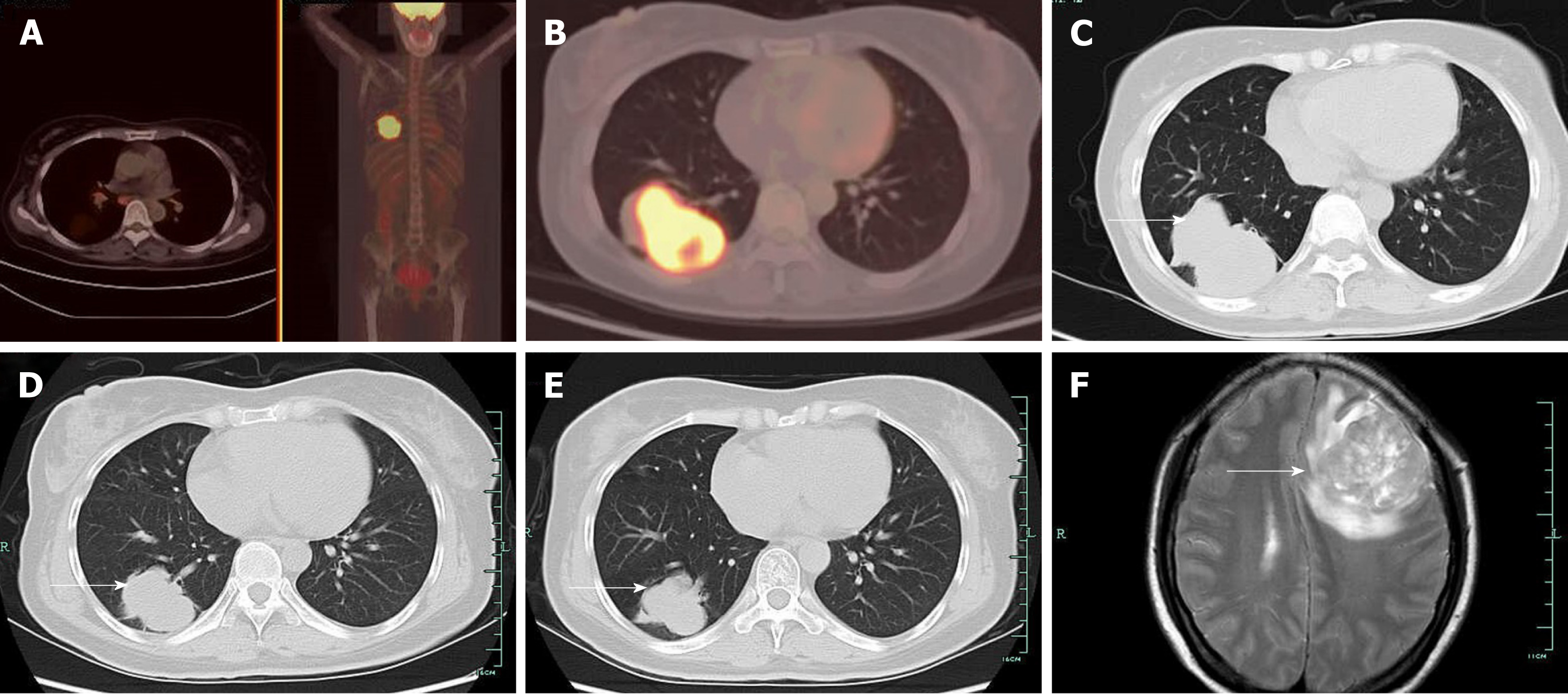
Case 1: Computed tomography (CT) showed a right pulmonary mass characterized by a solid region with contiguous ground-glass areas, stellate borders, and pleural puckering (Figure 1C). The result of endotracheal biopsy was unsatisfactory, although cytology revealed adenocarcinoma cells; however, there was insufficient sample for pathological examination and epidermal growth factor receptor (EGFR) gene testing. A positron emission tomography-CT (PET-CT) scan was performed before lung biopsy and showed hyper-metabolic activity in pulmonary lesions and the cervix uteri (Figure 1A and B).
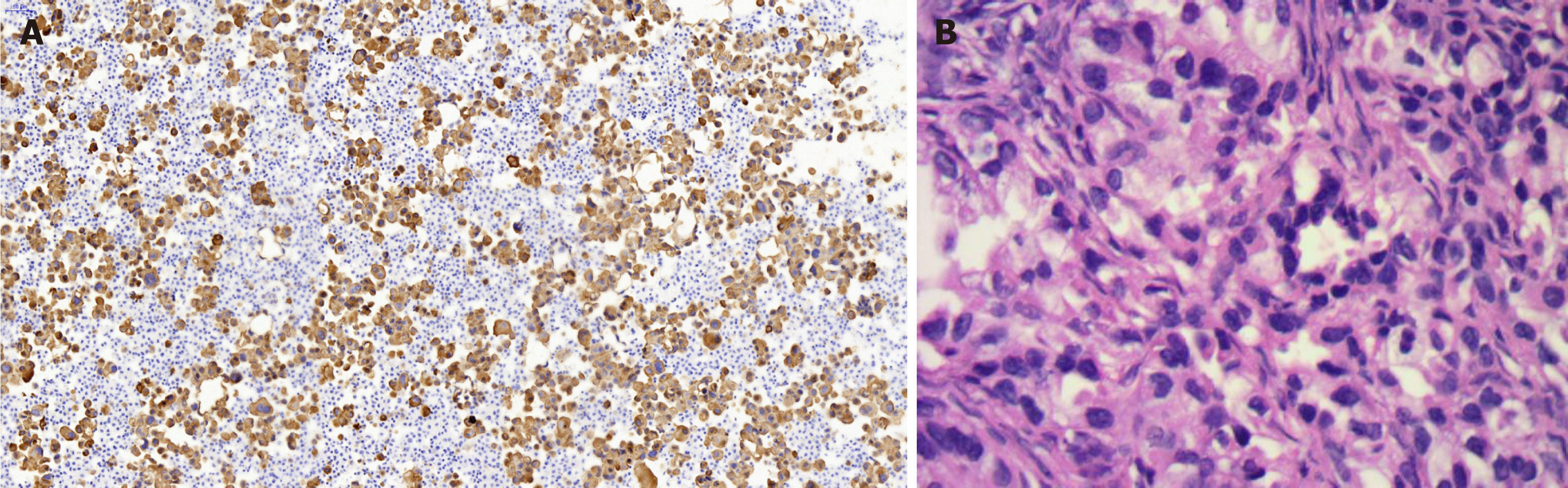
Lung biopsy was performed with CT guidance, after exclusion of relevant contraindications. Pathological examination showed adenocarcinoma with necrosis. Immunohistochemistry revealed thyroid transcription factor TTF-1(+) (Figure 2B), cytokeratin CK-7(+), and CK-20(-). EGFR mutational analysis could not be performed because we did not have sufficient biopsy specimen. Cervical biopsy was performed based on the hypermetabolic activity in the cervix uteri, and it showed poorly differentiated adenocarcinoma (Figure 2A). Immunohistochemistry showed TTF-1(+), CK-7(+), and CK-20(-). EFGR mutational analysis of the cervical biopsy specimen showed EGFR L858R mutation in exon 21. The patient displayed clinical symptoms including frequent micturition and hypogastralgia, and thus, pelvic magnetic resonance imaging (MRI) was performed, and it showed thickened cervical canal, without lymphadenectasis or pelvic effusion. Cervical cell cytology indicated negative results.
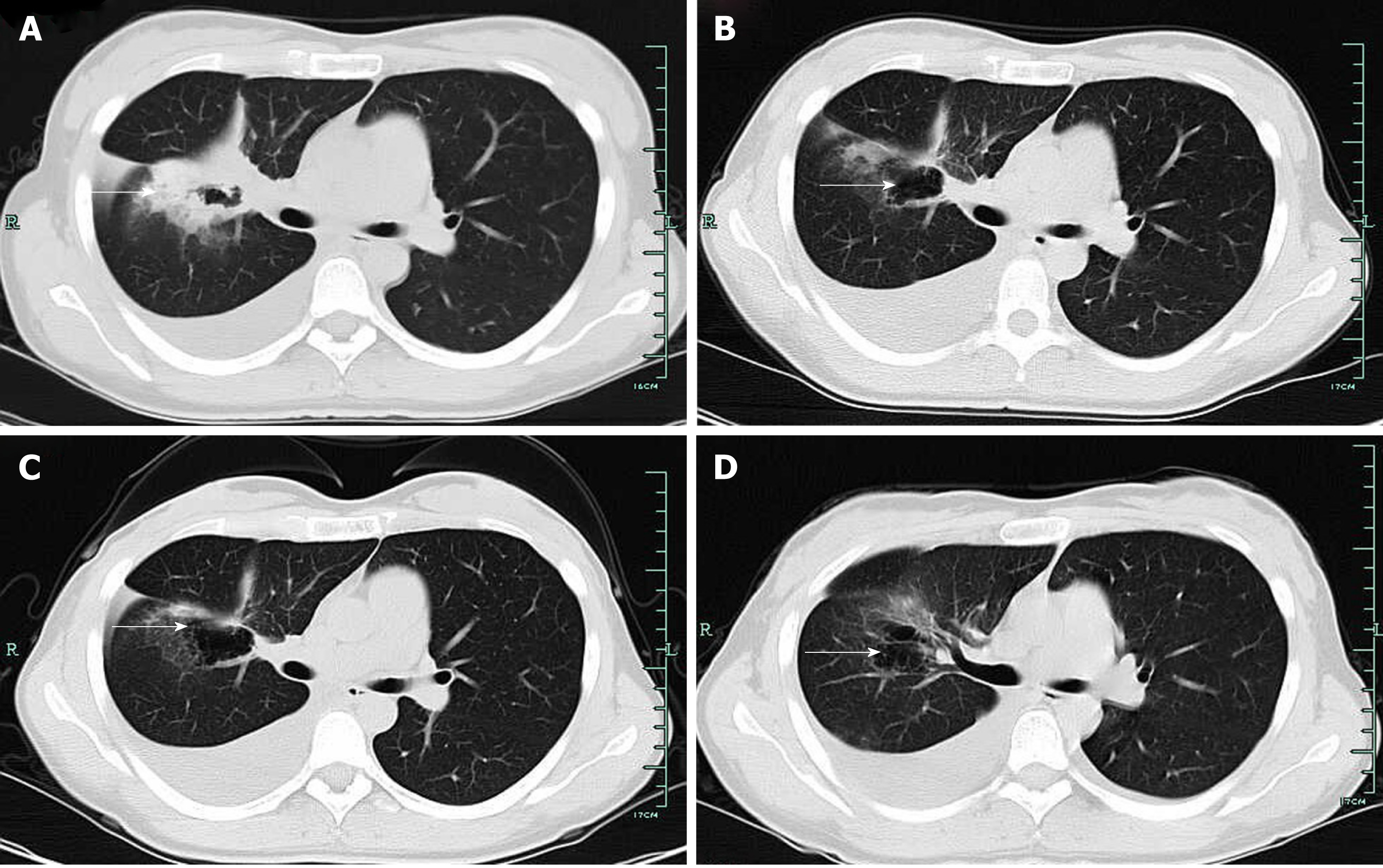
Case 2: Her initial CT scan showed lung lesions with cavity, as well as pleural effusion (Figure 3A). Ultrasound examination also showed pelvic effusion, although no ovarian masses were found. Pleural fluid cytology revealed adenocarcinoma, and immunohistochemistry revealed TTF-1(+) (Figure 4). EGFR mutational analysis of cell block showed EGFR 19-del mutation.
Lung adenocarcinoma with cervical metastasis, stage IV (cT4N3M1b), with EGFR 21 L858R mutation.
Lung adenocarcinoma with ovary metastasis, stage IV (cT2NxM1b), EGFR 19 deletion.
The patient received targeted therapy of gefitinib.
The patient was started on icotinib, which is an EGFR TKI, with a plan of sequential antiangiogenic therapy.
The patient had a positive response to gefitinib (Figure 1D and E), for both the pulmonary mass and cervical neoplasm. Routine examinations included chest CT scan and ultrasonography of the pelvic cavity and lymph nodes. Unfortunately, the patient was observed to have intracranial metastasis after 8 mo of gefitinib therapy (Figure 1F).
Targeted therapy resulted in a partial response after 3 mo (Figure 3B). Since the patient complained of repeated pleural effusion, close drainage had to be done every two months. Since March 1, 2017, the patient has been treated with bevacizumab (Avastin) and icotinib to reduce pleural effusion. Routine CT scan examination showed pleural effusion without enlargement of the tumor (Figure 3C and D). Ultrasound examination of the pelvis showed ovarian mass, as well as pelvic effusion. Ovarian biopsy was performed on September 15, 2017, which revealed adenocarcinoma. Immunohistochemistry revealed CDX2(-), TTF-1(+++), PAX8(-), CK-7(+++), CK-20(++), and Ki67(15%+) (Figure 4B). EFGR mutational analysis of the ovarian biopsy specimen showed EGFR 19-del mutation and T790M mutation in exon 20. Lung biopsy could not be performed because of obstructive pneumonia and pleural effusion. Since the EGFR TKI resistance mutation (T790M) appeared in the ovarian biopsy sample, osimertinib (Tagrisso) therapy was started (September 26, 2017).
Although metastases of lung adenocarcinoma may appear in any organ, they are more frequently observed in the bone, liver, adrenal gland, brain, and skin and seldom seen in the female genital tract, as metastasis in the female genital tract usually corresponds to small cell carcinomas[3,4]. We review the relevant studies both at home and abroad in recent years corresponding to patients with lung cancer metastasis to the female genital tract; ovarian metastases have been described in several articles[1-3,5-8], while rare cases have been reported for cervical metastasis.
Metastases to the female genital tract of lung neoplasm have not received enough attention. According to the principles of precision medicine, the initial tumor site should be considered when both pulmonary and female genital tract neoplasms exist. Immunohistochemistry and gene mutational analysis have greatly helped in locating the initial tumor site. Between 74% and 92% of lung adenocarcinoma cases exhibit TTF-1 nuclear expression, and almost 90% of patients with lung adenocarcinoma are positive for CK-7 and negative for CK-20[9]. Thus, a combination of TTF-1(+), CK-7(+), and CK-20(-) immunophenotypes is highly suggestive of primary adenocarcinoma of the lung (specificity, 100%)[5,10]. The immunohistochemical profiles of the two cases described here are indicative of metastatic lung adenocarcinoma.
In the first case, the patient did not have enough tissue sample in percutaneous lung puncture biopsy for gene mutational analysis; fortunately, cervical biopsy produced adequate tissue specimen on which we confirmed cervical metastasis by immunohistochemistry. PET-CT showed hypermetabolic activity in pulmonary lesions and the cervix uteri. According to clinical pathologists, the cervical neoplasm was a metastasis from lung cancer, which also confirmed by immunohistochemistry. Subsequent EGFR gene mutational analysis (using the Amplification Refractory Mutation System, ARMS) of the cervical tissue sample revealed EGFR L858R mutation in exon 21. The patient was then started on gefitinib and achieved partial remission after 4 mo of treatment.
In case 2, lung biopsy could not be performed because of obstructive pneumonia and pleural effusion. Pleural fluid cytology was performed on a cell block; thus, EGFR mutational analysis could be optimized. Although tissue biopsy still represents the gold standard for the diagnosis of lung cancer[11,12], it is not always possible to obtain high-quality specimens from all patients. In certain situations, liquid biopsy could be an essential tool for clinicians, especially for patients who cannot undergo invasive diagnostic procedures[11]. The patient’s disease progressed during treatment with TKI, accompanied by ovarian metastasis and bone metastasis. Since repeated biopsy of the lung mass is not recommended, analysis of the ovarian biopsy showed EGFR 19-del mutation and T790M mutation in exon 20, which represents one of the two confirmed mechanisms of drug resistance. Approximately half of the cancers that acquire resistance to EGFR TKIs develop a secondary mutation in EGFR (T790M), which abrogates the inhibitory activity of the TKI[13]. The patient was then started with osimertinib. As recently reported, osimertinib displays significantly greater efficacy than platinum therapy plus pemetrexed in patients with T790M-positive advanced non-small cell lung cancer (including cases with CNS metastases) in whom the disease has progressed during first-line EGFR TKI therapy[12,14]. Timely biopsy of the ovarian metastasis provided reference for the patient’s treatment, which greatly contributed to the patient’s prognosis.
According to the principles of precision medicine, the initial tumor site should be considered when both pulmonary and female genital tract neoplasms exist. Immunohistochemistry and gene mutational testing have greatly helped in locating the initial tumor site.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sipahi EY S-Editor: Ji FF L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Mazur MT, Hsueh S, Gersell DJ. Metastases to the female genital tract. Analysis of 325 cases. Cancer. 1984;53:1978-1984. [PubMed] [DOI] [Full Text] |
| 2. | Irving JA, Young RH. Lung carcinoma metastatic to the ovary: a clinicopathologic study of 32 cases emphasizing their morphologic spectrum and problems in differential diagnosis. Am J Surg Pathol. 2005;29:997-1006. [PubMed] |
| 3. | Fujiwara A, Higashiyama M, Kanou T, Tokunaga T, Okami J, Kodama K, Nishino K, Tomita Y, Okamoto I. Bilateral ovarian metastasis of non-small cell lung cancer with ALK rearrangement. Lung Cancer. 2014;83:302-304. [PubMed] [DOI] [Full Text] |
| 4. | Howell NR, Zheng W, Cheng L, Tornos C, Kane P, Pearl M, Chalas E, Liang SX. Carcinomas of ovary and lung with clear cell features: can immunohistochemistry help in differential diagnosis? Int J Gynecol Pathol. 2007;26:134-140. [PubMed] [DOI] [Full Text] |
| 5. | Lee KA, Lee JS, Min JK, Kim HJ, Kim WS, Lee KY. Bilateral Ovarian Metastases from ALK Rearranged Non-Small Cell Lung Cancer. Tuberc Respir Dis (Seoul). 2014;77:258-261. [PubMed] [DOI] [Full Text] |
| 6. | Min KW, Paik SS, Han H, Kim WS, Jang K. Tumour-to-tumour metastasis of lung adenocarcinoma to ovarian serous cystadenoma. J Obstet Gynaecol. 2014;34:650-651. [PubMed] [DOI] [Full Text] |
| 7. | Mushi RT, Yang Y, Cai Q, Zhang R, Wu G, Dong X. Ovarian metastasis from non-small cell lung cancer with ALK and EGFR mutations: A report of two cases. Oncol Lett. 2016;12:4361-4366. [PubMed] [DOI] [Full Text] |
| 8. | Giordano G, Cruz Viruel N, Silini EM, Nogales FF. Adenocarcinoma of the Lung Metastatic to the Ovary With a Signet Ring Cell Component. Int J Surg Pathol. 2017;25:365-367. [PubMed] [DOI] [Full Text] |
| 9. | Rossi G, Cavazza A, Righi L, Sartori G, Bisagni A, Longo L, Pelosi G, Papotti M. Napsin-A, TTF-1, EGFR, and ALK Status Determination in Lung Primary and Metastatic Mucin-Producing Adenocarcinomas. Int J Surg Pathol. 2014;22:401-407. [PubMed] [DOI] [Full Text] |
| 10. | Losito NS, Scaffa C, Cantile M, Botti G, Costanzo R, Manna A, Franco R, Greggi S. Lung cancer diagnosis on ovary mass: a case report. J Ovarian Res. 2013;6:34. [PubMed] [DOI] [Full Text] |
| 11. | Hiley CT, Le Quesne J, Santis G, Sharpe R, de Castro DG, Middleton G, Swanton C. Challenges in molecular testing in non-small-cell lung cancer patients with advanced disease. Lancet. 2016;388:1002-1011. [PubMed] [DOI] [Full Text] |
| 12. | Lam DC, Tam TC, Lau KM, Wong WM, Hui CK, Lam JC, Wang JK, Lui MM, Ho JC, Ip MS. Plasma EGFR Mutation Detection Associated With Survival Outcomes in Advanced-Stage Lung Cancer. Clin Lung Cancer. 2015;16:507-513. [PubMed] [DOI] [Full Text] |
| 13. | Xu Y, Liu H, Chen J, Zhou Q. Acquired resistance of lung adenocarcinoma to EGFR-tyrosine kinase inhibitors gefitinib and erlotinib. Cancer Biol Ther. 2010;9:572-582. [PubMed] [DOI] [Full Text] |
| 14. | Goss G, Tsai CM, Shepherd FA, Bazhenova L, Lee JS, Chang GC, Crino L, Satouchi M, Chu Q, Hida T, Han JY, Juan O, Dunphy F, Nishio M, Kang JH, Majem M, Mann H, Cantarini M, Ghiorghiu S, Mitsudomi T. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016;17:1643-1652. [PubMed] [DOI] [Full Text] |