Published online Jun 26, 2019. doi: 10.12998/wjcc.v7.i12.1393
Peer-review started: March 13, 2019
First decision: March 19, 2019
Revised: April 13, 2019
Accepted: May 10, 2019
Article in press: May 11, 2019
Published online: June 26, 2019
Processing time: 107 Days and 4.6 Hours
Acute right colonic diverticulitis (ARCD) is an important differential diagnosis of acute appendicitis (AA) in Asian countries because of the unusually high prevalence of right colonic diverticula. Due to qualitative improvement and the high penetration rate of computed tomography (CT) scanning in Japan, differentiation of ARCD and AA mainly depends on this modality. But cost, limited availability, and concern for radiation exposure make CT scanning problematic. Differential findings of ARCD from AA are based on several small studies that used univariate comparisons from Korea and Taiwan. Previous studies on clinical and laboratory differences between AA and ARCD are limited.
To determine clinical differences between AA and ARCD for differentiation of these two diagnoses by creating a logistic regression model.
We performed an exploratory single-center retrospective case-control study evaluating 369 Japanese patients (age ≥ 16 years), 236 (64.0%) with AA and 133 (36.0%) with ARCD, who were hospitalized between 2012 and 2016. Diagnoses were confirmed by CT images. We compared age, sex, onset-to-visit interval, epigastric/periumbilical pain, right lower quadrant (RLQ) pain, nausea/vomiting, diarrhea, anorexia, medical history, body temperature, blood pressure, heart rate, RLQ tenderness, peritoneal signs, leukocyte count, and levels of serum creatinine, serum C-reactive protein (CRP), and serum alanine aminotrans-ferase. We subsequently performed logistic regression analysis for differentiating AA from ARCD based on the results of the univariate analyses.
In the AA and ARCD groups, median ages were 35.5 and 41.0 years, respectively (p=0.011); median onset-to-visit intervals were 1 [interquartile range (IQR): 0-1] and 2 (IQR: 1-3) days, respectively (P < 0.001); median leukocyte counts were 12600 and 11500/mm3, respectively (P = 0.002); and median CRP levels were 1.1 (IQR: 0.2-4.1) and 4.9 (IQR: 2.9-8.5) mg/dL, respectively (P < 0.001). In the logistic regression model, odds ratios (ORs) were significantly high in nausea/vomiting (OR: 3.89, 95%CI: 2.04-7.42) and anorexia (OR: 2.13, 95%CI: 1.06-4.28). ORs were significantly lower with a longer onset-to-visit interval (OR: 0.84, 95%CI: 0.72-0.97), RLQ pain (OR: 0.28, 95%CI: 0.11-0.71), history of diverticulitis (OR: 0.034, 95%CI: 0.005-0.20), and CRP level > 3.0 mg/dL (OR: 0.25, 95%CI: 0.14-0.43). The regression model showed good calibration, discrimination, and optimism.
Clinical findings can differentiate AA and ARCD before imaging studies; nausea/vomiting and anorexia suggest AA, and longer onset-to-visit interval, RLQ pain, previous diverticulitis, and CRP level > 3.0 mg/dL suggest ARCD.
Core tip: Right colonic diverticulitis is an important differential diagnosis of appendicitis in Asian countries because of the unusually high prevalence of right colonic diverticula; however, studies reporting clinical differentiation between appendicitis and right colonic diverticulitis are still limited. Our case-control study using a logistic regression model shows that nausea/vomiting [odds ratio (OR): 3.89] and anorexia (OR: 2.13) suggest that appendicitis is more likely. On the other hand, longer onset-to-visit interval (OR: 0.84), right lower quadrant pain (OR: 0.28), history of diverticulitis (OR: 0.034), and CRP level > 3.0 mg/dL (OR: 0.25) suggest that right colonic diverticulitis is more likely.
- Citation: Sasaki Y, Komatsu F, Kashima N, Sato T, Takemoto I, Kijima S, Maeda T, Ishii T, Miyazaki T, Honda Y, Shimada N, Urita Y. Clinical differentiation of acute appendicitis and right colonic diverticulitis: A case-control study. World J Clin Cases 2019; 7(12): 1393-1402
- URL: https://www.wjgnet.com/2307-8960/full/v7/i12/1393.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i12.1393
Because of the unusually high prevalence of right colonic diverticulosis in Asian countries[1-3], acute right colonic diverticulitis (ARCD) is a very important differential diagnosis of acute appendicitis (AA) in Asian countries[4,5]. Thanks to qualitative improvement and the high penetration rate of computed tomography (CT) scanning in Japan[6], differentiation of ARCD and AA mainly depends on this modality. However, cost, limited availability in primary care settings[7], and concern for radiation exposure in young patients[8] make CT scanning problematic. Although prolonged pain, initial right lower quadrant (RLQ) pain, lack of migration of pain, leukocytosis, nausea/vomiting, constipation, and systemic toxic signs have been proposed as differential findings of ARCD from AA[4,9-12], these findings are based on several small studies using univariate comparisons from Korea and Taiwan[9-12]; we could not find any previous published studies from Japan or confounder-adjusted studies. Therefore, this study aimed to reveal useful clinical differentiation points between AA and ARCD using a logistic regression model that adjusted for confounders based on Japanese data. Given the limitations of CT scanning described above[7,8], evidence on the clinical differences between ARCD and AA may be useful to clinicians.
In this exploratory single-center retrospective case-control study, we evaluated medical records from patients of the Toho University Medical Center Omori Hospital, which has 948 beds and is located in Tokyo, Japan. The ethics committee of Toho University Medical Center Omori Hospital approved the study’s protocol (M17057). Patients were enrolled if they were ≥ 16 years old and hospitalized for AA or ARCD between January 2012 and December 2016. All patients were Japanese (immigrants or tourists were not included). Diagnoses were confirmed by CT scans in all cases and for both groups. We included both simple and complicated appendicitis in the AA group. Patients with a history of appendectomy were excluded. We included both simple and complicated right colonic diverticulitis in the ARCD group.
The patients’ medical records were searched to collect data from their first visit, such as age, sex, time interval from the onset of symptoms until the time of the visit (onset-to-visit interval), epigastric/periumbilical pain, RLQ pain, nausea/vomiting, diarrhea, anorexia, medical history (of previous AA treated without appendectomy, previous acute diverticulitis (including any parts of the colon), diabetes, hypertension, hyperlipidemia, liver cirrhosis, hemodialysis, chronic lung diseases, malignant tumors, immunosuppressant use, and antiplatelet use), body temperature, blood pressure, heart rate, RLQ tenderness, peritoneal signs, leukocyte count, levels of serum creatinine, serum C-reactive protein (CRP), and serum alanine aminotransfe-rase (ALT), and findings of CT and ultrasonography at admission. Body temperature was measured at the axilla with an electric thermometer (Terumo, Tokyo, Japan). We reviewed whether the patients had a history of acute appendicitis that was treated without appendectomy because previous appendicitis is a well-known risk factor of recurrent appendicitis if appendectomy was not performed[13]. Although ALT has not been reported as a potential confounder in any previous studies, we collected and evaluated the ALT level to ensure that liver function abnormality was not a confoun-der in this study.
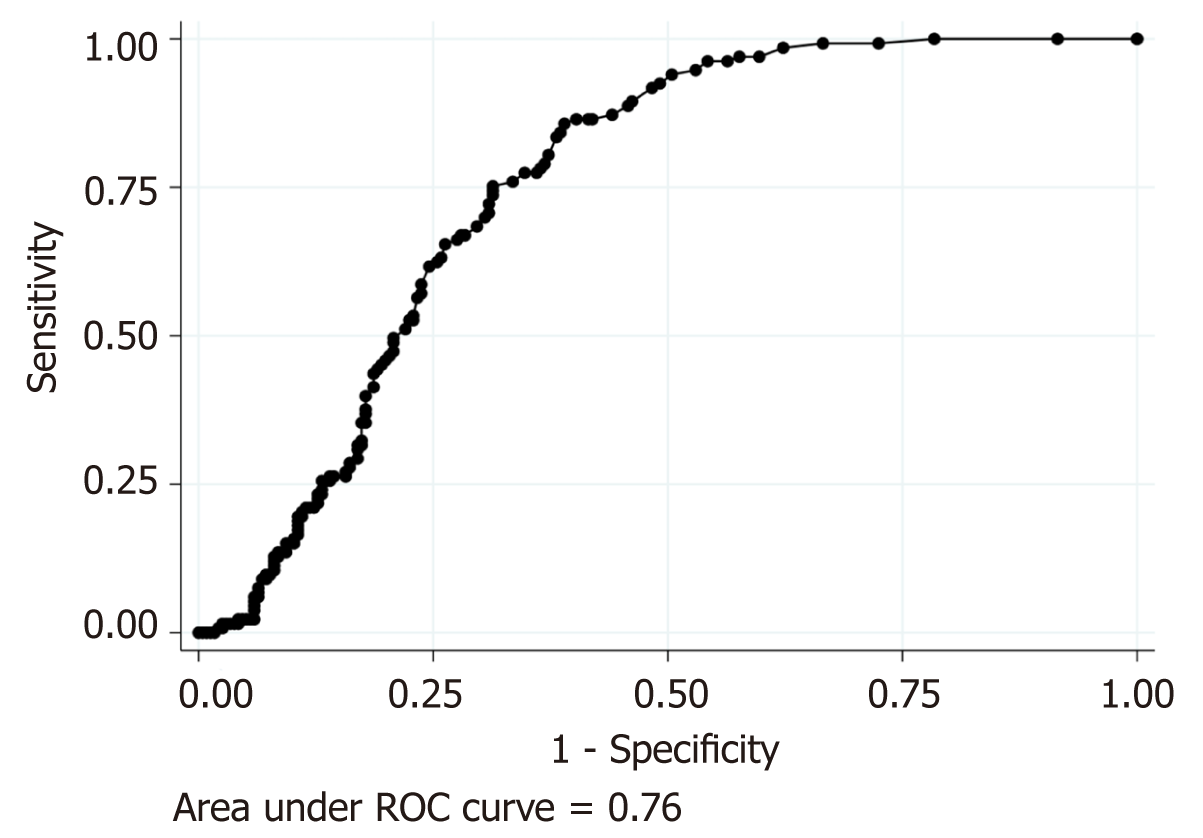
All continuous variables except for onset-to-visit interval were categorized for statistical analyses as follows. Fever was defined as an axillary measured body temperature ≥ 38.0°C[9]. Shock was defined as systolic blood pressure < 12.0 kPa (< 90 mmHg)[14]. Tachycardia was defined as heart rate ≥ 100 beats per minute. Leukocytosis was defined as a leukocyte count > 11000/mm3[15]. Elevated liver enzyme was defined as ALT > 29 IU/L[16]. Renal dysfunction was defined as a serum creatinine level > 1.2 mg/dL because of difficulty with retrospectively obtaining the estimated glomerular filtration ratio. Because we could not find previous studies that defined a specific cut-off of age groups, we divided the patients into age groups based on the median age of the patients as follows: young, ≤ 40 years and old, > 40 years; we did not use receiver operator characteristic (ROC) analysis of age for predicting ARCD because it was poorly accurate [area under the curve (AUC) was 0.41]. CRP was categorized as low: ≤ 3.0 mg/dL or high: > 3.0 mg/dL because ROC analysis of CRP for predicting ARCD showed that a CRP level of 3.0 mg/dL had the best corrective classification as much as 71.0% (AUC, 0.76; sensitivity, 75.2%; specificity, 68.6%; Figure 1).
Univariate comparisons: We compared all evaluated patient characteristics with AA and ARCD to select candidates of independent variables of logistic regression. The chi-square test was used for all dichotomous/categorical variables, while the Wilcoxon rank-sum test was used for continuous variables because of their skewed distributions.
Logistic regression model: Logistic regression analysis was subsequently performed based on the results of the univariate analyses. As mentioned above, we converted all continuous variables, except for onset-to-visit interval, into categorized variables for logistic regression. We examined the variance inflation factors (VIF) to evaluate multicollinearity of the regression model.
Discrimination, calibration, and internal validation of the regression model: We performed discrimination of the regression model by creating an ROC curve. We also calibrated the model using the Hosmer-Lemeshow (HL) goodness of fit test. Finally, we performed internal validation by bootstrap methods with 100 samples for 5 times.
All statistical analyses were performed using Stata/IC software (version 15.1; Stata Corp., College Station, TX, USA). A P-value < 0.05 was considered statistically significant. The statistical methods of this study were reviewed by Takuhiro Moro-mizato from the Internal Medicine Department, Renal and Rheumatology Division of the Okinawa Nanbu Medical Center and Children's Medical Center.
The manuscript was written according to the STROBE Statement—checklist of items.
The 369 eligible patients consisted of 236 patients (64.0%) with AA and 133 patients (36.0%) with ARCD. The median age was 38 years and 212 patients (57.5%) were men. Patient characteristics and the results of the univariate analyses are shown in Table 1. In 236 patients with AA, 38 patients (16.1%) were diagnosed with complicated appendicitis. On the other hand, 10/133 patients (7.5%) with ARCD were diagnosed with complicated right colonic diverticulitis. In 41 patients with a history of appendicitis, only 6 patients with a history of appendectomy were excluded before the study because conservative treatment for AA was the de facto standard treatment for appendicitis in our hospital; 32 and 3 patients with a history of appendicitis were eventually included in the AA group and ARCD group, respectively (P < 0.001, Table 1). The univariate analyses revealed that patients aged > 40 years were significantly less prevalent in the AA group; the median ages were 35.5 years [interquartile range (IQR), 25.0-50.5 years] in the AA group and 41.0 years (IQR, 31.0-51.0 years) in the ARCD group (P = 0.011). The onset-to-visit interval was 1 day longer in the ARCD group; the median interval was 1 d (IQR, 0-1) in the AA group and 2 d (IQR, 1-3) in the ARCD group (P < 0.001). The numbers of patients with epigastric/periumbilical pain, nausea/vomiting, anorexia, and history of unresected appendicitis were significantly higher in the AA group. Conversely, the numbers of patients with RLQ pain, history of diverticulitis, leukocytosis, and high CRP levels were significantly higher in the ARCD group. Median leukocyte counts in the AA and ARCD groups were 12600/mm3 (IQR, 10100-15200) and 11500/mm3 (IQR, 9500-13500), respectively (P = 0.002). Median CRP levels in the AA and ARCD groups were 1.1 mg/dL (IQR, 0.2-4.1) and 4.9 mg/dL (IQR, 2.9-8.5), respectively (P < 0.001). Although RLQ pain was significantly prevalent in the ARCD group (AA 72.5% vs ARCD 94.0%, P < 0.001), the prevalence of RLQ tenderness was not different between the groups (AA 97.5% vs ARCD 95.5%, P = 0.31). In 65 patients with AA without RLQ pain, 61 (93.9%) had RLQ tenderness. Prevalence of fever (body temperature ≥ 38.0°C) was not significantly different between the AA and ARCD groups (AA 14.4% vs ARCD 15.0%, P = 0.87). Because the definition of fever differs among previous studies[9,11,17], we also evaluated the prevalence of fever with the definition of fever as a body temperature > 37.2°C[11] or > 37.3°C[17]. However, all results showed that the prevalence of fever was not significantly different between AA and ARCD (P = 0.77-0.78).
| Characteristics | Appendicitis (n = 236) | Diverticulitis (n = 133) | P-value |
| Age > 40 (yr) | 96.0 (40.7) | 70.0 (52.6) | 0.027a |
| Age (years) | 35.5 [25-50.5] | 41.0 [31.0-51.0] | 0.011a |
| Male sex | 129 (54.7) | 83 (62.4) | 0.149 |
| Onset-to-visit interval (d) | 1 [0-1] | 2 [1-3] | < 0.001a |
| Epigastric/periumbilical pain | 119 (50.4) | 47 (35.3) | 0.005a |
| RLQ pain | 171 (72.5) | 125 (94.0) | < 0.001a |
| Nausea/vomiting | 123 (52.1) | 19 (14.3) | < 0.001a |
| Diarrhea | 46 (19.5) | 25 (18.8) | 0.871 |
| Anorexia | 64 (27.1) | 21 (15.8) | 0.013a |
| History of unresected appendicitis | 32 (13.6) | 3 (2.3) | < 0.001a |
| History of diverticulitis | 2 (0.9) | 22 (16.5) | < 0.001a |
| Diabetes mellitus | 11 (4.7) | 2 (1.5) | 0.114 |
| Hypertension | 24 (10.2) | 14 (10.5) | 0.914 |
| Dyslipidemia | 21 (8.9) | 15 (11.3) | 0.459 |
| Liver cirrhosis | 0 | 1 (0.8) | 0.182 |
| Hemodialysis | 0 | 1 (0.8) | 0.182 |
| Chronic lung diseases | 2 (0.9) | 1 (0.8) | 0.922 |
| Malignancy | 1 (0.4) | 2 (1.5) | 0.267 |
| Immunosuppressant use | 1 (0.4) | 3 (2.3) | 0.103 |
| Antiplatelet use | 0 | 0 | N/A |
| Fever | 34 (14.4) | 20 (15.0) | 0.869 |
| Shock | 6 (2.5) | 3 (2.3) | 0.864 |
| RLQ tenderness | 230 (97.5) | 127 (94.5) | 0.306 |
| Peritoneal signs | 137 (58.1) | 72 (54.1) | 0.466 |
| Leukocytosis | 159 (67.4) | 72 (54.1) | 0.012a |
| Leukocyte count (103/mm3) | 12.6 [10.1-15.2] | 11.5 [9.3-13.5] | 0.002a |
| High CRP level (> 3.0 mg/dL) | 74 (31.4) | 98 (73.7) | < 0.001a |
| CRP level (mg/dL) | 1.1 [0.2-4.1] | 4.9 [2.9-8.5] | < 0.001a |
| Creatinine level > 1.2 (mg/dL) | 7 (3.0) | 5 (3.8) | 0.68 |
| ALT level > 29 (IU/L) | 38 (16.1) | 24 (18.1) | 0.632 |
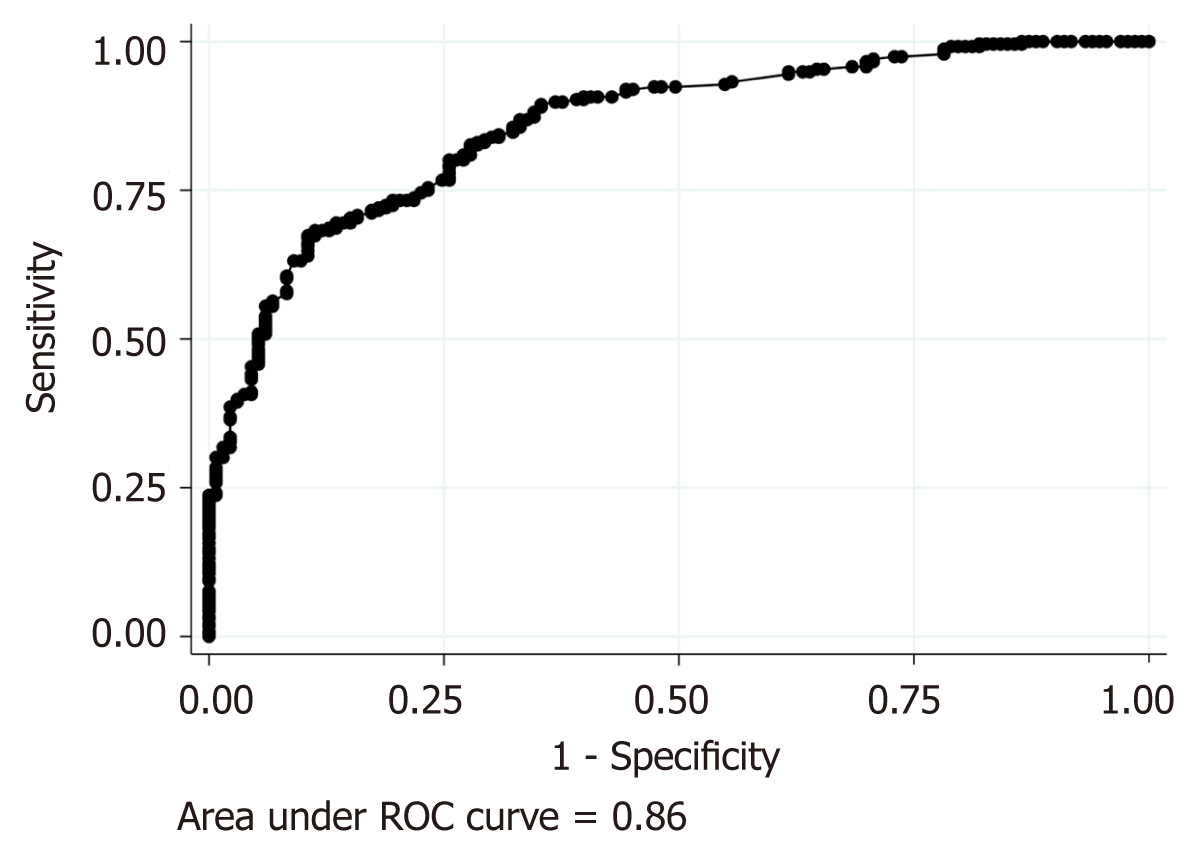
On the basis of the results of univariate analysis, we performed logistic regression analysis to compare AA and ARCD using the following ten factors as explanatory factors: Age, onset-to-visit interval, epigastric/periumbilical pain, RLQ pain, nausea/ vomiting, anorexia, history of unresected appendicitis, history of acute appendicitis, leukocytosis (leukocyte >11000/mm3), and high CRP level (> 3.0 mg/dL). As shown in Table 2 and Figure 2, the logistic regression revealed that nausea/vomiting and anorexia had significantly high odds ratios (ORs), suggesting that AA is more likely. On the other hand, longer onset-to-visit interval, RLQ pain, history of diverticulitis, and high CRP level had significantly low ORs, suggesting that ARCD is more likely. Age, epigastric/periumbilical pain, history of unresected appendectomy, and leukocytosis were not significant. The regression model showed good calibration (HL chi-square: 8.14, P = 0.42) and good discrimination (AUC = 0.86, Figure 3), and there was no multicollinearity because the VIF of all explanatory variables were 1.2 or less and the mean VIF was 1.10. Optimism, as calculated by the bootstrap method, was 0.00003.
| OR [95%CI] | P-value | |
| Age >40 (yr) | 0.62 [0.35-1.08] | 0.093 |
| Onset-to-visit interval (d) | 0.84 [0.72-0.97] | 0.021a |
| Epigastric/periumbilical pain | 1.14 [0.65-2.00] | 0.64 |
| RLQ pain | 0.28 [0.11-0.71] | 0.007a |
| Nausea/vomiting | 3.89 [2.04-7.42] | < 0.001a |
| Anorexia | 2.13 [1.06-4.28] | 0.033a |
| History of unresected appendicitis | 3.09 [0.82-11.63] | 0.095 |
| History of diverticulitis | 0.034 [0.0059-0.20] | < 0.001a |
| Leukocytosis | 1.50 [0.86-2.60] | 0.15 |
| High CRP level (>3.0 mg/dL) | 0.25 [0.14-0.43] | < 0.001a |
Previous studies have reported prolonged pain and higher age as predictors of ARCD and nausea/vomiting and leukocytosis as predictors of AA; most of our results were consistent with the results of previous studies[9-12]. On the other hand, history of diverticulitis, RLQ pain, and high serum CRP levels have not been previously established as predictors of ARCD.
In order to discuss the clinical differences of AA and ARCD, we would like to begin with discussing the association between clinical findings and the pathophysiologies of AA and ARCD, especially the differences between them, because we think that clinical differences are based on differences in pathologies of AA (localized peritonitis following intraluminal pressure elevation of the appendix) and diverticulitis (localized peritonitis due to microperforation of the affected diverticulum).
While contradicting evidence has been proposed[18], most cases of AA are traditionally thought to be initiated by elevation of the intraluminal pressure of the appendix (with concurrent inflammation), which can be caused by luminal obstruction associated with fecalith, enlarged lymphoid tissue, barium, worms, tumors, or appendiceal ulcer due to unknown etiology[18,19]. These conditions cause poorly localized visceral epigastric/periumbilical pain that is conducted by slow-conducting C fibers that enter the spinal cord at T8-T10[19,20]. Anorexia, nausea, and vomiting soon follow as the distension exacerbates[21]. Persistent elevation of intraluminal pressure of the appendix causes ischemia and subsequently proceeds to necrosis of the appendix and localized peritonitis around the adjacent parietal peritoneum, which cause somatic pain localized to the RLQ that is conducted by A delta fibers (fast-conducting and unilateral)[19]. Previous studies comparing AA and ARCD reported that the prevalence of the migration of pain in AA was 39.2%-82.0%, which is a significantly higher prevalence than that in ARCD (15.4%-54.0%)[9-11]. Another review on AA reported that the sensitivity and specificity of the migration for the diagnosis of AA were 64% and 82%, respectively[21]. Although we lacked the prevalence of the migration of pain in the present study, our result indicating a significantly high OR of nausea/vomiting and anorexia in the logistic regression model for differentiating AA from ARCD is compatible with the generally accepted pathophysiology described above and that discussed in previous studies. In four previous studies, two studies showed a significantly high proportion of nausea/ vomiting in AA cases; the proportions were 8%-16% in ARCD groups and 32%-72% in AA groups[10,12]. The proportions were insignificant in the other two studies[9,11].
On the other hand, acute diverticulitis is thought to be caused by localized peritonitis around the diverticulum due to micro-macroperforation from invasion or ischemia of the affected diverticulum via fecalith[22,23]. Therefore, acute diverticulitis generally presents as “localized peritonitis” without the preceding phase of symptoms caused by visceral nerve stimulation. This difference of pathophysiology from AA may explain the reason why our study showed that RLQ pain, a typical symptom of localized peritonitis around ascending colonic diverticula, was more prevalent in ARCD at the time of visit while some patients with AA visited the hospital due to other symptoms associated with elevated intraluminal pressure, such as nausea/vomiting or epigastric/periumbilical pain before complaining of RLQ pain. The results of comparing the prevalence of RLQ pain between AA and ARCD patients differs among previous studies[9,11,12]. Our logistic regression model showed a significantly lower OR, implying that RLQ pain was a better predictor of ARCD.
Although a previous study showed that ARCD had prolonged symptoms compared to AA (ARCD 68.4 ± 23.3 h vs AA 29.8 ± 20.2 h, P < 0.01)[9], we could not find the cause of the difference. We think that rapid progression of AA may explain the shorter onset-to-visit interval in AA cases; approximately 90% of patients with AA reportedly developed localized inflammation or necrosis within 24 h after onset of symptoms[24]. Namely, rapid exacerbation and changing symptoms might motivate patients to visit the hospital early.
Diagnostic ability of CRP in AA patients has been extensively studied[25]. Although it is frequently elevated, recent reviews have concluded that serum CRP has insufficient diagnostic utility of simple appendicitis, especially in the early phase[8,25]. Some studies showed that an elevated CRP level and persistent elevation of the CRP level reportedly serve as predictors of perforated appendicitis or appendicitis complicated with an intra-abdominal abscess[8,25]. On the other hand, recent reviews concluded that the CRP level is associated with diverticulitis severity[26]. Similar to appendicitis, the CRP level was also significantly higher in patients who required surgery or cases with perforated diverticulitis compared to mild or simple diverticulitis[27]. Although previous studies suggested that lack of systemic signs of toxicity and fever make ARCD more likely compared to AA[4,11], they did not mention CRP. Therefore, to the best of our knowledge, this is the first study to discuss differences of CRP between appendicitis and diverticulitis. We think our result aligned with previously explained CRP elevation in proportion to progression and severity of peritonitis or intra-abdominal inflammation[8,28]. Namely, the CRP value was lower in the AA group because it included patients with simple appendicitis who had a low CRP level due to the absence of peritoneal inflammation, while most patients with ARCD had an elevated CRP level associated with peritonitis. Considering the chronological elevation of CRP levels in AA[8,29], a longer onset-to-visit interval may confound the higher CRP at the time of visit in ARCD cases. However, our logistic regression indicated that the CRP level was independent from the onset-to-visit interval.
Previous studies have proposed leukocytosis as an important differential factor of AA rather than ARCD based on univariate analyses[9,10,12]. Our study also showed a higher leukocyte count in the AA group than in the ARCD group. However, it was not a significant predictive factor in our logistic regression model (Table 2 and Figure 2).
We believe that our findings will provide new evidence on the utility of CRP and leukocytosis for diagnosing acute abdomen. However, considering the inconsistent clinical significance of CRP for diagnosing acute abdomen in previous studies and the discrepancy of significance of leukocytosis between previous studies and the present study, a cautious attitude is required when applying our results to individual patients. We will separately discuss the heterogeneity of cases (simple and complicated) in the present study in the following paragraphs about our study limitations.
Similar to a previous study[9], our univariate comparison showed that a higher median age of patients in the ARCD group was comparable to that in the AA group. Yet, it was insignificant in logistic regression. Increase of diverticulosis in proportion to patients’ age[23] and higher prevalence of AA in younger patients[21] may explain the difference of age. On the other hand, the age of the patients with ARCD is reportedly younger than the age of patients with left colonic diverticulitis[5]. Given that our study included only patients with ARCD, this age difference among the site of diverticulum may explain the insignificance in our logistic regression.
Our study has some limitations. First, because the present study was a retrospective case-control study that used medical records, we could not collect some previously reported important information, such as parameters included in the Alvarado score[17], e.g., migration of abdominal pain or neutrophilia/left shift, despite previous studies clearly showing a high prevalence of pain migration and higher Alvarado score in AA compared to ARCD[9,11,12]. Furthermore, because the patient groups in the present study were limited to patients with AA and ARCD instead of including all patients with differential diagnoses of RLQ pain, our results should be cautiously applied to patients with RLQ pain.
As mentioned earlier, we diagnosed AA and ARCD based on the findings of CT because it has been the most common diagnostic tool of AA and ARCD in Japanese clinical practice thanks to the fact that CT is the most available modality[6]. We believe that we could appropriately diagnose AA and ARCD by CT because its respective sensitivities and specificities are reportedly 90%-100% and 91%-99% for diagnosing AA[20], and 94% and 99% for diagnosing colonic diverticulitis[30]. Of note, we lacked the confirmation of diverticula by colonoscopy or barium enema examination in the present retrospective study.
Second, the cut-off value of the CRP level in our study can be regarded as arbitrarily defined because it was not previously defined. However, we determined 3.0 mg/dL as the cut-off value based on the result of ROC analysis (Figure 1) because of the lack of commonly used cut-off values of the CRP level; the cut-off values for diagnosing AA or ARCD vary widely between 3.0 and 20 mg/dL depending on the study[8,25-27].
Third, we included 38 (16.1%) patients with complicated appendicitis in the AA group. As discussed, symptoms, physical findings, and laboratory data of uncomplicated cases should be different from those of complicated appendicitis; complicated appendicitis should manifest similarly to ARCD because of the progression of peritonitis. Thus, the mixed results in the AA group might affect the study results. We initially considered dividing the AA group into simple appendicitis and complicated appendicitis groups, as done in a previous study[10]. However, we eventually took priority in examining our simple question, “Does this patient have appendicitis or diverticulitis?” Therefore, we eventually included all cases in one AA group, regardless of complications/stages. We will evaluate the clinical differences of simple and complicated appendicitis in a separate clinical study. We also included 10 (7.5%) patients with complicated right colonic diverticulitis in the ARCD group. Although we took priority in examining appendicitis or diverticulitis in the present study, further studies on the clinical difference of simple and complicated ARCD are required. Because this study included potentially different cases in the same groups, we have to be cautious in applying our results to individual cases. Because previous studies focused on differences between each single parameter[9-12], further multivariable analysis or scoring system studies that address the limitations of the present study are warranted.
In conclusion, our logistic regression model for differentiating AA from ARCD showed that nausea/vomiting and anorexia increase the probability of AA rather than ARCD. Conversely, longer onset-to-visit interval, RLQ pain, history of diverticulitis, and CRP level > 3.0 mg/dL at the time of visit increase the probability of ARCD rather than AA (Figure 2). Because of the lack of previous studies on the clinical differences between AA and ARCD (especially from Japan), and the cost, limited availability, and concern for radiation exposure of CT scanning, we believe that our findings provide important evidence for many physicians managing acute abdominal pain.
Because of the high prevalence of right colonic diverticulosis in Asian countries, acute right colonic diverticulitis (ARCD) is an important differential diagnosis of acute appendicitis (AA) in Asian countries. However, studies on the clinical differentiation of AA and ARCD are limited.
Given the cost, limited availability in primary care settings and concern for radiation exposure in young patients of computed tomography (CT) scan, evidence on the clinical differentiation of ARCD and AA based on history, physical signs, and easily available laboratory data will be useful for clinicians who care for Asian patients with acute abdomen.
This study aimed to reveal clinical findings, such as symptoms, physical signs, and widely available laboratory data that are useful for differentiating AA from ARCD.
We performed a single-center retrospective case-control study that evaluated 236 patients with AA and 133 patients with ARCD, who were hospitalized in Toho University Medical Center Omori Hospital between 2012 and 2016. We compared patients’ characteristics, symptoms, physical signs, and widely available laboratory data. We performed logistic regression for clinical differentiation between AA and ARCD.
Median ages were 35.5 and 41.0 years in the AA and ARCD groups, respectively (P = 0.011). Median onset-to-visit intervals were 1 and 2 days in the AA and ARCD groups, respectively (P < 0.001). Prevalences of epigastric/periumbilical pain, nausea/vomiting, anorexia, and history of unresected appendicitis were significantly higher in the AA group, whereas RLQ pain and history of diverticulitis were more prevalent in the ARCD group. Median leukocyte counts in the AA and ARCD groups were 12600 and 11500/mm3, respectively (P = 0.002). Median CRP levels in the AA and ARCD groups were 1.1 and 4.9 mg/dL, respectively (P < 0.001). The logistic regression model showed a significantly high odds ratio (OR) in nausea/vomiting (OR: 3.89) and anorexia (OR: 2.13). ORs were significantly lower with a longer onset-to-visit interval (OR: 0.84), RLQ pain (OR: 0.28), history of diverticulitis (OR: 0.034), and CRP level > 3.0 mg/dL (OR: 0.25), suggesting that ARCD was more likely.
Our logistic regression model for differentiating AA from ARCD showed that nausea/vomiting and anorexia increase the probability of AA rather than ARCD. Conversely, longer onset-to-visit interval, RLQ pain, history of diverticulitis, and CRP level > 3.0 mg/dL at the time of visit increase the probability of ARCD rather than AA. Our study suggests that clinical findings can differentiate AA and ARCD based on clinical information in advance of imaging studies.
Given the lack of previous study on clinical differences between AA and ARCD, and the cost, limited availability, and concern for radiation exposure of CT scanning, our findings will provide useful evidence for physicians managing Asian patients with acute abdomen.
We acknowledge the excellent assistance of the staff of Harvard Medical School Introduction to Clinical Research Training Japan and Okinawa Asia Clinical Investigation Synergy (OACIS). Moreover, we thank Dr. Takuhiro Moromizato (Internal Medicine Department, Renal and Rheumatology Division at the Okinawa Nanbu Medical Center and Children's Medical Center) for providing assistance and reviewing the statistical analysis.
Conflicts-of-interest statement: No conflicts-of-interest.
STROBE Statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Augustin G, Wijaya R S-Editor: Wang JL L-Editor: A E-Editor: Xing YX
| 1. | Markham NI, Li AK. Diverticulitis of the right colon--experience from Hong Kong. Gut. 1992;33:547-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Narasaka T, Watanabe H, Yamagata S, Munakata A, Tajima T. Statistical analysis of diverticulosis of the colon. Tohoku J Exp Med. 1975;115:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Chan CC, Lo KK, Chung EC, Lo SS, Hon TY. Colonic diverticulosis in Hong Kong: distribution pattern and clinical significance. Clin Radiol. 1998;53:842-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 4. | Lee IK. Right colonic diverticulitis. J Korean Soc Coloproctol. 2010;26:241-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Matsushima K. Management of right-sided diverticulitis: A retrospective review from a hospital in Japan. Surg Today. 2010;40:321-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Organisation for Economic Cooperation and Development. Health at a Glance 2017: OECD indicators; 2017. Available from: https://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance-2017_health_glance-2017-en. |
| 7. | Wu HP, Lin CY, Chang CF, Chang YJ, Huang CY. Predictive value of C-reactive protein at different cutoff levels in acute appendicitis. Am J Emerg Med. 2005;23:449-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | March B, Leigh L, Brussius-Coelho M, Holmes M, Pockney P, Gani J. Can CRP velocity in right iliac fossa pain identify patients for intervention? A prospective observational cohort study. Surgeon. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Chen SC, Chang KJ, Wei TC, Yu SC, Wang SM. Can cecal diverticulitis be differentiated from acute appendicitis? J Formos Med Assoc. 1994;93:263-265. [PubMed] |
| 10. | Shin JH, Son BH, Kim H. Clinically distinguishing between appendicitis and right-sided colonic diverticulitis at initial presentation. Yonsei Med J. 2007;48:511-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Lee IK, Kim SH, Lee YS, Kim HJ, Lee SK, Kang WK, Ahn CH, Oh ST, Jeon HM, Kim JG, Kim EK, Chang SK. Diverticulitis of the right colon: Tips for preoperative diagnosis and treatment strategy. J Korean Soc Coloproctol. 2007;23:223-231. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Lee IK, Jung SE, Gorden DL, Lee YS, Jung DY, Oh ST, Kim JG, Jeon HM, Chang SK. The diagnostic criteria for right colonic diverticulitis: prospective evaluation of 100 patients. Int J Colorectal Dis. 2008;23:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Salminen P, Tuominen R, Paajanen H, Rautio T, Nordström P, Aarnio M, Rantanen T, Hurme S, Mecklin JP, Sand J, Virtanen J, Jartti A, Grönroos JM. Five-Year Follow-up of Antibiotic Therapy for Uncomplicated Acute Appendicitis in the APPAC Randomized Clinical Trial. JAMA. 2018;320:1259-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 312] [Article Influence: 44.6] [Reference Citation Analysis (2)] |
| 14. | Edelman DA, White MT, Tyburski JG, Wilson RF. Post-traumatic hypotension: should systolic blood pressure of 90-109 mmHg be included? Shock. 2007;27:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Riley LK, Rupert J. Evaluation of Patients with Leukocytosis. Am Fam Physician. 2015;92:1004-1011. [PubMed] |
| 16. | Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol. 2017;112:18-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 710] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 17. | Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med. 1986;15:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 811] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 18. | Arnbjörnsson E, Bengmark S. Role of obstruction in the pathogenesis of acute appendicitis. Am J Surg. 1984;147:390-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Silen W, Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL. Acute appendicitis and peritonitis. Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL. Harrison’s principles of internal medicine. 18th ed. New York: McGraw-Hill 2012; 2516-2519. |
| 20. | Birnbaum BA, Wilson SR. Appendicitis at the millennium. Radiology. 2000;215:337-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 343] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 21. | Wagner JM, McKinney WP, Carpenter JL. Does this patient have appendicitis? JAMA. 1996;276:1589-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Gearhart SL, Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL. Diverticular disease and common anorectal disorders. Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL. Harrison’s principles of internal medicine. New York: McGraw-Hill 2012; 2502–2510. |
| 23. | Touzios JG, Dozois EJ. Diverticulosis and acute diverticulitis. Gastroenterol Clin North Am. 2009;38:513-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Temple CL, Huchcroft SA, Temple WJ. The natural history of appendicitis in adults. A prospective study. Ann Surg. 1995;221:278-281. [PubMed] |
| 25. | Shogilev DJ, Duus N, Odom SR, Shapiro NI. Diagnosing appendicitis: evidence-based review of the diagnostic approach in 2014. West J Emerg Med. 2014;15:859-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 26. | Tan JP, Barazanchi AW, Singh PP, Hill AG, Maccormick AD. Predictors of acute diverticulitis severity: A systematic review. Int J Surg. 2016;26:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Käser SA, Fankhauser G, Glauser PM, Toia D, Maurer CA. Diagnostic value of inflammation markers in predicting perforation in acute sigmoid diverticulitis. World J Surg. 2010;34:2717-2722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Kechagias A, Sofianidis A, Zografos G, Leandros E, Alexakis N, Dervenis C. Index C-reactive protein predicts increased severity in acute sigmoid diverticulitis. Ther Clin Risk Manag. 2018;14:1847-1853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Clyne B, Olshaker JS. The C-reactive protein. J Emerg Med. 1999;17:1019-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 438] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 30. | Laméris W, van Randen A, Bipat S, Bossuyt PMM, Boermeester MA, Stoker J. Graded compression ultrasonography and computed tomography in acute colonic diverticulitis: Meta-analysis of test accuracy. Eur Radiol. 2008;18:2498-2511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |