Published online Jan 6, 2019. doi: 10.12998/wjcc.v7.i1.89
Peer-review started: August 10, 2018
First decision: October 9, 2018
Revised: November 2, 2018
Accepted: November 7, 2018
Article in press: November 7, 2018
Published online: January 6, 2019
Processing time: 147 Days and 7.7 Hours
Cardiac toxic effect of tegafur (S-1) is extremely rare, and there has been no report on this issue so far.
We herein report a typical case of single S-1 administration after radical operation for colon cancer. The patient had no background or medical history of acute coronary syndrome (ACS), and only aortic and coronary atherosclerosis was revealed by computed tomography (CT) before surgery. He complained of sternum pain during the fifth cycle of S-1 treatment. Electrocardiogram (ECG) and serum cardiac marker cardiac troponin T (cTnT) strongly suggested ACS, which was possibly caused by S-1 cardiotoxicity.
Monitoring protocols based on ECG, CT, and cTnT should be performed in real time to evaluate cardiac function during S-1 administration.
Core tip: Cardiac toxic effect of tegafur (S-1) is extremely rare, and there has been no report on this issue so far. We herein report a typical case and review the literature. The case might contribute to improving our understanding of the pharmacology and mechanism of S-1 in treating colon cancer. This report also emphasizes the group of cancer patients who have already been diagnosed with cardiovascular atherosclerosis and serves as a reminder to gastroenterologists that delicate monitoring protocols should be carried out in real time to evaluate the cardiac function of S-1-onging patients.
- Citation: Zhang SC, Yu MY, Xi L, Zhang JX. Tegafur deteriorates established cardiovascular atherosclerosis in colon cancer: A case report and review of the literature. World J Clin Cases 2019; 7(1): 89-94
- URL: https://www.wjgnet.com/2307-8960/full/v7/i1/89.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i1.89
Colon cancer is a common malignancy originating from the digestive tracts. Its worldwide incidence is increasing year by year, and it ranks the third among gastric and intestinal tumors nowadays[1]. Surgical operation is the cure, and chemotherapy is considered as a general adjuvant therapy to reduce recurrence and improve survival rate postoperatively. Tegafur/gimeracil/oteracil (S-1) is a new generation oral fluorouracil drug against gastric and colorectal cancer. Clinical data indicate that myelosuppression, gastrointestinal reaction, peripheral neurotoxicity, and liver damage are the main dose limiting toxicities. Reports on its cardiac injury are rare. In this case, non-ST segment elevation acute coronary syndrome (NSTE-ACS) was confirmed by electrocardiography (ECG) as well as the serum cardiac marker cardiac troponin T (cTnT) in a colon cancer patient during the fifth cycle of single S-1 chemotherapy.
Recurrent abdominal pain for half a month.
An 87-year-old male patient was admitted to our hospital on May 1, 2017 for recurrent abdominal pain with anal exhaust and defecation cessation for half a month.
He had a history of right inguinal herniorrhaphy 20 years ago and he denied histories of trauma, smoking or drinking, hyperlipemia, or chronic diseases such as hypertension and diabetes.
Upon physical examination, a 2 cm solid mass was found in his right lower abdomen.
Fecal occult blood test was positive. Albumin was 31.1 g/L, indicating low albumin. Serum CEA was 93.00 ng/mL (normal range: < 5.00 ng/mL) and CA199 was 113.7 U/mL (normal range: < 39.0 U/mL).
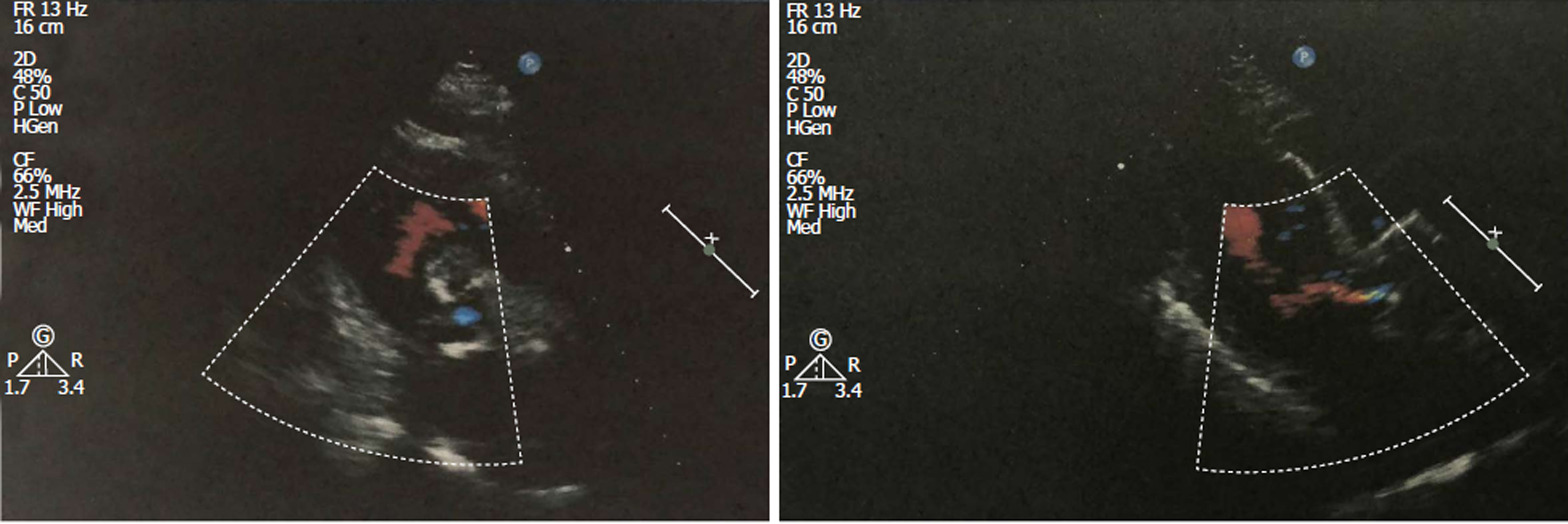
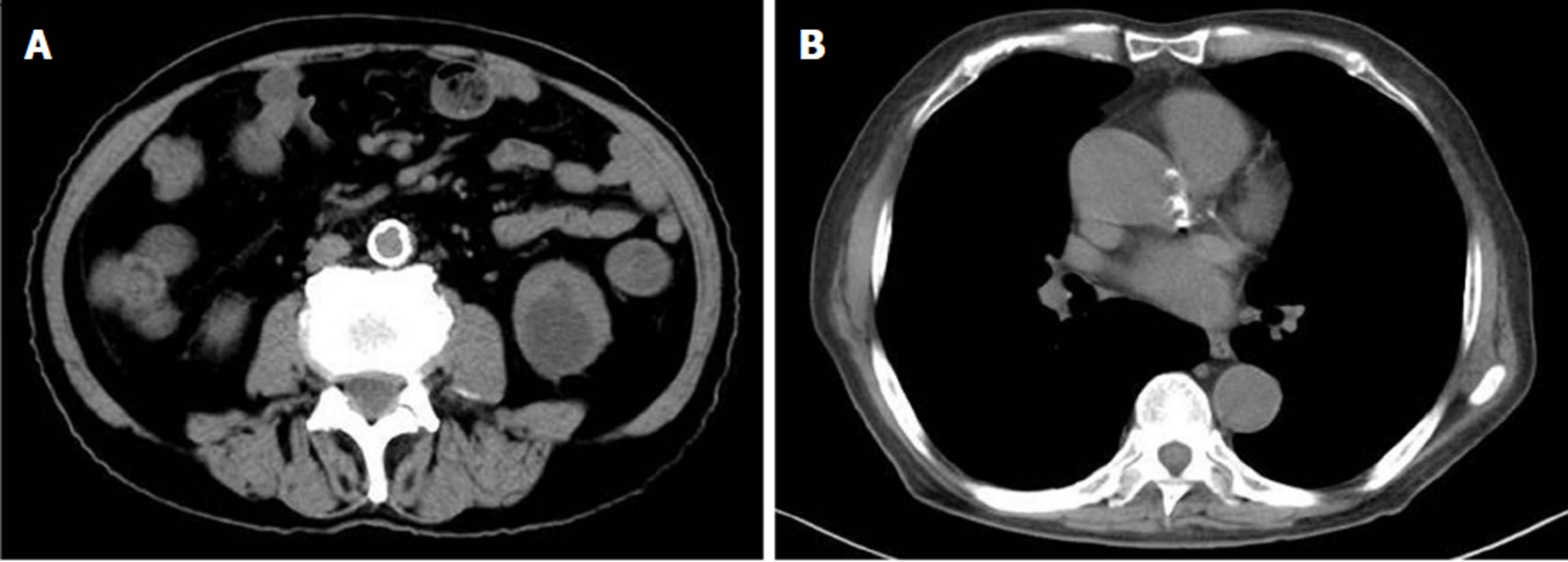
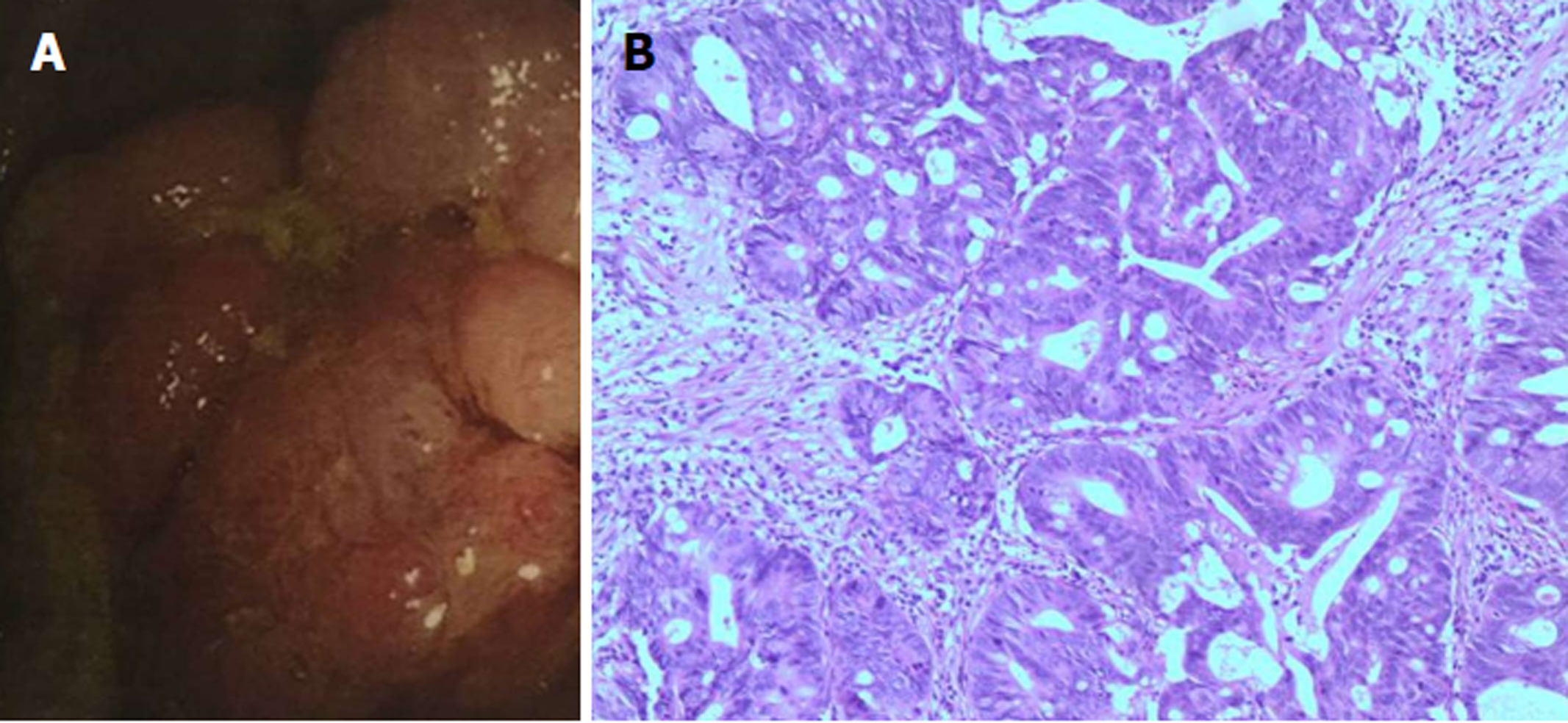
Echocardiographic examination revealed mild tricuspid and aortic regurgitation insufficiency (Figure 1). Chest and abdomen computed tomography (CT) showed a soft tissue shadow in the ileocecal junction and adjacent ileum that was surrounded by multiple swollen lymph nodes, which was highly indicative of a tumor lesion (Figure 2A). Aortic and coronary atherosclerosis was also detected (Figure 2B). Colonoscopy further confirmed an ileocecal cauliflower-like space-occupying lesion (Figure 3A), followed by a pathological diagnosis of adenocarcinoma (Figure 3B).
Ileocecal adenocarcinoma and ACS.
The patient underwent radical resection on May 4, 2017 and began S-1 capsule administration at one month after the operation.
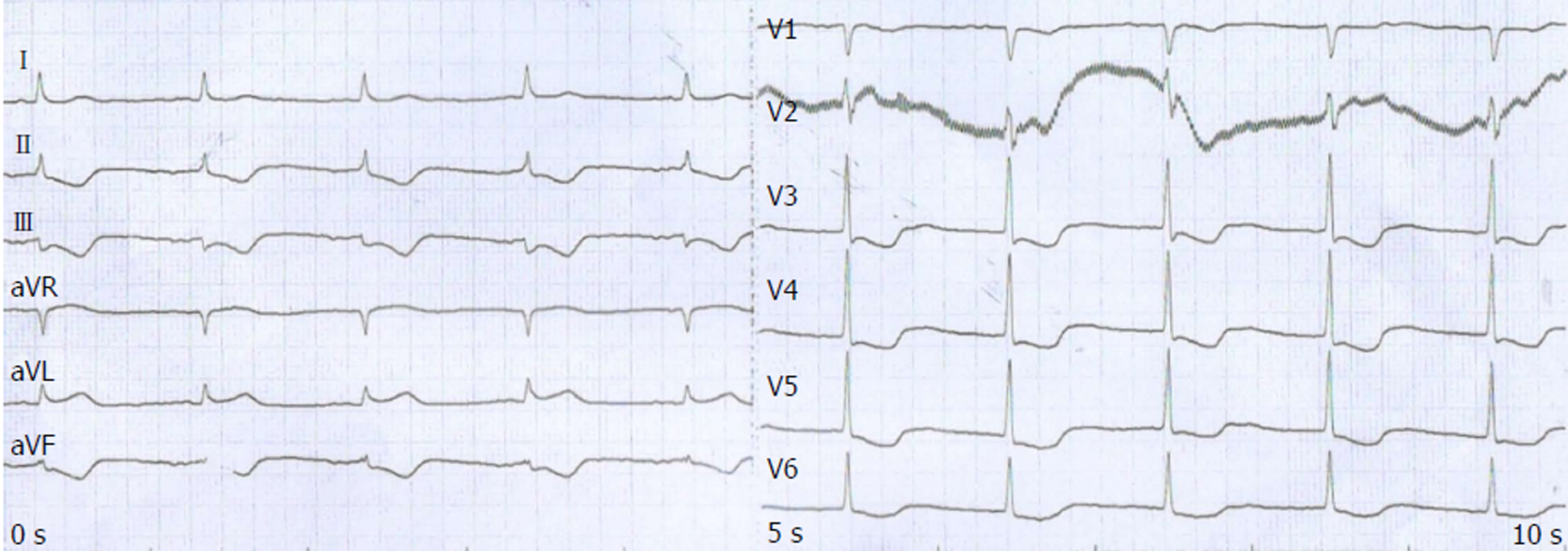
The patient periodically went back to our hospital for review. He had no documented complaints of discomfort and showed no obvious adverse reaction until the fifth cycle of single S-1 chemotherapy on October 28, 2017. He developed retrosternal pain. ECG showed extensive ST-T segment depression along with inversed T wave (Figure 4). A serial of cardiac markers were continuously detected during the onset (Table 1), among which serum cTnT and pro-brain natriuretic peptide levels were extremely elevated. In retrospect of CT scan of the heart and after consultation with cardiologists, the patient was diagnosed with NSTE-ACS. Considering D-dimer was elevated after the outbreak of ACS (1.66 mg/L; normal range: < 0.55 mg/L), antiplatelet and anticoagulant protocols were applied to stabilize plaque. Improvement of myocardial metabolism and nutritional support were also applied.
| Date | cTnI | cTnT (ng/L) | CK-MB (U/L) | Mb (μg/L) | PRO-BNP (pg/mL) | PCT (ng/mL) |
| 28/10 | - | 146.2 | 43.0 | 230 | / | / |
| 29/10 | - | 1256.0 | 50.0 | 54 | 1077 | 0.059 |
| 01/11 | - | 1102.0 | / | / | / | / |
| 06/11 | ± | 507.3 | / | / | / | / |
Adjuvant chemotherapy, such as 5-fluorouracil (5-FU), has been proven to reduce the high proportion of recurrence and metastasis of colon cancer, which are still the main cause of death after surgical resection. However, every coin has two sides. The common side effects of 5-FU are myelosuppression, diarrhea, mucositis, and hand-foot syndrome. In recent years, S-1 has become a new trend of anticancer first-line drug due to its better tolerance and fewer toxicity than 5-FU. It is a synergetic and modified agent of 5-FU consisting of the active ingredient tegafur (FT) and other two biological regulators, gimeracil (CDHP) and oteracil potassium (Oxo)[2]. As a pro-5-FU, FT preserves bioavailability and can be converted into 5-FU through oral uptake, thus interfering DNA, RNA, and protein synthesis in tumor cells. The regulator CDHP inhibits the catabolism of 5-FU released from FT under the action of dihydropyrimidine dehydrogenase. Therefore, it contributes adequate concentration and therapeutic effect of 5-FU in peripheral blood and tumor tissue, which is similar to that of continuous intravenous infusion of 5-FU. Oxo, another regulator of S-1, concentrates in gastrointestinal tissue after oral administration. Oxo blocks 5-FU phosphorylation and reduces local toxicity[3].
The patient in our case had confirmed aortic and coronary atherosclerosis by CT examination before surgery. Considering no other obvious triggers within 4 months except single S-1 administration, ACS onset was considered to be related to cardiotoxicity of S-1. According to some reports, cardiotoxicity is of less frequency but more lethal during 5-FU treatment, with a classical manifestation of angina-like chest pain. The exact pathophysiological mechanism of cardiotoxicity of 5-FU has not yet been fully elucidated. One hypothesis is that 5-FU and its metabolites (e.g., fluoroacetate) induce coronary vasospasm. This has been demonstrated in both animal models and human vascular samples during 5-FU infusion[4,5]. Another theory is that 5-FU is catabolized to alpha-fluoro-beta alanine and subsequently to fluoroacetate, which is known to be highly cardiotoxic and neurotoxic[6]. The original records on the side effects of tegafur date back to the last century. However, whether cardiotoxicity of 5-FU also applies to S-1 is barely documented.
Coronary CT angiography (CCTA) provides both detailed information on the cavity and wall of the coronary artery and the dynamic signal of blood flow in it, emphasizing its important diagnostic value for ACS. Its high-resolution images can exhibit the main branch stenosis of the coronary artery. In addition, CCTA is also applied for morphological evaluation of atherosclerotic plaque in the main coronary artery as well as its main branches[7].
ECG is a first-line diagnostic technique for patients with chest pain. It is of great clinical significance in reducing disability and mortality of ACS by early diagnosis and in patients’ better prognosis. ECG is more operable than the interventional examination and coronary angiography in ACS diagnosis. The characteristic of NSTE-ACS, ST-T alternation, such as depressed ST without ST elevation, is one of the most important indicators[8].
cTnT is a structural protein of cardiac myocytes. When cardiac cells are injured, increased membrane permeability occurs, followed by cell apoptosis or necrosis. The cytoplasmic and structural cTnT will be released into the blood. Therefore, it is considered to be the “gold standard” for diagnosing myocardial injury, especially myocardial infraction, due to its rapid increase in blood to achieve the detection sensitivity[9].
Individual susceptibility to cardiotoxicity is unpredictable when taking S-1. For those who have already been diagnosed with cardiovascular atherosclerosis and are highly likely to have a lethal strike, we recommend that delicate monitor protocols, based on ECG, CT and cTnT, should be carried out in real time to evaluate the cardiac function of S-1-onging patients. As such, high-risk patients may truly benefit from S-1 treatment since it may improve their prognosis for myocardial infarction prevention.
Individual susceptibility to cardiotoxicity is unpredictable in patients with gastric and colorectal cancer when they take S-1. Cardiotoxicity of S-1 should be considered in patients with cardiovascular atherosclerosis during anticancer therapy. Monitoring protocols based on ECG, CT, and cTnT should be carried out in real time to evaluate cardiac function during S-1 administration.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ueda H S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Bian YN
| 1. | García-Alfonso P, Grande E, Polo E, Afonso R, Reina JJ, Jorge M, Campos JM, Martínez V, Angeles C, Montagut C. The role of antiangiogenic agents in the treatment of patients with advanced colorectal cancer according to K-RAS status. Angiogenesis. 2014;17:805-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Benson AB 3rd. S-1: another oral agent for patients with colorectal cancer. Lancet Oncol. 2013;14:1244-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Kobayakawa M, Kojima Y. Tegafur/gimeracil/oteracil (S-1) approved for the treatment of advanced gastric cancer in adults when given in combination with cisplatin: a review comparing it with other fluoropyrimidine-based therapies. Onco Targets Ther. 2011;4:193-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Deboever G, Hiltrop N, Cool M, Lambrecht G. Alternative treatment options in colorectal cancer patients with 5-fluorouracil- or capecitabine-induced cardiotoxicity. Clin Colorectal Cancer. 2013;12:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Südhoff T, Enderle MD, Pahlke M, Petz C, Teschendorf C, Graeven U, Schmiegel W. 5-Fluorouracil induces arterial vasocontractions. Ann Oncol. 2004;15:661-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Leong LEX, Khan S, Davis CK, Denman SE, McSweeney CS. Fluoroacetate in plants - a review of its distribution, toxicity to livestock and microbial detoxification. J Anim Sci Biotechnol. 2017;8:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Lubbers MM, Dedic A, Kurata A, Dijkshoorn M, Schaap J, Lammers J, Lamfers EJ, Rensing BJ, Braam RL, Nathoe HM, Post JC, Rood PP, Schultz CJ, Moelker A, Ouhlous M, van Dalen BM, Boersma E, Nieman K. Round-the-clock performance of coronary CT angiography for suspected acute coronary syndrome: Results from the BEACON trial. Eur Radiol. 2018;28:2169-2175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Mueller C. Biomarkers and acute coronary syndromes: an update. Eur Heart J. 2014;35:552-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 9. | Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525-2538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1372] [Cited by in RCA: 1356] [Article Influence: 79.8] [Reference Citation Analysis (0)] |