Published online Jan 6, 2019. doi: 10.12998/wjcc.v7.i1.58
Peer-review started: July 13, 2018
First decision: October 8, 2018
Revised: November 20, 2018
Accepted: November 30, 2018
Article in press: December 1, 2018
Published online: January 6, 2019
Processing time: 175 Days and 22.9 Hours
A 43-year-old woman with an associated history of gynecological pathology and breast cancer with only one cryopreserved embryo wished to be a mother. Several factors that influenced the success of the pregnancy in this case were analyzed. Favorable factors included: triple positive breast cancer [positive hormone receptors and positive human epidermal growth factor receptor 2], which is more hormosensitive and chemosensitive; absence of metastasis; correct endometrium preparation; and the patient’s optimistic attitude and strict health habits. In contrast, the factors against success were: breast cancer; adjuvant breast cancer therapy gonadotoxicity; the age of the patient (> 40-year-old); endometriosis; ovarian cyst; hydrosalpinx; submucosal fibroids and the respective associated surgery done for the above-mentioned pathology (all resolved prior to the embryo transfer); and a low quantity of ovules (low ovarian reserve) after ovarian stimulation. This is a very special clinical case of a patient with theoretically low pregnancy success probability due to the consecutive accumulation of gynecological and oncological pathologies, who nonetheless became pregnant and delivered a full-term infant and was able to provide adequate breastfeeding.
Core tip: Considering age, endometriosis, a hyperplastic endometrium, the presence of myomas, amenorrhea, breast cancer, chemo- and radiotherapy and in vitro fertilization with a single cryopreserved embryo, the likelihood of a successful pregnancy was low. This case report details how a 43-year-old woman was able to overcome these negative aspects and become the mother of a healthy baby.
- Citation: Garrido-Marín M, Argacha PM, Fernández L, Molfino F, Martínez-Soler F, Tortosa A, Gimenez-Bonafé P. Full-term pregnancy in breast cancer survivor with fertility preservation: A case report and review of literature. World J Clin Cases 2019; 7(1): 58-68
- URL: https://www.wjgnet.com/2307-8960/full/v7/i1/58.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i1.58
There is a growing trend of longer postponement of first pregnancy from age 30 to 40. This tendency may be the result of socioeconomic factors (e.g., level of education, economic dependence on parents, employment instability, lack of a stable partner, etc.), and it has been reported to be correlated with a simultaneous increase in the incidence of breast cancer in women who have not completed their families[1]. Currently, nearly 25% of women diagnosed with breast cancer are premenopausal[2]; yet no clear recommendations are available for counselling this population on the safety of becoming pregnant after breast cancer treatment[3].
Breast cancer accounts for one-third of all neoplasms seen in reproductive-age women; it is the most commonly diagnosed cancer for this age group[4]. Among the tens of thousands of women affected each year, most are candidates for chemotherapy treatment. The most commonly used adjuvant drug treatment regimens involve a combination of chemotherapy and hormone therapy. Although this approach has improved both disease-free survival and overall survival rates in young breast cancer patients[5], the agents are well-known for their negative impact on fertility due to gonadotoxicity, which may even lead to premature ovarian failure[6]. Therefore, a diagnosis of breast cancer in a young woman represents a threat to fertility. Oncofertility counseling is of great importance in this regard[7]. These cases should be managed in a multi-disciplinary manner, involving different health care specialists - oncologists, breast surgeons, gynecologists, reproductive specialists, breast nurses, psychologists - with the aim of ensuring that an educated team can keep up with the progress of fertility preservation knowledge[8]. Providing consultation with a reproductive specialist and appropriate information on fertility preservation for these women should be an essential component of their supportive care[9].
The offspring of patients who became pregnant after completion of chemotherapy have shown no adverse effects or congenital anomalies from the treatment, but sometimes high rates of miscarriage (29%) and premature deliveries with low birth weight (40%) have been demonstrated[1]. Thus, it is important to offer early referral to a reproductive specialist to those patients at risk of infertility who are interested in fertility preservation.
There have been recent advances in the field of fertility preservation in young women undergoing breast cancer therapy that have allowed many of these breast cancer survivors to have children in the future. The most common option is embryo and oocyte cryopreservation (providing a pregnancy rate of 25%-35%), even though the suitable strategy to be chosen depends also on age, type of chemotherapy, partner status, and the patient’s motivation. Once oocytes are removed, women undergo in vitro fertilization (IVF). The IVF process involves stimulation of multiple follicles and eggs for development (ovarian induction with synthetic gonadotropins), transvaginal ultrasound-guided oocyte retrieval, fertilization of the eggs in the laboratory, and, finally, embryo transfer to the uterus, limiting the number of embryo transfers to maximum of two or three to avoid multiple gestations (which is the most frequent complication of this procedure)[10]. The most serious complication after ovarian stimulation is ovarian hyper-stimulation syndrome (characterized by ovarian enlargement due to multiple ovarian cysts and an acute fluid shift into the extravascular space)[11].
Broadly speaking, we can say that the chance of giving birth after one IVF cycle is approximately 20%-25% in women less than 35 years old, 15%-20% for those 35-39, and 6%-10% for those over 40[12]. Therefore, the age of the patient is one of the most influential factors in assisted reproductive outcomes. Nowadays, there are also other strategies for fertility preservation, some still experimental, such as ovarian tissue cryopreservation and ovarian suppression with gonadotrophin-releasing hormone agonists (GnRHa) during chemotherapy[7].
As to adjuvant radiotherapy in breast cancer, it has been shown to have no impact on the rate or clinical outcome of pregnancy, and no anatomical defects have been observed in the offspring[13]. Nevertheless, diminished lactation from the irradiated breast has been reported in those women who underwent radiotherapy following breast-conserving surgery, presumably due to atrophy of the breast lobules[14].
The following case is interesting because it is about a 43-year-old woman with an associated history of gynecological pathology, including ovarian cysts, endometriosis, hyperplastic endometrium, hydrosalpinx, myomas, and amenorrhea. On top of such negative factors for motherhood, she developed breast cancer (pT1bpN1a, stage IIA), and after chemo- and radiotherapy, she became pregnant following fertility preservation of a single cryopreserved embryo. After just one IVF cycle, she delivered a full-term infant with adequate breastfeeding.
The patient is a Caucasian woman born in 1972, middle class, with a university degree, a base body mass index of 19 kg/m² and strictly healthy hygienic-dietary lifestyle without unhealthy habits, and no relevant clinical family history. She is currently premenopausal, with regular periods and a personal history of laparoscopy due to endometriosis (endometrioma in the left ovary) at age 29. To facilitate visual understanding of the case, the chronologically ordered medical history constructed from the patient’s reporting and the information from three medical centers is presented in Figure 1. The patient has private health insurance, so she had no problem attending any of the three centers.
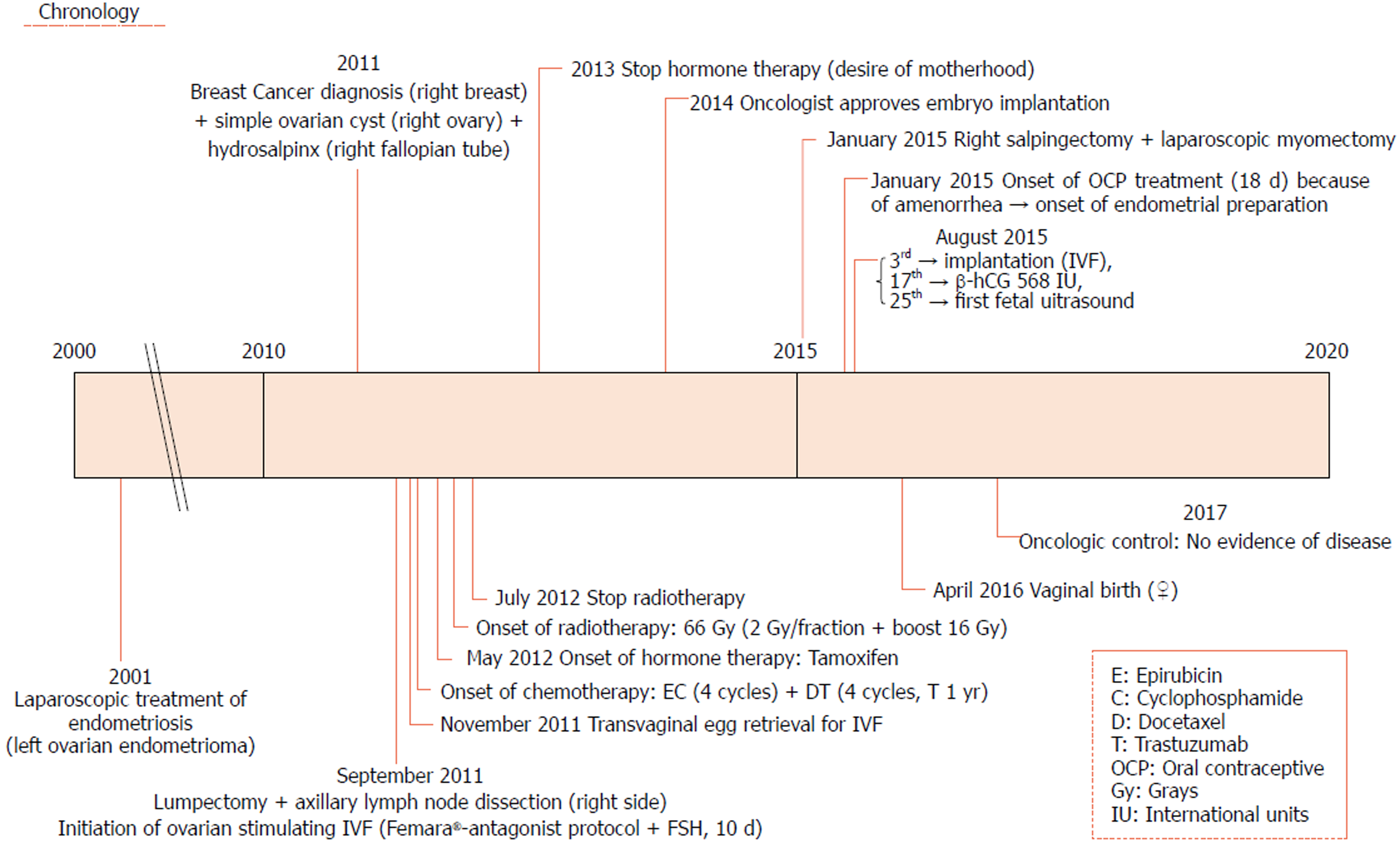
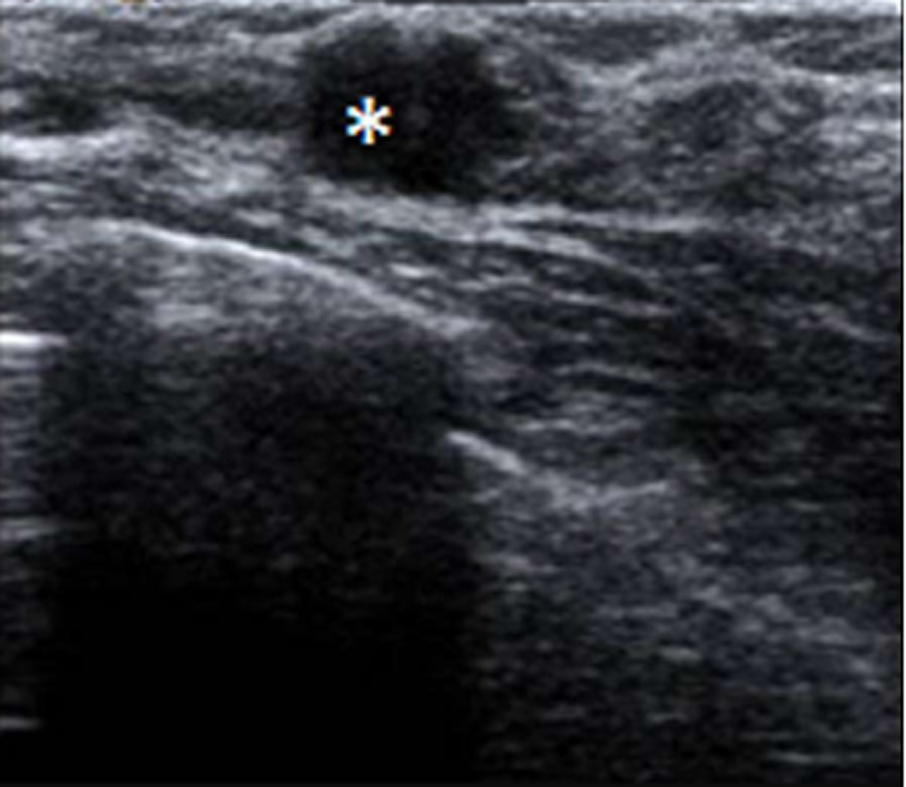
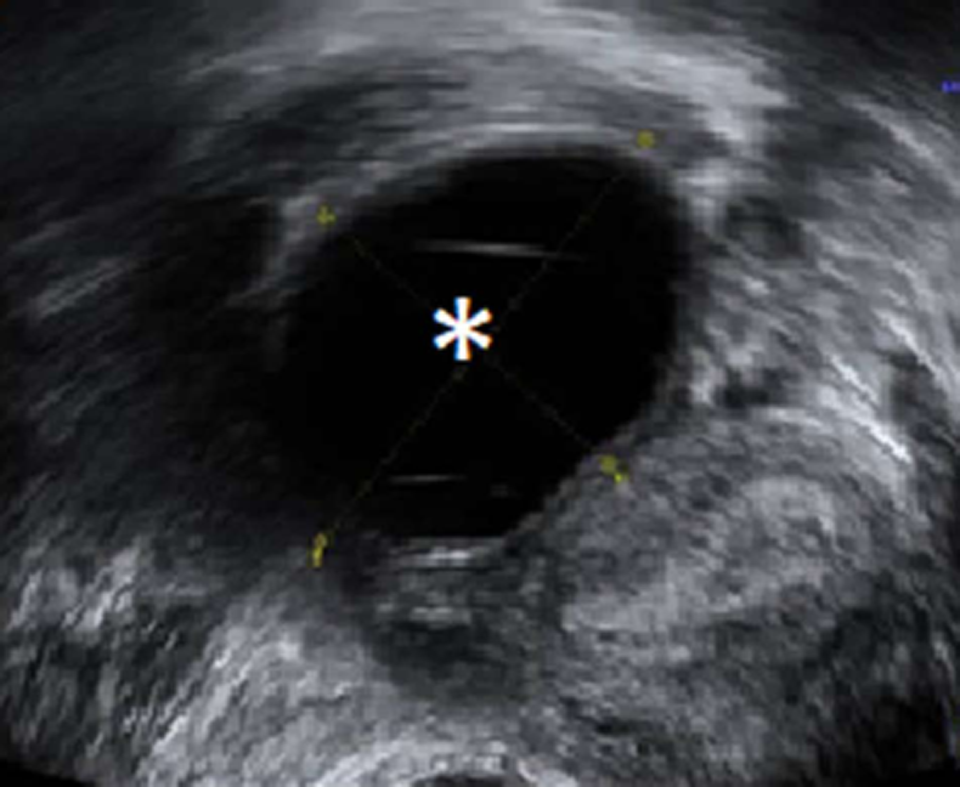
In July 2011, she went to an initial gynecological examination visit at the Hospital General de Catalunya due to self-palpation of a superior-external quadrant lump in the right breast, where a 1 cm mammary nodule compatible with a fibroadenoma was clinically evidenced. A mammography exam and a breast ultrasound were requested. These revealed a 10 mm × 10 mm nodule with radiological signs of suspected malignancy (Figure 2). Consequently, a targeted biopsy was performed evidencing a human epidermal growth factor receptor 2 (HER2)-positive hormone-dependent (80% estrogenic receptors, 70% progestational receptors) CD56 (marker for detecting neuroendocrine differentiation) negative high-grade invasive ductal carcinoma. In the gynecological visit, a transvaginal ultrasound was also performed showing an anechoic cystic image of 39 mm diameter in the right adnexal area compatible with a simple ovarian cyst versus follicular cyst (Figure 3); the rest of the exploration was anodyne. Tumor markers were studied as well with a CA-125 of 82.2 U/mL (carbohydrate antigen 125; it has been associated mainly with ovarian cancer when values are > 35 U/mL but also with other tumors such as breast cancer), with the rest of the markers normal. Subsequently, a new transvaginal ultrasound was performed evidencing a persistence of a 5 cm anechoic cyst and diagnosing a 38 mm right hydrosalpinx (Figure 4).
In September 2011, the patient underwent a lumpectomy with a sentinel lymph node dissection at the Corporació Sanitària Parc Taulí, which confirmed the histologic diagnosis of high grade (III) invasive ductal carcinoma with two axillary sentinel lymph nodes affected, classifying the tumor as pT1bpN1a, stage IIA.
With these results, staging was completed with a right axillary lymphadenectomy, without evidence of other affected lymph nodes. Thus, the patient was a candidate to receive adjuvant treatment with chemotherapy, radiotherapy, and hormone therapy for 5 years. In addition, the ovarian cyst disappeared, although the hydrosalpinx, measuring 33 mm, persisted.
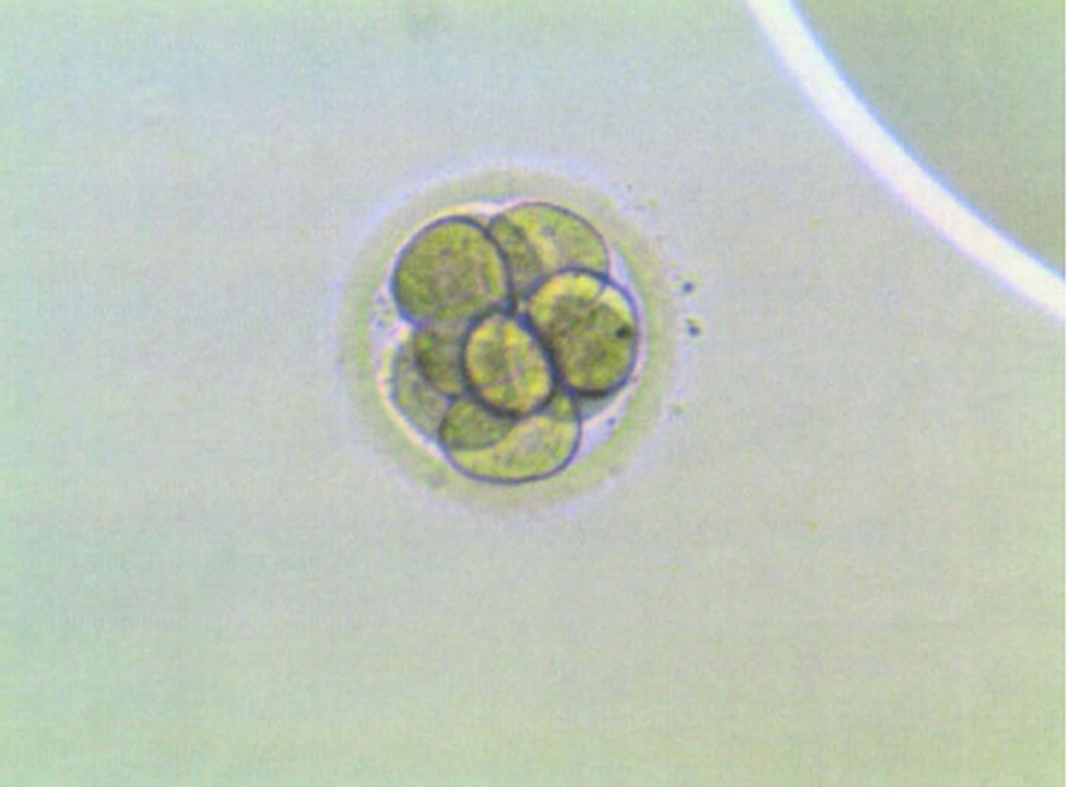
Before starting the treatment, oocyte preservation was carried out after ovarian stimulation treatment because of the patient’s desire for maternity. The patient started medication with Letrozole (Femara®) Protocol (2.5 mg/daily administration, oral route) and 225 mg of recombinant follicle-stimulating hormone (rFSH) (225 UI daily) for 10 d. Four follicles developed in the right ovary, but during the pickup it was found that they were empty. Two ovules that developed in the contralateral ovary, harder to reach, were extracted in a puncture performed on November 4, 2011 at the Cefer Institute, and after IVF, only a single day 3 embryo (type A) was available for freezing.
The patient started adjuvant treatment with chemotherapy following a scheme based on Epirubicin 90 mg/m2 and Cyclophosphamide 600 mg/m2 every 21 d up to four cycles, followed by Docetaxel 75 mg/m2 and Trastuzumab every 21 d. She completed four cycles with good patient tolerance.
In May 2012, the patient started hormone therapy with Tamoxifen 20 mg daily for 5 years, followed by subcutaneous Trastuzumab 600 mg every 3 wk until completing 1 year of treatment. Next, she received radiotherapy (complementing breast conserving surgery) with a total dose of 66 Gy (2 Gy/fraction, 5 d/wk) followed by a boost of 16 Gy over the surgical site, ending in June 2012.
In December 2013, she ended treatment with Tamoxifen due to her desire for pregnancy, considering that this drug could hinder correct embryo implantation. In total, the patient underwent eight sessions of chemotherapy, 18 sessions of monoclonal antibodies (Trastuzumab), 33 sessions of radiotherapy, and 2 years of hormone therapy.
At the end of 2014, her oncologist approved transfer of the single cryopreserved embryo. Prior to this, an ultrasound was requested that showed a hyperplastic endometrium and the hydrosalpinx, so doctors decided to carry out a hysteroscopy with uterine cavity dilation and curettage, and laparoscopic surgery for exeresis/occlusion of the affected fallopian tube.
In January 2015, a diagnostic hysteroscopy showed two small submucosal myomas, for which the patient underwent right salpingectomy and hysteroscopic myomectomy to facilitate a potential pregnancy. Having reached this point, the patient was ready to begin the embryo transfer. However, the patient began having menstrual cycle alterations (amenorrhea), so in June 2015 she started combined oral contraception with Suavuret® (Fix combination of Desogestrel 0.15 mg and 0.02 mg EthinylEstradiol, daily) for 18 d to regulate her menstrual cycle.
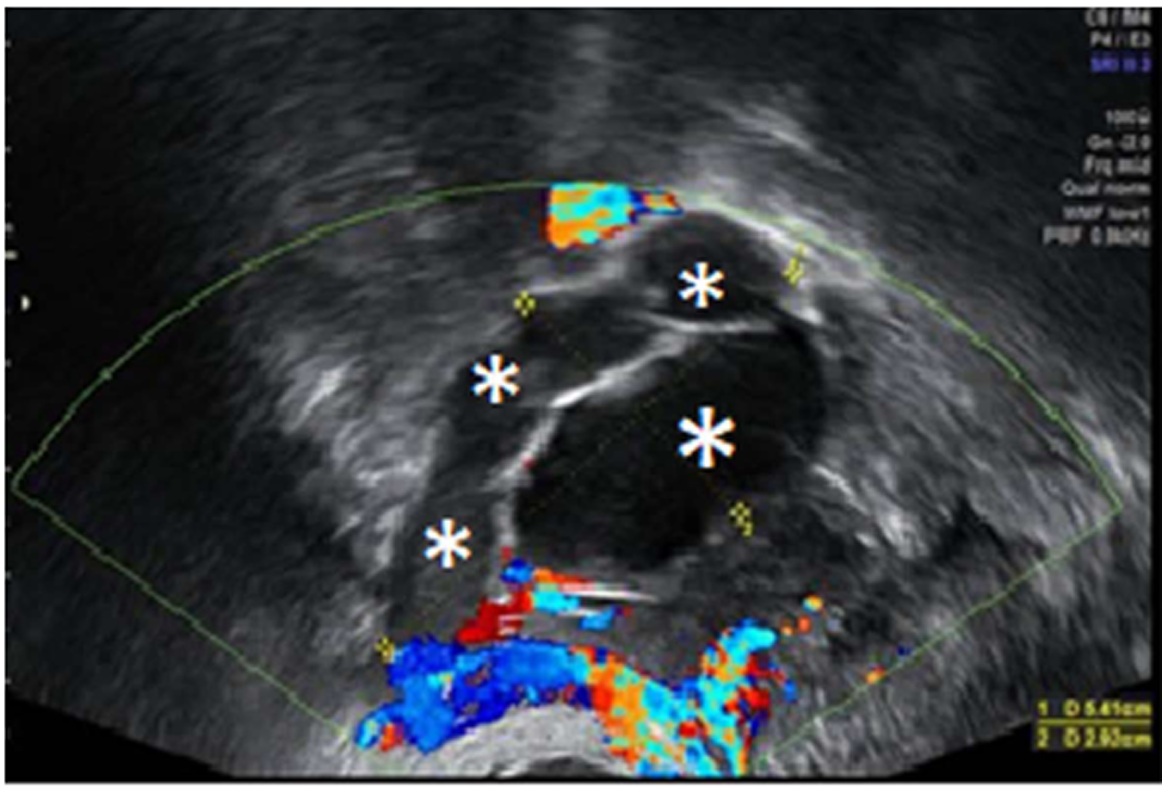
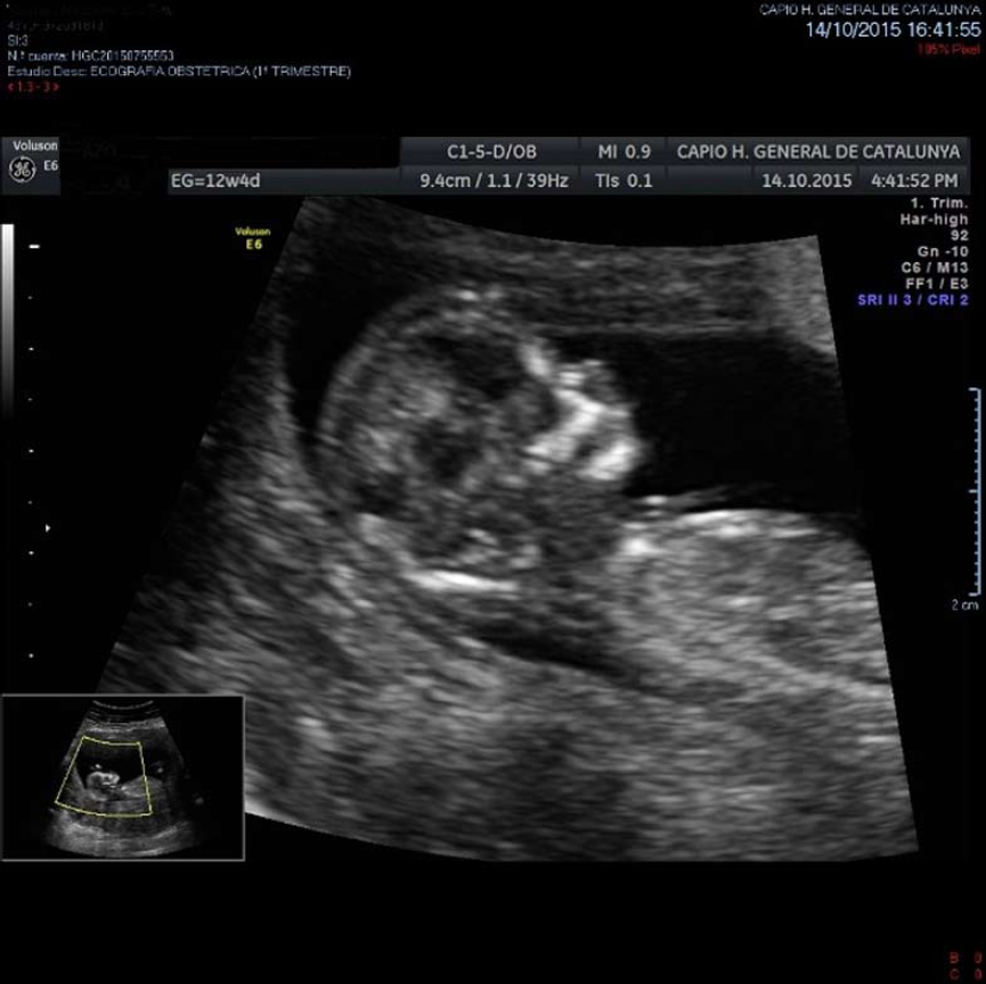
To prepare the uterine endometrium before embryo transfer and to maintain the pregnancy, on July 15 she started treatment with Progynova® 4 mg/d for 3 mo (equivalent to Estradiol Valerate; standard-dose is 6 mg/d, but due to low body mass index, the dose was lowered). Endometrium was correctly prepared, so the transfer was carried out on August 3 (when the patient was 43 years old) using an IVF reproduction technique with the vitrified embryo from the age of 39 (type A embryo transfer, with A being the best quality for an embryo to be transferred, B not as good, C poor quality, and D the worst) (Figure 5). On August 17, the biochemical pregnancy test result was positive [human chorionic gonadotropin was 568 IU]. On the 25th of the same month, the patient underwent her first gestational ultrasound showing the gestational sac with a sketch of vitelline vesicle. On September 8, at wk 7 of pregnancy, fetal heartbeat was positive, so the patient was referred to the obstetrician. Regular controls made by the obstetrician showed a correctly developing pregnancy (Figure 6).
Pregnancy was completed without complications and with a vaginal delivery (assisted Thierry’s spatulas) in April 2016, with a healthy girl born (full term pregnancy at wk 37, 2800 kg weight, and 46.5 cm length). Puerperium was normal, including 9 mo of exclusive breastfeeding. The last oncological control was in January 2017, without evidence of disease.
Hormone-dependent carcinomas, such as breast cancer, express estrogen and/or progesterone receptors and are the most hormone-sensitive tumors (response to treatment is 55% in tumors with positive hormone receptors and < 10% in tumors without them). Hormone therapy is very useful, especially in premenopausal women, so in this clinical case our patient was a candidate to receive Tamoxifen. This drug also has beneficial effects on the cardiovascular and skeletal systems (it acts as an estrogen receptor antagonist in the mammary glands but as an agonist on endometrial and bone tissue). Nevertheless, Tamoxifen has relevant side effects that include thromboembolism, ocular toxicity, and even endometrial alterations (endometrial carcinoma)[15]. Recently, aromatase inhibitor drugs (such as Letrozole) have been developed, which are basically used in postmenopausal women because they prevent synthesis of estrogen from the adrenal gland (the main source of estrogen in postmenopausal women)[16]. Compared to Tamoxifen, aromatase inhibitors induce fewer uterine cancers and thrombotic phenomena, but they increase the risk of sexual dysfunction, arthralgia, osteoporosis, and cardiocirculatory events[17].
Another relevant aspect is the presence of HER2 gene overexpression. It is important to determine whether a breast cancer is positive for HER2 or not, because nowadays there are targeted therapies[18] for this cancer type using HER2 antagonist drugs, such as Trastuzumab (the first anti-HER2 antibody). Only about 15%-20% of all breast cancer patients are positive for HER2[19]; that is to say, these are cancers that have this oncogene amplified, and, in approximately 20% of HER2 cases, tumors present hormone receptors too. Even so, this subtype of breast cancer tends to have fairly high cell proliferation rates (measured by the percentage of ki-67), which is the reason why these cancers tend to be more aggressive than tumors that are only hormone-dependent. Moreover, they are often more undifferentiated (grade 3) and present ductal invasion more frequently than tumors without overexpression of HER2. For this reason, Trastuzumab has had an enormous impact on the prognosis of the presence of HER2 in breast cancer (regardless of tumor size, lymph node involvement, presence of hormone receptors, and age of the patient)[20], since it improves the survival rate in both advanced and localized disease, and its inclusion in adjuvant chemotherapy reduces relapses by 50%. Over the years, multiple HER2-targeting drugs have entered clinical practice, for the curative as well as the metastatic situation. Nowadays, there are several advances in treatment of these patients, and new drugs have been developed such as Lapatinib, T-DM1 (Trastuzumab-Emtansina), and Pertuzumab[18].
Regarding breast cancer treatment in general and its relation to potential infertility, it is important to mention that the main risk comes from the chemotherapy regimens used in these patients. It is well known that breast surgery, radiotherapy (which is mandatory to complete organ preservation surgery), and lymphadenectomy do not affect conception ability, but lymphadenectomy is the main cause of chronic morbidity in breast surgery. Regarding chemotherapy, the current preference is to administer monotherapy-based regimens or sequential two-drug therapy. For patients who are positive for HER2, the most commonly used regimen is anthracycline + Cyclophosphamide followed by a taxane + Trastuzumab. It is important to highlight the potential cardiotoxicity of both Trastuzumab and Epirubicin (the anthracycline used in our patient), so in this case strict control of cardiovascular function is necessary. In reference to the agents commonly used for breast cancer, alkylating agents (such as Cyclophosphamide) are those with the highest gonadotoxic potential risk; Epirubicin can cause fertility problems in 10% of cases (it affects men as well as women), and taxanes can cause intermediate ovarian damage[21]. Moreover, both hormone therapy and some chemotherapeutic agents have potential teratogenic risk, so the most widely accepted recommendation is to avoid pregnancy for a minimum of 2 years after completing cancer treatment[22]. This further compromises the chance of future biological childbirth by pushing conception attempts to the later stages of a woman’s reproductive lifetime.
In addition to breast cancer, the patient also had other pregnancy-impeding factors, such as endometriosis, an ovarian cyst, hydrosalpinx, submucosal myomas, and their associated surgeries, all previously resolved before embryo transfer. Our patient underwent laparoscopic treatment of endometriosis. It has been reported that in women with minimal, mild, or severe endometriosis, surgical excision or ablation of endometriosis is recommended as a first line, with doubling of the pregnancy rate[23]. Concerning hydrosalpinx, there are cases where unilateral salpingectomy for hydrosalpinx in the presence of a contralateral healthy tube could result in spontaneous pregnancy[24]. Regarding myomas, it is worth mentioning that the number of myomas removed during myomectomy significantly affects fertility: women with > six myomas removed are less likely to become pregnant, more likely to require fertility treatment, and less likely to have a term birth when compared with women with less than six myomas removed, as was the case with our patient, from whom only two myomas were removed[25]. Moreover, it is important to highlight the age of the patient (over 40-years-old). It should be remembered that females are born with a maximum number of oogonia, and these will undergo apoptosis throughout life. To get an idea of this process, we find about 2 million oogonia at birth, and at the beginning of puberty there are only about 400000 left. Therefore, the age of the patient conditions her low ovarian reserve, making a successful pregnancy even more difficult.
Another factor against successful pregnancy is the low number of ovules produced by a patient after stimulation with Femara® Protocol and rFSH. This protocol is based on ovarian stimulation with Letrozole (an aromatase inhibitor), which produces estrogen suppression both peripherally and intratumorally. Negative feedback produced by estrogens on the hypothalamic-pituitary-gonadal axis is diminished because of its synthesis reduction, increasing gonadotropin release (FSH increases and ovulation is stimulated)[26]. Moreover, it is believed that in increasing ovarian androgens (with accumulation of androstenedione and testosterone, which are not transformed into estrogen), FSH receptor genes are amplified, with a resulting follicular sensitization to follicular development stimulus. Letrozole is combined with rFSH in patients expected to have poor response to hyperstimulation (as in this case, as the patient already had a low baseline ovarian reserve due to her age). Regarding the fact that increased estrogen levels may potentially be risky in hormone-dependent breast cancer patients (they could stimulate breast tissue growth again), recently developed ovarian stimulation protocols with aromatase inhibitors seem to provide safer ovarian stimulation with endogenous estrogen.
On the other hand, the patient had factors that favor pregnancy: she was a person with strict healthy habits, she had a triple positive breast cancer (positive hormone receptors and HER2 that made it more hormosensitive and more chemosensitive), there was no metastasis present at diagnosis, and she had a very positive attitude towards life. Regarding attitude, it is known that dispositional optimism is a predictor of quality of life preservation in women with breast cancer[27], and it favors greater adherence to treatment and better patient follow-up. Moreover, there is also a correlation between positive attitude and pregnancy success; some studies have concluded that being pessimistic may be a risk factor for IVF treatment failure, independent of other factors that may negatively affect treatment outcomes of fertility (such as age, for example)[28].
Another factor that favored a full-term pregnancy in this patient was that, despite her gynecological history, she correctly prepared the endometrium. In accordance with the endometrial preparation protocol for embryo transfer, for IVF in a natural cycle the treatment started on the 1st-2nd d of menstruation with Progynova® 2 mg every 8 h for 15 d. After these 2 wks, the patient underwent both ultrasound (to assess thickness and appearance of the endometrium) and a blood test to check estrogen levels. If everything was correct in these checks, Progesterone was added (two vaginal ovules every 8 h), and after 3-4 d, embryo transfer performed (outpatient procedure under ultrasound control). Treatment with Progesterone + Progynova® was maintained until the pregnancy test and, in case of pregnancy, until week 11-12 of pregnancy[29].
In general, the probability of natural pregnancy in a fertile woman is approximately 20%[30], and it decreases as female age increases. This fertility reduction is more pronounced from the middle of the third decade of life[31]. In addition, the chance of giving birth after one IVF cycle is approximately 6%-10% in women ≥ 40 years old, as we noted earlier.
To calculate an approximation of the probability of our patient’s having a full-term gestation; several factors had to be considered. First, there was endometriosis and surgery for it. According to some recent studies, the pregnancy rate after laparoscopic surgery for endometriosis is about 55%. However, once pregnancy is established, the prematurity risk is only slightly higher than it is for the general population, since there are no significant differences either in spontaneous abortion rate or in other maternal and neonatal complications[32]. Moreover, nowadays it is known that the presence of endometriomas has no impact on clinical IVF outcomes, and that the optimal time to perform IVF appears to be 7-25 mo after endometriosis surgery[33].
Regarding submucosal fibroids (which correspond to 5%-10% of uterine fibroids, producing more symptoms than the other fibroid types, such as metrorrhagia and infertility), myomectomy is the indicated therapeutic technique, and it is used more frequently in women with symptomatic myomas and future fertility desire. Pregnancy rates following this surgery, both via laparoscopy and laparotomy, are in the 50%-60% range[34]. However, myomectomy may not permanently eliminate the symptoms, and it is associated with risks (post-operative adhesions, for example, can negatively affect fertility) and surgical complications. Patients who undergo myomectomy for submucosal fibroid resection have higher clinical pregnancy rates compared to patients with non-operated myomas (43.3% vs 27.2%, respectively)[35].
Moreover, IVF pregnancy rates in women who undergo a salpingectomy for hydrosalpinx are about 40%, and the risk of having an ectopic pregnancy in IVF in patients who have been operated on is 2.2% (comparing with non-treated patients: 8.6%)[36].
In addition, we must also consider breast cancer and its adjuvant treatment with chemotherapy and hormone therapy. Fertility preservation for breast-cancer patients using unstimulated in vitro maturation followed by oocyte or embryo vitrification has shown calculated pregnancy rates per vitrified oocyte and embryo of 3.8% and 8.1%, respectively[37]. In some studies, it has been shown that, after chemotherapy and hormone therapy, the incidence of amenorrhea increases to 68%[1]. Up to 5% of women suffer from secondary amenorrhea during their lifetime; that is, the absence of menstrual period in a woman of reproductive age for ≥ 6 mo, for example, produced by a common cause such as stress. The importance of this is that woman suffering from secondary amenorrhea have greater difficulties becoming pregnant, although this is not a cause of infertility itself, unless the period loss is evident or is not treated for a long time. In fact, in 85% of cases, a simple change in diet or in lifestyle can rebalance the hormonal system, permitting easy and healthy gestation.
It is not possible to make a statistical calculation of the overall probability of getting pregnant (including birth of a live baby) by combining different probabilities of the discussed pathologies suffered by the patient, because data sources for each pathology are independent. Even so, this clinical case shows that despite the low probabilities of successful motherhood (due to the consecutive accumulation of gynecological and oncological pathologies), the patient eventually gave birth to a healthy baby.
In summary, we have presented a special clinical case of full-term pregnancy and successful exclusive breastfeeding during the first 9 mo of the baby’s life. This is of interest given several factors (both positive and negative) discussed in this case report, since the chances of patient pregnancy success were theoretically low. Currently, the first choice for premenopausal breast cancer patients is adjuvant therapy, and although cytotoxic drugs and/or hormone therapy can significantly reduce mortality, questions arise regarding long-term toxicity, such as menopause induction and fertility deterioration, of these treatments. Thus, in these women it is essential to propose therapies to preserve fertility before starting potentially gonadotoxic treatment as well as to refer them early on to a specialized reproduction center if there is a wish for a future pregnancy.
Full-term pregnancy success in a 43-year-old woman treated with chemotherapy and hormonotherapy for breast cancer who also had having several gynecological problems.
Endometriosis, ovarian cyst, hydrosalpinx, submucosal myomas, breast cancer, and in vitro fertilization.
Laparoscopy, lymphadenectomy, chemotherapy, radiotherapy, and hormonotherapy.
Biopsy evidencing a human epidermal growth factor receptor 2-positive hormone-dependent (80% estrogenic receptors, 70% progestational receptors) CD56 negative high grade invasive ductal carcinoma.
Ultrasound and Doppler showing ovarian cystic tumors, hydrosalpinx, and breast cancer tumor.
Pathological diagnosis showed a pT1bpN1a, stage IIA breast cancer tumor. Diagnostic hysteroscopy showed two small submucosal myomas.
Chemotherapy based on Epirubicin and Cyclophosphamide, followed by Docetaxel and Trastuzumab; Hormonotherapy based on Tamoxifen followed by subcutaneous Trastuzumab; Radiotherapy; Oral contraception based on Suavuret® (Desogestrel and EthinylEstradiol); Uterine endometrium preparation based on Progynova® (equivalent to EstradiolValerate).
The probability of natural pregnancy in a fertile woman is approximately 20%. With age and health problems such as gynecological or/and oncological, this percentage drops significantly. This case serves to maintain hope in women in a similar situation who wish for motherhood.
Successful pregnancy achieved (with healthy baby born) with only one in vitro fertilization procedure, using a single vitrified embryo at age 39, and transferred at age 43.
This is a special clinical case of a patient with theoretically low pregnancy success, probably due to the consecutive accumulation of gynecological and oncological pathologies, who became pregnant and delivered a full-term infant with adequate breastfeeding.
We thank Aram Ehsan and Teresa Pernía for comments that greatly improved the manuscript. We would also like to thank Tom Johannan for language assistance, as well as to Jennifer van Velkinburgh for her valuable help. Finally, we would also like to express our gratitude to the patient for sharing her medical history with the community. The publication of this paper has been supported by the Vicerrectorat de Política Científica of the University of Barcelona.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Khajehei M, Zhang XQ S- Editor: Dou Y L- Editor: Filipodia E- Editor: Bian YN
| 1. | Kasum M, Beketić-Orešković L, Peddi PF, Orešković S, Johnson RH. Fertility after breast cancer treatment. Eur J Obstet Gynecol Reprod Biol. 2014;173:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 536] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 3. | Raphael J, Trudeau ME, Chan K. Outcome of patients with pregnancy during or after breast cancer: a review of the recent literature. Curr Oncol. 2015;22:S8-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Sonmezer M, Oktay K. Fertility preservation in young women undergoing breast cancer therapy. Oncologist. 2006;11:422-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 696] [Cited by in RCA: 776] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 6. | Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17:2365-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 383] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 7. | Tomasi-Cont N, Lambertini M, Hulsbosch S, Peccatori AF, Amant F. Strategies for fertility preservation in young early breast cancer patients. Breast. 2014;23:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Snyder KA, Pearse W. Discussing fertility preservation options with patients with cancer. JAMA. 2011;306:202-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Carneiro MM, Cota AM, Amaral MC, Pedrosa ML, Martins BO, Furtado MH, Lamaita RM, Ferreira MCF. Motherhood after breast cancer: can we balance fertility preservation and cancer treatment? A narrative review of the literature. JBRA Assist Reprod. 2018;22:244-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Academia de estudios MIR SL. Manual AMIR de ginecología y obstetricia. 2017;72-73 Available from: http://www.academia.edu/14265215/Manual_AMIR_Ginecolog%C3%ADa_y_Obstetricia_6a_Edici%C3%B3n. |
| 11. | Busso CE, Soares SR, Pellicer A. Management of ovarian hyperstimulation syndrome. Accessed 21 Sept 2017. Available from: https://www.uptodate.com/contents/management-of-ovarian-hyperstimulation-syndrome/print. |
| 12. | Rodrigo A. Reproducción Asistida ORG: Porcentaje de éxito de la fecundación in vitro (FIV). 2016. Available from: http://www.reproduccionasistida.org/resultados-de-fiv/. |
| 13. | Malamos NA, Stathopoulos GP, Keramopoulos A, Papadiamantis J, Vassilaros S. Pregnancy and offspring after the appearance of breast cancer. Oncology. 1996;53:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Surbone A, Petrek JA. Childbearing issues in breast carcinoma survivors. Cancer. 1997;79:1271-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Bernstein L, Deapen D, Cerhan JR, Schwartz SM, Liff J, McGann-Maloney E, Perlman JA, Ford L. Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst. 1999;91:1654-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 247] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Breast International Group (BIG) 1-98 Collaborative Group, Thürlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M, Smith I, Wardley A, Price KN, Goldhirsch A. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747-2757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1162] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 17. | Krásenská M. [Treatment with Aromatase Inhibitors in Postmenopausal Women with Breast Cancer and the Possibility of Influencing Side Effects]. Klin Onkol. 2016;29 Suppl 3:S39-S49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Schramm A, De Gregorio N, Widschwendter P, Fink V, Huober J. Targeted Therapies in HER2-Positive Breast Cancer - a Systematic Review. Breast Care (Basel). 2015;10:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Zhu X, Joy AA. Targeting HER2 in Advanced Breast Cancer. Methods Mol Biol. 2017;1652:63-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay MA, Riva A, Crown J. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1817] [Cited by in RCA: 1995] [Article Influence: 142.5] [Reference Citation Analysis (0)] |
| 21. | Okanami Y, Ito Y, Watanabe C, Iijima K, Iwase T, Tokudome N, Takahashi S, Hatake K. Incidence of chemotherapy-induced amenorrhea in premenopausal patients with breast cancer following adjuvant anthracycline and taxane. Breast Cancer. 2011;18:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Cardoso F, Loibl S, Pagani O, Graziottin A, Panizza P, Martincich L, Gentilini O, Peccatori F, Fourquet A, Delaloge S, Marotti L, Penault-Llorca F, Kotti-Kitromilidou AM, Rodger A, Harbeck N. The European Society of Breast Cancer Specialists recommendations for the management of young women with breast cancer. Eur J Cancer. 2012;48:3355-3377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 23. | Rizk B, Turki R, Lotfy H, Ranganathan S, Zahed H, Freeman AR, Shilbayeh Z, Sassy M, Shalaby M, Malik R. Surgery for endometriosis-associated infertility: do we exaggerate the magnitude of effect? Facts Views Vis Obgyn. 2015;7:109-118. [PubMed] |
| 24. | Aboulghar MA, Mansour RT, Serour GI. Spontaneous intrauterine pregnancy following salpingectomy for a unilateral hydrosalpinx. Hum Reprod. 2002;17:1099-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Shue S, Radeva M, Falcone T. Comparison of Long-Term Fertility Outcomes after Myomectomy: Relationship with Number of Myomas Removed. J Minim Invasive Gynecol. 2018;25:1002-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Requena A, Herrero J, Landeras J, Navarro E, Neyro JL, Salvador C, Tur R, Callejo J, Checa MA, Farré M, Espinós JJ, Fábregues F, Grańa-Barcia M. Use of letrozole in assisted reproduction: a systematic review and meta-analysis. Hum Reprod Update. 2008;14:571-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Valor-Segura I, Expósito F, Moya M. Victim blaming and exoneration of the perpetrator in domestic violence: the role of beliefs in a just world and ambivalent sexism. Span J Psychol. 2011;14:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Bleil ME, Pasch LA, Gregorich SE, Millstein SG, Katz PP, Adler NE; Infertility Outcomes Program Project Group. Fertility treatment response: is it better to be more optimistic or less pessimistic? Psychosom Med. 2012;74:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | López IS. ClÃnica Esimer: Protocolo de preparación endometrial para tranferencia embrionaria. Accessed 10 Nov 2017. Available from: http://www.esimer.com/staff/dr-ignasi-segura-lopez/. |
| 30. | Evers JL. Female subfertility. Lancet. 2002;360:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 448] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 31. | Baird DT, Collins J, Egozcue J, Evers LH, Gianaroli L, Leridon H, Sunde A, Templeton A, Van Steirteghem A, Cohen J, Crosignani PG, Devroey P, Diedrich K, Fauser BC, Fraser L, Glasier A, Liebaers I, Mautone G, Penney G, Tarlatzis B; ESHRE Capri Workshop Group. Fertility and ageing. Hum Reprod Update. 2005;11:261-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 403] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 32. | Uccella S, Cromi A, Agosti M, Casarin J, Pinelli C, Marconi N, Bertoli F, Podesta’-Alluvion C, Ghezzi F. Fertility rates, course of pregnancy and perinatal outcomes after laparoscopic ureterolysis for deep endometriosis: A long-term follow-up study. J Obstet Gynaecol. 2016;36:800-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | AlKudmani B, Gat I, Buell D, Salman J, Zohni K, Librach C, Sharma P. In Vitro Fertilization Success Rates after Surgically Treated Endometriosis and Effect of Time Interval between Surgery and In Vitro Fertilization. J Minim Invasive Gynecol. 2018;25:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Goldberg J, Pereira L. Pregnancy outcomes following treatment for fibroids: uterine fibroid embolization versus laparoscopic myomectomy. Curr Opin Obstet Gynecol. 2006;18:402-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Casini ML, Rossi F, Agostini R, Unfer V. Effects of the position of fibroids on fertility. Gynecol Endocrinol. 2006;22:106-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 36. | Na ED, Cha DH, Cho JH, Kim MK. Comparison of IVF-ET outcomes in patients with hydrosalpinx pretreated with either sclerotherapy or laparoscopic salpingectomy. Clin Exp Reprod Med. 2012;39:182-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Shalom-Paz E, Almog B, Shehata F, Huang J, Holzer H, Chian RC, Son WY, Tan SL. Fertility preservation for breast-cancer patients using IVM followed by oocyte or embryo vitrification. Reprod Biomed Online. 2010;21:566-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |