Published online Sep 6, 2018. doi: 10.12998/wjcc.v6.i9.296
Peer-review started: March 10, 2018
First decision: April 18, 2018
Revised: May 4, 2018
Accepted: June 7, 2018
Article in press: June 8, 2018
Published online: September 6, 2018
Processing time: 181 Days and 12.3 Hours
Von Meyenburg complexes (VMCs) are a rare type of ductal plate malformation. We herein report two Chinese families with VMCs, and the suspicious gene mutation of this disease. Proband A was a 62-year-old woman with abnormal echographic presentation of the liver. She received magnetic resonance imaging (MRI) examination and liver biopsy, and the results showed she had VMCs. Histologically proved hepatocellular carcinoma was found 1 year after the diagnosis of VMCs. Proband B was a 57-year-old woman with intrahepatic diffuse lesions displayed by abdominal ultrasonography. Her final diagnoses were VMCs, congenital hepatic fibrosis, and hepatitis B surface e antigen-negative chronic hepatitis B after a series of examinations. Then, all the family members of both proband A and proband B were screened for VMCs by MRI or ultrasonography. The results showed that four of the 11 family members from two families, including two males and two females, were diagnosed with VMCs. DNA samples were extracted from the peripheral blood of those 11 individuals of two VMCs pedigrees and subjected to polymerase chain reaction amplification of the polycystic kidney and hepatic disease 1 (PKHD1) gene. Two different mutation loci were identified. Heterozygous mutations located in exon 32 (c.4280delG, p.Gly1427ValfsX6) in family A and exon 28 (c.3118C>T, p.Arg1040Ter) in family B were detected. We speculate that PKHD1 gene mutations may be responsible for the development of VMCs.
Core tip: Von Meyenburg complexes (VMCs) are a rare type of ductal plate malformation. Although generally benign, VMCs have been found to correlate with malignant diseases and progress towards adenocarcinomas. Mutations of the PKHD1 gene have been demonstrated to cause autosomal recessive polycystic kidney disease, a type of ductal plate malformation. In this study, mutations of the PKHD1 gene located in exon 28 and exon 32, respectively, were identified in two Chinese VMCs families, with four VMCs patients reported in total.
- Citation: Lin S, Shang TY, Wang MF, Lin J, Ye XJ, Zeng DW, Huang JF, Zhang NW, Wu YL, Zhu YY. Polycystic kidney and hepatic disease 1 gene mutations in von Meyenburg complexes: Case report. World J Clin Cases 2018; 6(9): 296-300
- URL: https://www.wjgnet.com/2307-8960/full/v6/i9/296.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i9.296
Von Meyenburg complexes (VMCs), or biliary hamartomas, are a rare type of ductal plate malformation (DPM). Although generally benign, VMCs have been found to correlate with malignant diseases and progress towards adenocarcinomas[1]. Family cluster of VMCs has been observed clinically, indicating a genetic background of this disease. However, the gene mutation associated with this rare disorder has not been reported until now.
Mutations of the polycystic kidney and hepatic disease 1 (PKDH1) gene are confirmed to incur autosomal recessive polycystic kidney disease (ARPKD), a severe type of DPMs. Epigenetic changes in the liver and bile ducts vary from different exon mutation regions of PKHD1. Herein, we reported the PKHD1 gene sequences in two families of VMCs.
Two probands with histology-proved VMCs were included in this study. None of the family members/offspring in pedigree died or had clinical full-blown ARPKD.
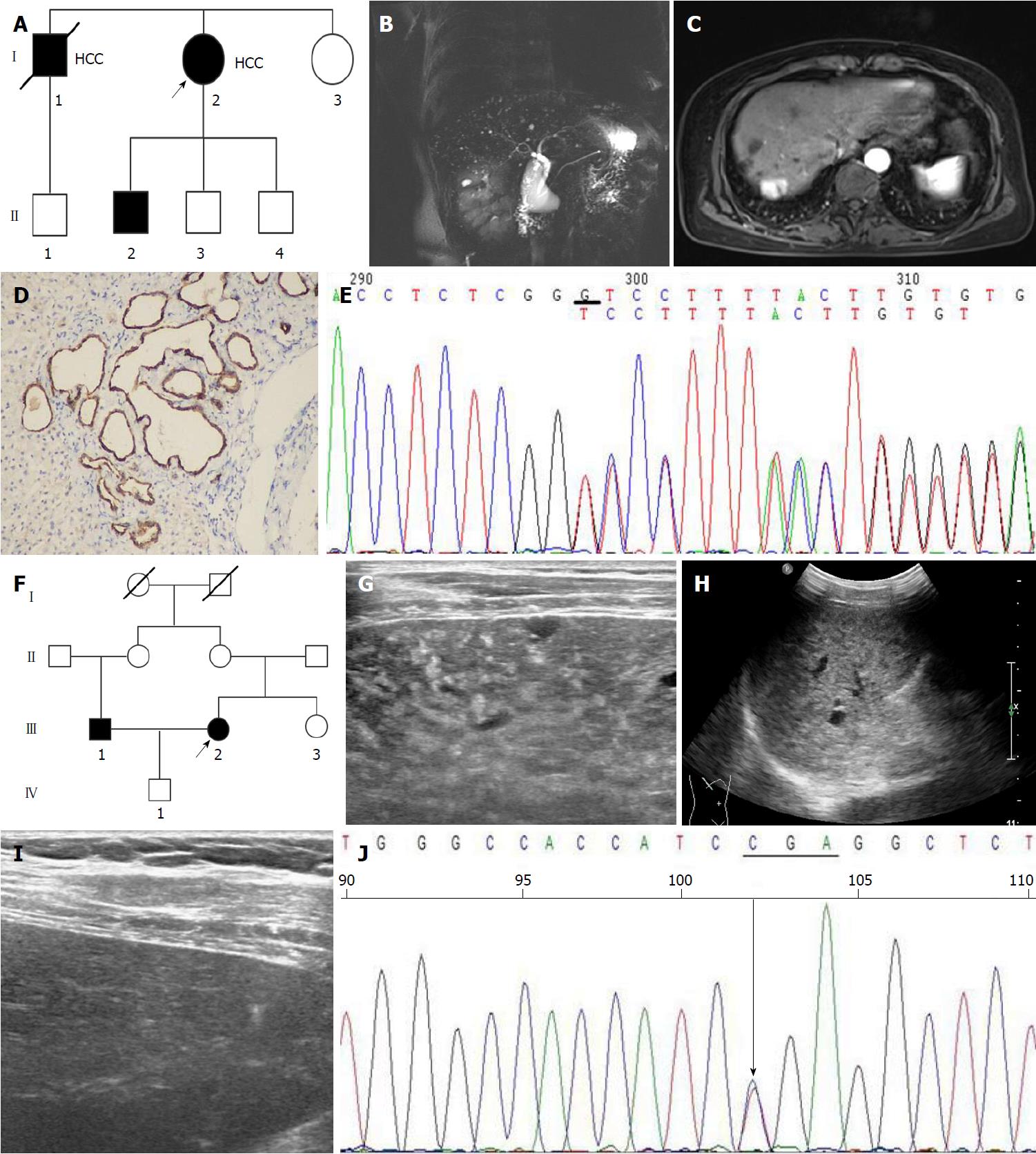
Pedigree 1 (VMC1): Proband A was a 62-year-old woman with no medical history, who was referred due to abnormal echographic presentation of the liver (Figure 1A, I: 2). Laboratory examinations showed 36 U/L alanine aminotransferase (ALT), 32 U/L aspartate aminotransferase (AST), 13.2 µmol/L total bilirubin, 2.22 ng/mL α-fetoprotein (AFP), positive hepatitis B surface antigen (HBsAg) and e antigen (HBeAg), and 3.13 × 106 copies/mL HBV DNA, and hepatitis C virus and human immunodeficiency virus antibodies were both negative. Magnetic resonance imaging (MRI) displayed a typical feature of VMCs (Figure 1B); however, no renal cysts were found. She denied a history of alcohol consumption or drug administration. Liver biopsy confirmed the diagnosis of VMCs and chronic hepatitis B. The woman was given entecavir for successive 6 mo, and a 1-year follow-up by MRI displayed carcinoma in the right lobe of liver (Figure 1C). The subsequent intraoperative pathology revealed a moderately differentiated hepatocellular carcinoma (HCC) and the existence of VMCs (Figure 1D). The woman reported that one of her siblings was also diagnosed with HCC and VMCs 2 years ago and died of HCC. Subsequently, her five family members received MRI scans, and VMCs were identified in two members, without renal kidney cysts seen.
Pedigree 2 (VMC2): Proband B was a previously healthy 57-year-old woman (Figure 1F, III: 2), and abdominal ultrasonography displayed intrahepatic diffuse lesions (Figure 1G and H) with no kidney cysts. Laboratory tests showed 34 U/L ALT, 32U/L AST, 10.8 µmol/L total bilirubin, 3.8 ng/mL AFP, 1310.95 IU/mL HBsAg, 0.02 s/co HBeAb, 11.57 s/co HBeAb, and < 500 copies/mL HBV-DNA viral load. She was finally diagnosed with VMCs, congenital hepatic fibrosis (CHF), and HBeAg-negative chronic hepatitis B after MRI and histopathological examinations. Liver ultrasonography was performed in three members of her family, and her husband, also her cousin (Figure 1F, III: 1), reported an abnormal echographic presentation (Figure 1H).
The method of proteinase K and phenol were applied to extracted genomic DNA from venous blood of the members. Seventy-two primers were designed (Shanghai Genesky Biotechnology Co., Ltd.; Shanghai, China), adopting an online software (http://frodo.wi.mit.edu/cgi-bin/primer3/ primer3_http://www.cgi) to amplify exons 1-67 and the ambient introns of the PKDH1 gene (supplementary Table 1).
Venous blood samples were collected for amplifying exons 1-67 and the ambient intronic sequences of the PKDH1 gene, and genomic DNA from the proband A and II: Two were sequenced for the entire coding region and splice sites of PKHD1. One heterozygous deletion mutation was detected at exon 32 in the PKHD1 gene (c.4280delG), leading to p.Gly1427ValfsX6 at protein level (Figure 1E). Further sequencing analysis of the entire family 1 revealed that other affected individuals (II: 1, II: 2) were also heterozygous for 4280delG, while unaffected siblings were wild type at the sequence position. Another mutation was located in exon 28 (p.Arg1040Ter) from proband B (Figure 1I), while mutations of PKHD1 of the remaining members of family 2 were not detected.
Ductal plate arises from single- or double-layered epithelial structures of hepatoblasts around the portal vein in the embryonic stage. Ductal plate undergoes remodeling (molding process from large bile ducts to microscopic bile ducts) and gives rise to the formation of bile ducts. DPM is defined as the developmental abnormalities considered to be resulted from the lack of ductal plate remodeling during bile duct morphogenesis. The congenital DPM diseases incorporate congenital hepatic fibrosis, autosomal dominant polycystic kidney disease (ADPKD), autosomal dominant polycystic liver disease (ADPLD), ARPKD, Caroli’s disease, Caroli’s syndrome, and VMCs[2]. ADPLD is a heritable disease characterized by the malformation of medium sizes ducts, which ultimately generate cysts full of fluid. This malformation is usually underdiagnosed and genetically distinct from polycystic liver disease associated with ADPKD but with similar pathogenesis, manifestations, and management[3]. Liver cysts are more frequently found in patients with ADPKD and adult-type polycystic liver disease, where the development of renal cysts precedes hepatic cysts[4]. Dissimilar to ADPLD, hepatic cysts in ADPKD are originated from peribiliary bile duct glands and dilated biliary microhamartomas[5]. By contrast, VMCs are caused from malformations of the small-sized intra-hepatic duct[4] and are considered a histopathological lesion that transforms into cysts[6].
Often found as small, symptomless and scattered cysts[7], VMCs are diagnosed accidentally upon their special radiologic appearance and sometimes in a manner of either not exact abdominal symptoms or the onset of liver sepsis[8]. A consecutive autopsy study indicates that the incidence in adults was about 5.6% and in children was 0.9%[9]. In another biopsy series, the incidence was only 0.6%[10]. Our previous study showed that the prevalence in patients subjected to diagnostic liver biopsy was 0.35%[11].
Mutations of the PKHD1 gene have been demonstrated to cause ARPKD, a type of DPM[12]. PKHD1 gene contains 76 exons and more than 300 types of mutation. PKHD1 exon 2-deficient mice exhibit hepatic, pancreatic, and renal abnormalities, grossly cystic and fibrotic livers, and progressive bile duct dilatation as well as structural abnormalities and shortening of primary cilia in the bile ducts relative to the wild-type animals[13]. Deletion of exon 40 on the PKHD1 gene resulted in bile duct abnormality in mice[14]. In addition, mutation of the PKHD1 gene by disrupting exon 4 down-regulated fibrocystin/polyductin (FPC) expression, resulting in intrahepatic bile duct proliferation with progressive cyst formation and associated periportal fibrosis in mice[15]. Herein, we examined the genetic mutations of two VMCs pedigrees. Two mutations (c.4280delG and c.3118C>T) of the PKHD1 gene located on exons 32 and 28 were detected, respectively, both of which led to early termination of synthesis. These heterozygous deletion mutations may result in a single-dose deficiency of the PKHD1 gene, which affects the development of bile ducts. The exon 28-32 of PKHD1 gene mainly encodes the IPT/TIG (Ig-like, plexins, transcription factors), IPT_PCSRP (plexins and cell surface receptors), and initial G8 (this domain is named G8 after its 8 conserved glycines) domains of the fibrocystin protein. These domains are involved in the regulation of cell growth, signal transduction, proliferation, and adhesion. Our previous study also showed that silencing of the PKHD1 gene promoted the proliferation, migration, and invasion of human intrahepatic cholangiocarcinoma HuCCT-1 cells via the PI3K/Akt signaling pathway, indicating that PKHD1 may contribute to the development and progression of intrahepatic cholangiocarcinoma[16]. It is therefore hypothesized that the protein component encoded by exon 28-32 of the PKHD1 gene may have a closer correlation with the development of bile duct than with renal tubules. The mutation in exon 28-32 may lead to the malformations of the ductal plate alone without kidney involvement.
In summary, our report provides four cases to the literature of VMCs associated with PKHD1 gene mutations. To be more persuasive, more clinicopathologic and molecular studies are needed to validate the findings and test the hypothesis from the present study.
A 62-year-old woman and a 57-year-old woman were both previously healthy, who were referred due to abnormal echographic presentation of the liver.
Liver cirrhosis, metastases, microabscesses, and simple liver cysts.
Proband A and B were diagnosed with chronic hepatitis B and HBeAg-negative chronic hepatitis B, respectively, by laboratory examinations of positive hepatitis B surface antigen (HBsAg), negative anti-hepatitis C virus and anti-human immuno-deficiency virus tests, and normal liver function tests.
Abdominal ultrasonography displayed intrahepatic diffuse lesions and magnetic resonance imaging (MRI) showed cystic lesions that shared no connection with the intra- and extra-hepatic bile duct system and were of normal size for von Meyenburg complexes (VMCs). A carcinoma in the right lobe of the liver was also found in proband A. No kidney cyst was seen in the imaging examinations of any family member.
Pathological diagnosis confirmed the diagnoses of hepatocellular carcinoma VMCs and chronic hepatitis B in proband A and of congenital hepatic fibrosis (CHF) and VMCs in proband B.
Proband A was given entecavir and complete surgical resection of the hepatocellular carcinoma lesion. Proband B did not receive any medication and was followed up regularly.
In most of cases, VMCs are incidentally detected, focusing on the location of the disease (liver surface, extrahepatic), relative symptoms, mimicking metastatic disease or malignancy, and association with liver tumor.
PKHD1 gene is located on chromosome 6p12. It encodes a protein named fibrocystin/polyductin (FPC). FPC protein is involved in the maintenance of the normal tubular structure of intrahepatic bile duct epithelial cells. Mutation of PKHD1 may cause the structural and functional disorder of FPC, leading eventually to the development of renal and hepatic cysts. VMCs are benign neoplasms characterized by the disorderly arrangement of biliary epithelium, which form abnormal biliary ducts surrounded by ample fibrous stroma.
The PKHD1 gene mutations were identified in two VMCs patients, providing new insights into the pathogenesis, diagnosis, and progression of the VMCs.
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Burgesser MV, Cheungpasitporn W, Rangan G S- Editor: Ji FF L- Editor: Filipodia E- Editor: Tan WW
| 1. | Song JS, Lee YJ, Kim KW, Huh J, Jang SJ, Yu E. Cholangiocarcinoma arising in von Meyenburg complexes: report of four cases. Pathol Int. 2008;58:503-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Desmet VJ. Congenital diseases of intrahepatic bile ducts: variations on the theme “ductal plate malformation”. Hepatology. 1992;16:1069-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 339] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 3. | Qian Q, Li A, King BF, Kamath PS, Lager DJ, Huston J 3rd, Shub C, Davila S, Somlo S, Torres VE. Clinical profile of autosomal dominant polycystic liver disease. Hepatology. 2003;37:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Venkatanarasimha N, Thomas R, Armstrong EM, Shirley JF, Fox BM, Jackson SA. Imaging features of ductal plate malformations in adults. Clin Radiol. 2011;66:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Kida T, Nakanuma Y, Terada T. Cystic dilatation of peribiliary glands in livers with adult polycystic disease and livers with solitary nonparasitic cysts: an autopsy study. Hepatology. 1992;16:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 62] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Karhunen PJ. Adult polycystic liver disease and biliary microhamartomas (von Meyenburg’s complexes). Acta Pathol Microbiol Immunol Scand A. 1986;94:397-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Tohmé-Noun C, Cazals D, Noun R, Menassa L, Valla D, Vilgrain V. Multiple biliary hamartomas: magnetic resonance features with histopathologic correlation. Eur Radiol. 2008;18:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Sinakos E, Papalavrentios L, Chourmouzi D, Dimopoulou D, Drevelegas A, Akriviadis E. The clinical presentation of Von Meyenburg complexes. Hippokratia. 2011;15:170-173. [PubMed] |
| 9. | Redston MS, Wanless IR. The hepatic von Meyenburg complex: prevalence and association with hepatic and renal cysts among 2843 autopsies [corrected]. Mod Pathol. 1996;9:233-237. [PubMed] |
| 10. | Thommesen N. Biliary hamartomas (von Meyenburg complexes) in liver needle biopsies. Acta Pathol Microbiol Scand A. 1978;86:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Lin S, Weng Z, Xu J, Wang MF, Zhu YY, Jiang JJ. A study of multiple biliary hamartomas based on 1697 liver biopsies. Eur J Gastroenterol Hepatol. 2013;25:948-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Bergmann C, Senderek J, Küpper F, Schneider F, Dornia C, Windelen E, Eggermann T, Rudnik-Schöneborn S, Kirfel J, Furu L. PKHD1 mutations in autosomal recessive polycystic kidney disease (ARPKD). Hum Mutat. 2004;23:453-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Woollard JR, Punyashtiti R, Richardson S, Masyuk TV, Whelan S, Huang BQ, Lager DJ, vanDeursen J, Torres VE, Gattone VH. A mouse model of autosomal recessive polycystic kidney disease with biliary duct and proximal tubule dilatation. Kidney Int. 2007;72:328-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Moser M, Matthiesen S, Kirfel J, Schorle H, Bergmann C, Senderek J, Rudnik-Schöneborn S, Zerres K, Buettner R. A mouse model for cystic biliary dysgenesis in autosomal recessive polycystic kidney disease (ARPKD). Hepatology. 2005;41:1113-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Gallagher AR, Esquivel EL, Briere TS, Tian X, Mitobe M, Menezes LF, Markowitz GS, Jain D, Onuchic LF, Somlo S. Biliary and pancreatic dysgenesis in mice harboring a mutation in Pkhd1. Am J Pathol. 2008;172:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Lin S, He C, Wang MF, Wu YL, Lin J, Liu Y, Zhu YY. shRNA-mediated silencing of PKHD1 gene promotes proliferation, migration and invasion of human intrahepatic cholangiocarcinoma HuCCT-1 cells. Int J Clin Exp Pathol. 2017;10:2496-2509. |