Published online Sep 6, 2018. doi: 10.12998/wjcc.v6.i9.274
Peer-review started: April 2, 2018
First decision: May 8, 2018
Revised: May 31, 2018
Accepted: June 7, 2018
Article in press: June 8, 2018
Published online: September 6, 2018
Processing time: 157 Days and 23.5 Hours
Systemic air embolism through a bronchovenous fistula (BVF) has been described in patients undergoing positive-pressure ventilation. However, no report has mentioned the potential risks of systemic air embolism through a BVF in patients undergoing extracorporeal membrane oxygenation (ECMO). Positive-pressure ventilation and ECMO support in patients with lung injury can increase the risk of systemic air embolism through a BVF. Increased alveolar pressure, decreased pulmonary venous pressure, and anticoagulation are thought to be the factors that contribute to this complication. Here, we present a case of systemic air embolism in a patient with ECMO and mechanical ventilator support.
Core tip: Sudden deterioration of patients during extracorporeal membrane oxygenation support is not unusual. Usually, it is thought to result from the critical illness of the patients. This report suggests that some such cases may be related to bronchovenous fistula, which causes cerebral and coronary air embolisms.
- Citation: Ryu SM, Park SM. Unexpected complication during extracorporeal membrane oxygenation support: Ventilator associated systemic air embolism. World J Clin Cases 2018; 6(9): 274-278
- URL: https://www.wjgnet.com/2307-8960/full/v6/i9/274.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i9.274
Air in the extracorporeal membrane oxygenation (ECMO) circuit (1.4%-4.6%) can lead to systemic air embolism[1-3]. In most cases, the air source is the venous system, and massive systemic air embolism is rare. The known origins of air embolism include the venous cannula, central venous catheter, membrane oxygenator, and cavitation[4]. In most studies, the lung is not considered a source of air embolism in ECMO support. However, massive systemic air embolism can occur in patients with positive pressure ventilation[5,6]. When there is an injury to the lung and the alveolar pressure exceeds pulmonary venous pressure, air can enter the systemic circulation through the pulmonary vein. This is known as bronchovenous fistula (BVF) and causes massive cerebral and myocardial air embolism[7,8]. No previous report has considered the possibility that ECMO can contribute to the development of air embolism through BVF. We present a case of systemic air embolism in a patient undergoing ECMO support and mechanical ventilation.
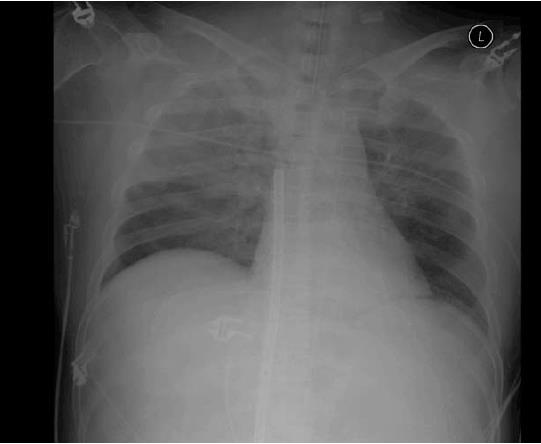
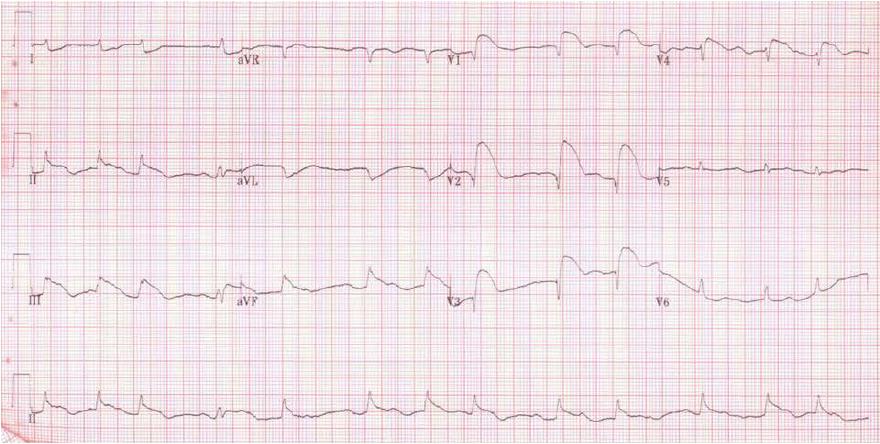
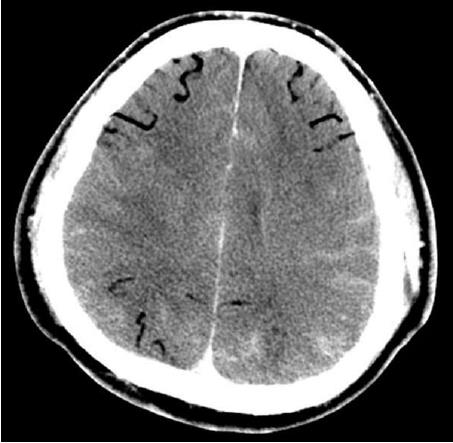
A 47-year-old man was admitted to the emergency room for chest pain. He had a medical history of hypertension and diabetes mellitus. His initial blood pressure and heart rate were 80/50 mmHg and 124/min, respectively. Electrocardiography (ECG) showed ST-segment elevation on leads V2-V4. The troponin Iconcentration was 102 ng/mL. Cardiac arrest developed and cardiac massage was initiated. The patient was intubated with a 7.5 Fr endotracheal tube and was manually ventilated with an ambu bag. During bagging, bloody secretion was observed in the endotracheal tube. An intra-aortic balloon pump was inserted through the left femoral artery. Because of severe cardiac dysfunction and ventricular arrhythmia, ECMO (Capiox EBS, Terumo Corp., Tokyo, Japan) was applied through the right femoral vein and artery. Emergency coronary angiography (CAG) revealed total occlusion of the proximal left anterior descending artery and up to 40% diffuse stenosis of the right coronary artery (RCA). A coronary artery stent was inserted into the left anterior descending artery. After the procedure, the patient was supported with mechanical ventilation. The ventilator was set in the pressure-control mode with an FiO2 of 0.8, peak end expiratory pressure of 6 cmH2O, peak pressure of 26 cmH2O, and respiratory rate of 12/min. The follow-up chest X-ray revealed haziness in the right upper lung field (Figure 1). The patient’s hemodynamic condition and consciousness level gradually improved; he became able to follow commands and open his eyes in response to stimulation. His hourly urine output increased, and the inotropic agent was withdrawn. ECMO flow was decreased from 3.5 L/min to 1.0 L/min. Five hours after percutaneous coronary intervention (PCI), he experienced a sudden decrease in blood pressure from 120/70 mmHg to 60/40 mmHg and bradycardia, as low as 15/min, which recovered after administration of atropine and epinephrine. The ECMO circuit was immediately examined for any flow disturbance, but no abnormal sign or dysfunction was found. ECMO flow was increased up to 3.0 L/min. The 12-lead ECG results suggested acute inferior and anteroseptal wall ischemia (Figure 2). The follow-up CAG showed no evidence of occlusion or significant stenosis of coronary vessels. Echocardiography did not show any evidence of an intracardiac shunt or pericardial tamponade, but severe dysfunction of the left ventricle was detected. Acute neurological deterioration was also present; his Glasgow Coma Scale score was 4. Because of the unexplained neurologic dysfunction, a computed tomographic brain scan was taken, revealing a massive cerebral air embolism (Figure 3). The patient was placed in the Trendelenburg position. Although he did not have a central venous catheter (Figure 1), all indwelling catheters, including the ECMO circuit, were inspected for a possible origin of the air embolism, but we found no defect. Despite resuscitation measures, the patient’s condition became aggravated and he died 10 h after the sudden deterioration.
Systemic air embolism is a dreaded complication in ECMO support. Several sources of air emboli are known: The venous cannula, central venous catheter, membrane oxygenator, and cavitation[4]. In this case, there was a massive cerebral air embolism. If such a large amount of air originated from the venous system, air should have been detected in the ECMO circuit. However, no air was detected in the ECMO circuit, including the oxygenator and the cone of the centrifugal pump. The systemic air embolism could not be explained until a pulmonary origin of the air embolism was suspected.
No previous report has mentioned the lung as a source of systemic air embolism in patients with ECMO support. However, systemic air embolism can result from the interface between the alveoli and pulmonary veins known as BVF[5-8]. BVF causes massive cerebral and coronary air embolism in neonates with mechanical ventilation and in adults who have lung injury and are supported by positive pressure ventilation[9,10]. The underlying mechanism is increased alveolar pressure exceeding pulmonary venous pressure and shift of air through the damaged pulmonary vasculature[6]. Loss of consciousness from cerebral air embolism and sudden bradycardia from RCA occlusion with air emboli are the prominent signs of air embolism caused by BVF[6-8]. These clinical features closely resemble those of our case.
There are a number of ECMO-related factors that might contribute to the increased risk of systemic air embolism originating from a BVF. One factor is decreased venous return to the heart. ECMO (VA mode) drains venous blood, thereby decreasing venous return, and lowers pulmonary venous pressure, which consequently increases the chances of alveolar air entering the vascular system. Many patients receive CPR before ECMO support. Manual ambu bagging with cardiac massage during CPR can cause lung injury, forcing air to enter the pulmonary vein[11]. The use of anticoagulation prevents sealing of the injured vascular bed of the lung, increasing the risk of air embolism. When these patients are supported by positive pressure ventilation, which is often the case, the air can enter the vascular system through the injured alveoli. LV diastolic pressure can fall below zero in mitral stenosis patients[12]. Under ECMO support where LV diastolic volume is reduced, the diastolic LV pressure may drop to negative pressure when the LV function returns to normal (e.g., after PCI). Consequently the risks of BVF air embolism will increase. Because of these clinical conditions, patients with ECMO support have increased risk of developing air embolism originating from BVF.
Although there are a number of factors that can increase the risk of air entrance into the pulmonary vein, it seems that the actual systemic air embolism does not occur until there is sufficient left ventricular blood flow. The patient did not show any sign of systemic air embolism when fully supported with ECMO. The systemic air embolism developed after we decreased ECMO flow. In a case report about ECMO-related systemic air embolism, the author described a large oscillating air bubble detected in the aortic root immediately after initiation of the IABP[13]. These clinical features suggest that the air embolism might take place in two phases. First, the air in the alveolar space enters into the pulmonary vein. It is trapped in the pulmonary vein, left atrium, or left ventricle depending on the position of the patient. Secondly, when there is enough left ventricular blood flow, the air bubbles move into the aorta and peripheral arteries, causing systemic air embolism.
Evidence of air entrance through BVF in patients with ECMO support has not been reported except in a pediatric patient who had total anomalous pulmonary venous return (TAPVR) and was supported with ECMO[2]. In that case, air was detected in the venous cannula during ambu bagging because there was a residual pulmonary vein-SVC connection. Air embolism through the lung during CPB has been reported[14,15]. These air embolisms were detected after left ventricular beating was started during open heart surgery. Common features of these cases include anticoagulation, lung injury, CPB, and positive pressure ventilation. We searched for cases of air embolism through BVF in ECMO support. There is one case report to compare with our case[13]. The case was similar to our case in that the patient had AMI and received CPR followed by ECMO support and PCI[13]. According to that report, IABP was inserted through the femoral artery in the intervention room to enhance coronary perfusion and decrease afterload. Immediately after IABP was started, a large air bubble was detected in the aortic root. The author admitted that the origin of the air was unknown and suggested the IABP sheath would be a possible source of the air. However, air entering the arterial system without a pressure gradient is unlikely. Considering the clinical conditions, which resembled those in our case, we believe that a BVF was the origin of the air embolism in that case.
One might wonder why there have been no reports about ECMO-related BVF air embolism. Gas in the systemic circulation is extremely difficult to document[5]. As is our experience, sudden deterioration of patients with AMI prompts a search for cardiac problems, and the possibility of cerebral air embolism might be overlooked. Even though scarce in the literature, there might be more ECMO-related BVF air embolisms than we think because CPR and ECMO support followed by PCI is common clinical practice. Sudden loss of consciousness and bradycardia in a patient with ECMO support might be a sign of cerebral and coronary air embolism caused by BVF. Avoiding high pressure ventilation setting might help to lower the risk of this complication. Because systemic air embolism is often lethal and there is no effective treatment available, prevention of this complication is of key importance. To reveal the true incidence of BVF air embolism in ECMO support and to prevent this devastating complication, clinicians should be aware of the possibility of air embolism from BVF in patients with ECMO support.
A 47-year-old male with extracorporeal membrane oxygenation (ECMO) support developed sudden cardiogenic shock and loss of consciousness.
The electrocardiography finding suggested acute inferior and anteroseptal wall ischemia, and the loss of consciousness was thought to be the consequence of the cardiogenic shock because the ECMO flow was low.
Differential diagnosis includes acute myocardial infarction, cerebral thromboembolism, and cerebral hemorrhage.
Brain CT showed massive cerebral air embolism.
The patient was placed in the Trendelenburg position.
Bronchovenous fistula (BVF) can cause systemic air embolism when the alveolar pressure exceeds pulmonary venous pressure.
BVF is a connection between alveolar and pulmonary vein caused by pulmonary injury.
ECMO support can increase the risk of systemic air embolism caused by BVF fistula, and this complication should be suspected when there is sudden bradycardia with loss of consciousness.
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Auzinger G, Ono M S- Editor: Ji FF L- Editor: Filipodia E- Editor: Tan WW
| 1. | Arbor A. ECLS Registry Report. Available from: https://www.elso.org/Registry/Statistics/InternationalSummary.aspx. |
| 2. | Timpa JG, O’Meara C, McILwain RB, Dabal RJ, Alten JA. Massive systemic air embolism during extracorporeal membrane oxygenation support of a neonate with acute respiratory distress syndrome after cardiac surgery. J Extra Corpor Technol. 2011;43:86-88. [PubMed] |
| 3. | Alghamdi AA, Coles JG, Holtby H, Al-Radi OO. Massive air embolism after the repair of obstructed total anomalous pulmonary venous drainage: an unusual complication. J Card Surg. 2010;25:582-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Allen S, Holena D, McCunn M, Kohl B, Sarani B. A review of the fundamental principles and evidence base in the use of extracorporeal membrane oxygenation (ECMO) in critically ill adult patients. J Intensive Care Med. 2011;26:13-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Marini JJ, Culver BH. Systemic gas embolism complicating mechanical ventilation in the adult respiratory distress syndrome. Ann Intern Med. 1989;110:699-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 67] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Durant TM, Oppenheimer MJ. Arterial air embolism. Am Heart J. 1949;38:481-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Gursoy S, Duger C, Kaygusuz K, Ozdemir Kol I, Gurelik B, Mimaroglu C. Cerebral arterial air embolism associated with mechanical ventilation and deep tracheal aspiration. Case Rep Pulmonol. 2012;2012:416360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Weaver LK, Morris A. Venous and arterial gas embolism associated with positive pressure ventilation. Chest. 1998;113:1132-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Kogutt MS. Systemic air embolism secondary to respiratory therapy in the neonate: six cases including one survivor. AJR Am J Roentgenol. 1978;131:425-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Ho AM, Ling E. Systemic air embolism after lung trauma. Anesthesiology. 1999;90:564-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Shiina G, Shimosegawa Y, Kameyama M, Onuma T. Massive cerebral air embolism following cardiopulmonary resuscitation. Report of two cases. Acta Neurochir (Wien). 1993;125:181-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Sabbah HN, Anbe DT, Stein PD. Negative intraventricular diastolic pressure in patients with mitral stenosis: evidence of left ventricular diastolic suction. Am J Cardiol. 1980;45:562-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Kim H, Baek SI, Kim HL. Entrapment of a large air bubble at aortic root associated with intra-aortic balloon pump insertion. Video J Cardiol. 2017;1:1-5. |
| 14. | Doshi HK, Thankachen R, Philip MA, Stephen T, Shukla V, Korula RJ. Bronchovenous fistula - leading to fatal massive systemic air embolism during cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 2005;4:440-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Hsaad AH, Bleich S, Nanda NC, Athanasuleas CL, Öz TK. Transesophageal echocardiographic diagnosis of bronchopulmonary vein fistula complicating mitral valve replacement. Echocardiography. 2013;30:850-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |