Published online Sep 6, 2018. doi: 10.12998/wjcc.v6.i9.249
Peer-review started: April 27, 2018
First decision: June 15, 2018
Revised: June 23, 2018
Accepted: July 31, 2018
Article in press: August 1, 2018
Published online: September 6, 2018
Processing time: 132 Days and 19.1 Hours
To determine if sorafenib, an antineoplastic agent, could prevent the development of spinal epidural fibrosis (EF).
The study used CD105 and osteopontin antibodies in an immunohistochemical approach to quantify EF that occurred as a consequence of laminectomy in rats. Wistar albino rats (n = 16) were divided into two groups: control (L1-2 level laminectomy only) and sorafenib treatment (L1-2 level laminectomy + topical sorafenib). The animals were euthanatized after 6 wk, and the EF tissues were examined for histopathological changes after immunohistochemical staining. The EF grades were assigned to the tissues, and the treatment and control groups were compared.
The EF thickness, inflammatory cell density, and arachnoid adherences determined by light microscopy were significantly higher in the control group compared to the sorafenib-treated group. Based on fibrosis scores, the extent of EF in the treatment group was significantly lower than in the controls. Immunohistochemical staining for CD105 to identify microvessels revealed that the EF grades based on vessel count were significantly lower in the treatment group. Staining for osteopontin did not show any significant differences between the groups in terms of the extent of EF. The staging of EF based on vascular counts observed after immunohistochemical staining for CD105, but not for osteopontin, was compatible with conventional staging methods. Neither toxic effects on tissues nor systemic side effects were observed with the use of sorafenib.
Local administration of sorafenib significantly reduced post-laminectomy EF. Decreased neovascularization in spinal tissue may be due to the sorafenib-induced inhibition of vascular endothelial growth factor.
Core tip: This study addressed the prevention of spinal epidural fibrosis (EF) by sorafenib, an antineoplastic agent, though immunohistochemical analyses of EF as a consequence of laminectomy in rats. The study demonstrated for the first time that the fibrosis thickness, inflammatory cell density, arachnoid adherences, fibrosis scores, and vessel count were significantly lower in the treatment group. These findings indicate that locally administered sorafenib may help reduce spinal EF after laminectomy without any significant complications or side effects.
- Citation: Tanriverdi O, Erdogan U, Tanik C, Yilmaz I, Gunaldi O, Adilay HU, Arslanhan A, Eseoglu M. Impact of sorafenib on epidural fibrosis: An immunohistochemical study. World J Clin Cases 2018; 6(9): 249-258
- URL: https://www.wjgnet.com/2307-8960/full/v6/i9/249.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i9.249
One of the complications that occurs most frequently after laminectomy is epidural fibrosis (EF)[1]. EF causes reoccurrence of symptoms because of post-operation nerve root tensioning or compression[2]. A second operation is often required to remove scar tissue, and it is both difficult and has a high rate of complications[1,3,4]. A majority of authors believe that to avoid surgical treatment, EF should be prevented before it occurs[5,6]. Several studies have previously addressed the effects of various compounds on EF after laminectomy in rat[2,7-10] and rabbit[11] models, on renal fibrosis[12], and on liver fibrosis[13]. Many of these drugs are still in the development stage, and some of them are intended to inhibit the mechanism underlying fibrosis development. The multi-kinase inhibitor sorafenib is an antineoplastic drug with secondary antifibrotic and antiangiogenic activity via inhibition of vascular endothelial growth factor (VEGF)[13]. Although the antifibrotic effectiveness of sorafenib is well known, there is no literature to our knowledge of its utilization in spinal surgery[12,13].
Chemotactic factors arising from the disintegration of erythrocytes and thrombocytes following bleeding in the epidural region after laminectomy, as well as fibroblast migration arising from paraspinal muscles, are the main causes of EF. Adhesions can also form due to fibrous connective tissue hyperplasia when fibroblasts are activated by inflammatory cytokines and growth factors, primarily transforming growth factor-β (TGF-β) and basic fibroblast growth factor, to repair the local defect in the vertebral lamina. With collagen fibril production, fibrous connective tissue is transformed into scar tissue, leading to the development of fibrositis[14].
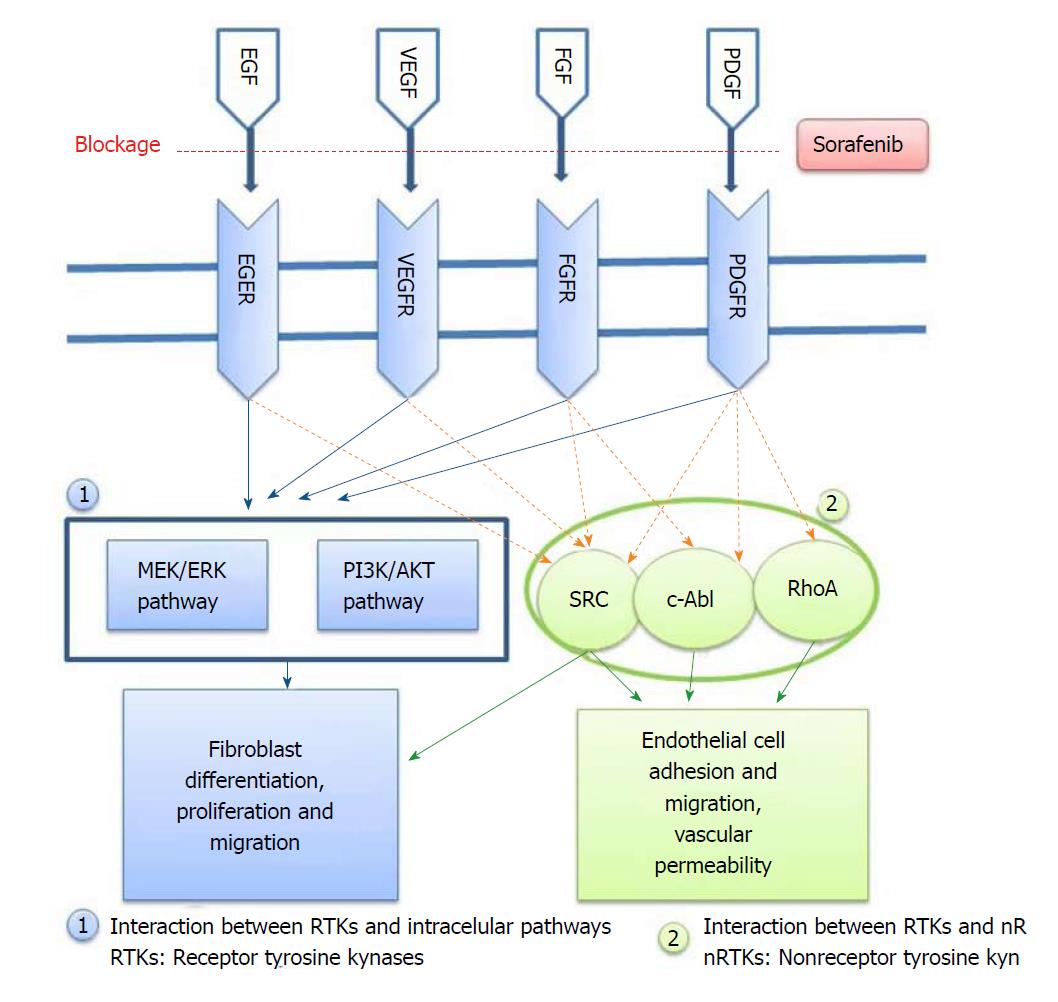
Tyrosine kinase (TK) inhibitors are a group of small molecules that have gained considerable importance in recent years for the prevention of angiogenesis. Sorafenib is one such antineoplastic drug, and it reduces the proliferation of tumor cells through its antiangiogenic effect via multi-kinase inhibition[15]. Its principal targets are the Raf/ERK, VEGFR and PDGFR-β pathways[13]. It has been shown that sorafenib can inhibit a great number of intracellular (c-CRAF, BRAF and mutant BRAF) and cell surface kinases (KIT, FLT-3, RET, VEGFR-1, VEGFR-2, VEGFR-3 and PDGFR-B) (Figure 1). It is therefore thought that some of these kinases may take part in tumor cell signal transmission, angiogenesis and apoptosis. Sorafenib selectively inhibits the VEGF receptor tyrosine kinase (TK). Due to this antiangiogenic effect, it has become one of the most effective drugs in cancer treatment[16]. Moreover, in recent years, sorafenib has conspicuously improved liver transplantation and conventional treatment methods of all other non-malignant fibrotic diseases like fibrosis caused by end-stage liver cirrhosis[13,17,18]. In the current study, we have investigated whether the antiangiogenic effect of sorafenib via VEGF receptor blockade could also prevent the development of experimental EF without toxic effects when applied locally to peripheral tissues.
CD105, also known as endoglin, is a membrane glycoprotein that regulates the signaling of TGF-β1[19]. It was first defined as a human endothelial cell marker produced by pre-B cell leukemia cell lines[20]. Further studies have indicated that this cell surface antigen is also expressed by macrophages, erythroid precursors, syncytiotrophoblasts of terminal placenta and stromal cells[20-23]. Previous studies have revealed that in various models of EF, CD105 expression could prevent the fibrogenic impact of TGF-β1[19,24]. In biopsies of patients with kidney and liver fibrosis, an excessive amount of CD105 expression was seen, and its role in regulating fibrogenesis was thus realized[20,25]. In the light of the above information, CD105 levels may be useful in determining the density of microvessels and the extent of angiogenesis in fibrosis. Unfortunately, there is no literature available to our knowledge on the use of CD105 immunostaining for the examination of EF after laminectomy. Given the role of CD105 in angiogenesis and its expression in fibrosis and in macrophages, we examined its use as a marker of EF post-laminectomy at different stages in the development of EF.
Osteopontin is a glycophosphoprotein that was first isolated from bone tissue and possesses a number of functions affecting various cells including macrophages, epithelial cells, smooth muscle cells and endothelial cells. High levels of osteopontin have been found in bone tissue, blood, milk, urine and seminal fluid[26]. It is secreted from embryonic stroma and from fibroblasts during wound healing[27]. Since osteopontin is secreted from inflammatory cells, fibroblasts and endothelial cells, it may be helpful in determining the presence of fibrosis[28]. We therefore analyzed whether osteopontin expression post-laminectomy could be used for the immunohistochemical detection of EF and if its use was compatible with conventional methods of EF assessment.
In the present study, we have investigated the preventive effect of sorafenib on the development of EF after laminectomy. In addition to conventional methods showing the number of fibroblasts and the prevalence of fibrosis, we employed immunohistochemical staining for CD105 and osteopontin to analyze the extent of EF and assessed whether these methods were compatible with conventional EF staging methods.
The study was performed at the Marmara University Experimental Animals Laboratory (İstanbul, Turkey) and was approved by the Marmara University Animal Experiments Research Ethics Committee (04.01.2016/001). All necessary precautions were taken to minimize pain and discomfort in animals throughout the procedures of the study. Male Wistar albino rats (n = 16, weight 250 g ± 30 g, 10-12 mo) were housed in an air-conditioned room with an average temperature of 23 ± 2°C and an average humidity of 65%-70%, under a 12-h light-dark cycle. Each animal was kept in a separate cage throughout the study with food provided ad libitum and without any medical treatment after the initial operation. Surgical intervention was performed using standard microsurgical instruments.
A prophylactic dose of 50 mg/kg ceftriaxone (Rocephine; Roche, İstanbul, Turkey) was intraperitoneally injected 30 min before surgery. General anesthesia was applied by intraperitoneal administration of 100 mg/kg ketamine hydrochloride (Ketalar, 50 mg/mL; Parke-Davis, Eczacıbaşı, İstanbul, Turkey) + 10 mg/kg xylazine hydrochloride (2% Rompun; Sigma-Aldrich, Merck, İstanbul). After fastening the rat to the operating table, the operating area was disinfected with povidone iodine (Poviod, 10% polyvinyl pyrrolidone-iodine complex; Saba, İstanbul, Turkey). The operating area was covered with sterile cloths. Upon determining the L1-2 level, an approximately 2-2.5 cm central line skin incision was made. Para-spinal muscles were stripped by blunt dissection. Under a surgical microscope, total laminectomy was performed within an approximately 4 mm2 area between L1 and L2, and the dural sac was revealed. Throughout the operation, the wound was irrigated with saline and hemostasis was ensured. Bipolar cautery, bone wax, surgical or other hemostasis materials were not used. Experimental animals were divided equally (n = 8 per group) between the control group (Group I) with laminectomy performed at the L1-2 level and physiologic saline applied, and the treatment group (Group II) with laminectomy performed at the L1-2 level and topical sorafenib (Nexavar; Bayer and Onyx Pharmaceutical, Berlin, Germany) applied. Because sorafenib is produced in the form of 200 mg tablets and its injectable type was not available, we administered sorafenib in the form of a diluted topical treatment. Following the advice of the manufacturer, a 200 mg tablet was dissolved in 5 cc saline and applied via impregnated cotton sponge to the laminectomy area for 5 min. The incision area was then sutured with 3/0 silk in conformity with its anatomy. Experimental and control animals were euthanatized by cervical dislocation 6 wk later. The motor functions of all animals was normal at the time of euthanasia, and no postoperative infection or other systematic pathology was evident. The relevant vertebral columns were removed as a block together with the paraspinal muscles. The tissue was fixed in 10% formalin (4% formaldehyde) solution.
Histopathological examination was performed by a qualified pathologist. The amount of fibrosis in the laminectomy area in relation to dura mater was assessed in conformity with standard immunohistochemical criteria and classifications in the literature. Comparisons were made within groups and between control and treatment groups, and results were compiled and statistically interpreted.
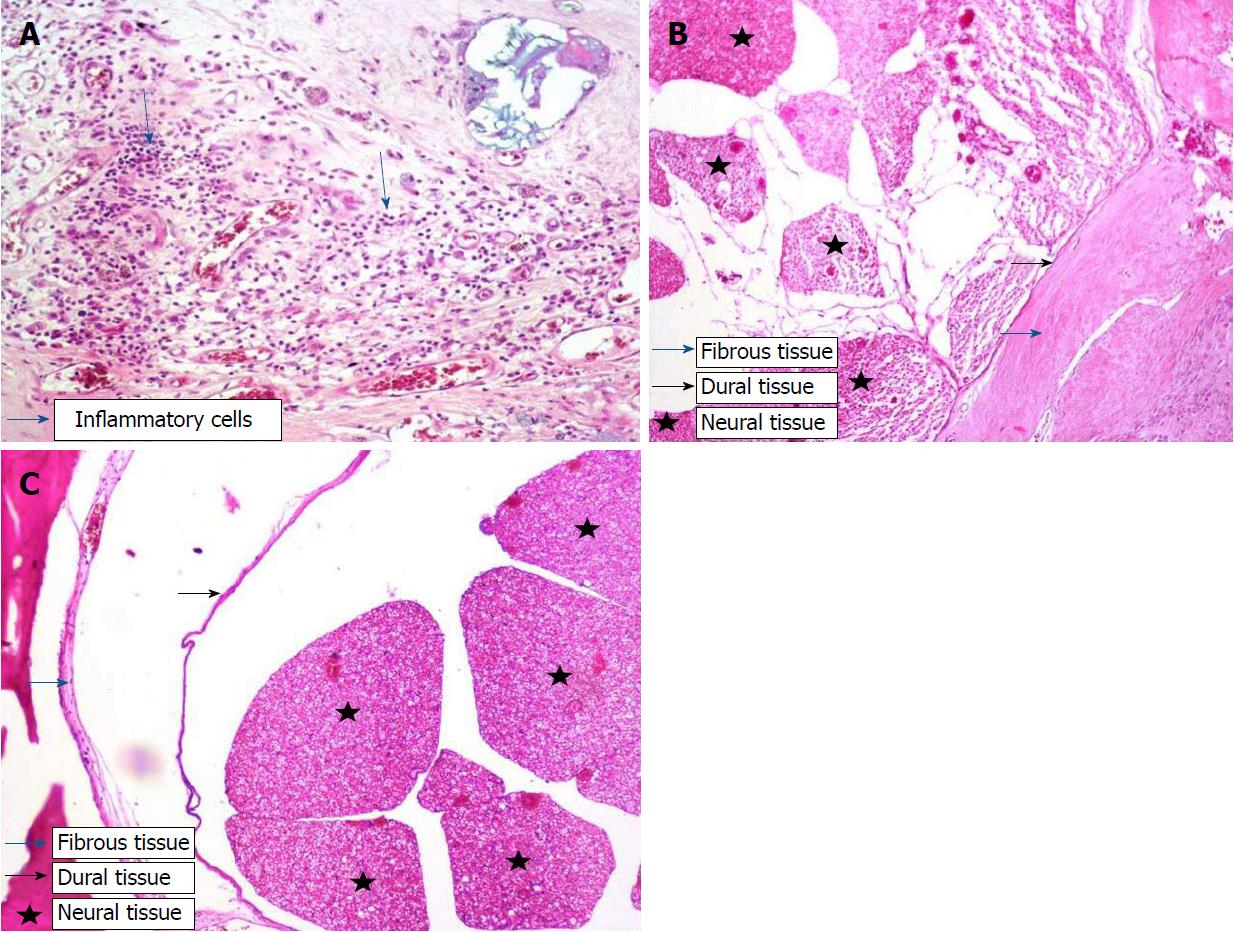
After fixation for 24 h in 10% buffered formalin, the vertebral column was removed as a block and decalcified in 10% formic acid for 2 d. Three specimens were taken from the area in which laminectomy was performed, and these specimens were subjected to Leica 300S “autotechnikon” routine tissue monitoring after washing for 6 h under running water. Paraffin blocks were sectioned at a thickness of 3 mm, and sections were stained with hematoxylin-eosin (HE) for inspection under a light microscope with respect to the prevalence of fibrosis, cellular density and arachnoid fibrosis. Fibrosis staging at the dura was performed according to He et al[29] (Table 1). Fibroblasts at 100 X magnification were counted by pathologists in three fields per specimen, one in the middle and two at the edge of the laminectomy (Figure 2A). The average number of fibroblasts and inflammatory cells in the three fields were graded as follows: Grade 1, less than 100 fibroblasts/inflammatory cells per field; Grade 2, 100–150 fibroblasts/inflammatory cells per field; Grade 3, more than 150 fibroblasts/inflammatory cells per field. To verify the quantification of fibroblast numbers, immunohistochemistry was performed using monoclonal anti-vimentin antibody 1:100 (Vimentin clone: V9, LEICA Biosystems Newcastle Upon Tyne, United Kingdom), and the density of vimentin was evaluated[6]. Similarly, inflammatory cell density was assessed at 40 X (Figure 2B and C). Furthermore, we evaluated the sections for bone renewal, winding of the nerve root and adhesions between dura and arachnoid.
| Grade | Width of the scar tissue |
| 0 | No scar tissue in dura mater |
| 1 | Thin fibrosis bands present between scar tissue and dura mater |
| 2 | Adhesions holding 2/3 of the laminectomy defect present |
| 3 | Widespread scar. More than 2/3 of the laminectomy defect affected |
Microvessel density in the specimens was determined based on immunostaining with anti-osteopontin and anti-CD105 monoclonal antibodies. Paraffin-embedded tissues fixed in 10% formalin were sectioned at 3-5 mm and mounted onto poly-L-lysine-coated slides. The immunohistochemical study was done in the Leica Bond III. The slides were kept at 80°C in the incubator for 3 h and installed in the Bond III device. For the primary antibodies, 1/400 dilution was prepared from CD105 (Clone EP274, catalog number AC-0243A, Epitomics, Burlingame, CA, United States) and 1/100 dilution was prepared for osteopontin (Clone EP106, catalog number AC-0102RUO; Epitomics, Burlingame, CA, United States). After automatized coloring, the slides were kept in xylene for 3 min and sealed.
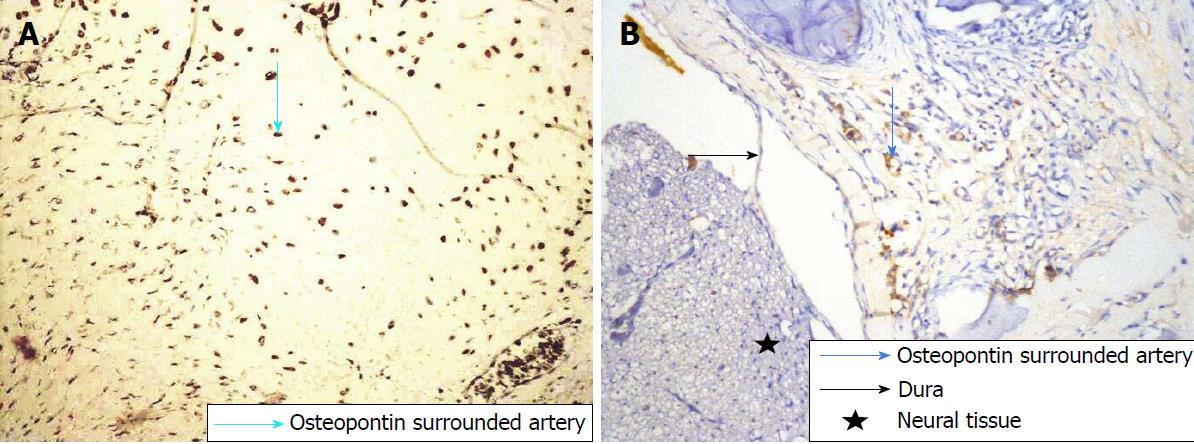
Using an Olympus (Shinjuku, Japan) CX41 light microscope, tissue sections immunostained with anti-CD105 and anti-osteopontin were scanned at 40X and 100X in three different areas where the density of neovascularization was the highest (hot spot). A single brown-stained endothelial cell or interconnected endothelial cell cluster separate from fibrosis and other connective tissue components at 400 X magnification, irrespective of lumens, was considered a countable microvessel (Figures 3A and B; 4A and B). Every experimental animal was classified in accordance with the number of stained vessels (Table 2) observed at 400 X magnification as follows: Grade 1, ≤ 3 vessels; grade 2, 4-6 vessels; and grade 3, ≥ 7 vessels. Vessels in the muscle layer were not included in the counts. The average number of vessels in three areas was calculated as the vessel density.
| Grade | Average number of microvessels (400 x) |
| 1 | ≤ 3 |
| 2 | 4-6 |
| 3 | ≥ 7 |
SPSS 15.0 for Windows (IBM, Armonk, NY, United States) was used for statistical analysis. Descriptive statistics were given as percentages for categorical variables. Differences in the proportional rates of categorical variables between groups were tested by chi-square analysis. In cases where the conditions could not be ensured, Monte Carlo simulation was implemented. Statistical significance was accepted as P < 0.05. All statistical procedures were carried out by an experienced biostatistician at Arat Biostatistics LLC (Istanbul, Turkey) through outsourcing.
Significant differences (P < 0.001) in fibrosis score, fibroblast density, inflammatory cell density and CD105 immunostaining were observed between the sorafenib and control groups (Table 3). All control animals (Group I) received fibrosis scores of grade 3, while scores for sorafenib-treated animals (Group II) did not exceed grade 1 (P < 0.001) (Figure 2A and B, Table 3). Fibroblast density was graded the highest in 100% of Group 1 animals but remained at grade 1 in Group II. Scores for inflammatory cell density were lowest in Group II but highest in Group I, as were scores for microvessel density assessed by CD105 immunostaining (P < 0.001) (Figure 3A and B, Table 3). There was no significant difference in microvessel density scores between groups after osteopontin staining (P = 0.355) (Figure 4A and B, Table 3). By light microscopy, it could be seen that EF thickness, inflammatory cell density and arachnoidal adhesions were greater in control animals compared to Group II (Table 3).
| Control (Group 1) | Sorafenib (Group 2) | |||||
| Assessment measure | Grade | n | % | n | % | P |
| Fibrosis score | 0 | 0 | 0 | 4 | 50 | < 0.001 |
| 1 | 0 | 0 | 4 | 50 | ||
| 3 | 8 | 100 | 0 | 0 | ||
| Fibroblast density | 1 | 0 | 0 | 8 | 100 | < 0.001 |
| 3 | 8 | 100 | 0 | 0 | ||
| Inflammatory cell density | 1 | 0 | 0 | 6 | 75 | < 0.001 |
| 2 | 2 | 25 | 2 | 25 | ||
| 3 | 6 | 75 | 0 | 0 | ||
| CD105-stained microvessels | 1 | 0 | 0 | 6 | 75 | < 0.001 |
| 2 | 2 | 25 | 2 | 25 | ||
| 3 | 6 | 75 | 0 | 0 | ||
| Osteopontin-stained microvessels | 1 | 4 | 50 | 7 | 87.5 | 0.355 |
| 2 | 2 | 25 | 1 | 12.5 | ||
| 3 | 2 | 25 | 0 | 0 | ||
EF is a major cause of failed back/lumbar surgery syndrome, which is often witnessed post-laminectomy[1,2]. Because treatment of EF is difficult and complication rates are high, ongoing studies have examined how to prevent its development. The role of angiogenesis in the development of fibrosis is known, with VEGF acting as a potent angiogenic agent[30]. Sorafenib inhibits VEGF in neoplastic cells and blocks angiogenesis, causing the death of tumor cells. The same mechanism is also applicable in the development of EF, and it has been shown that sorafenib prevents or inhibits the development of fibrosis in the liver and the kidney[13,19,31].
In the present study, the level/degree of EF was classified separately based on fibrosis scores, the density of fibroblasts and inflammatory cells, and counts of microvessels immunostained for either CD105 or osteopontin. Our results showed that EF developed to various extents in all control animals, indicating that experimental EF was successfully produced. The local application of sorafenib reduced the level and degree of EF compared to the control animals. We also found that the scoring of EF based on counts of osteopontin-stained microvessels did not agree with other measures used.
EF is a normal process with respect to the local reparation of surgical laminectomy, but its role in the production of epidural scar tissue has been controversial[2,10,14]. Clinical studies and results from the animal experiments have shown that many factors are related to the development of post-operative peridural scar tissue including postoperative hematoma, laminectomy technique, amount of bone removed and the relation of lamina to other anatomic structures[2,32]. However, the main factor in the creation of peridural scar tissue is fibroblast migration to the surgical area[32]. Theoretically, the augmentation of vascular permeability in the early phase of wound repair allows for the accumulation of fibrin-rich matrix required for cellular migration and proliferation[2,33]. The angiogenic response is characteristic of the early wound-repair phase and ensures vascularity, which nourishes newly formed granulation tissue. The most potent stimulant of angiogenesis is VEGF. In addition, other growth factors including FGF, PDGF, angiopoietin 1 and 2 and hepatocyte growth factor stimulate angiogenesis through TK receptors[30]. Furthermore, it is known that VEGF is helpful for vascularization in the postoperative damaged area and plays a key role in adhesion formation[8,10].
Sorafenib, a TK inhibitor, has been certified by the FDA for use as an antineoplastic agent demonstrating VEGF inhibitory and antiangiogenic effects. In work carried out by Ma et al[12], it was reported that sorafenib exerted an antifibrotic effect on the kidney and that this effect occurred as the result of its prevention of macrophage migration. In the present study, we showed through immunohistochemical staining that sorafenib applied locally to the surgical area prevented neovascularization in damaged postoperative tissue, thereby inhibiting or preventing EF. Furthermore, sorafenib did not produce any toxic effects on the dura, paraspinal muscles or other tissues.
One method to determine the level of angiogenesis in a tumor and/or fibrotic lesion is to directly measure the density of microvessels and veins in the tissue. An increase in the number of microvessels indicates that angiogenesis is strong[34]. Detection of microvessels in fibrotic tissue is possible by making the vessels visible after staining for proteins in the membranes of endothelial cells using immunohistochemical methods. CD105 is a potential immunohistochemical marker for the detection of microvessel density, which is associated with endothelial cells in angiogenic tissues and usually produces little or only weak color when used for immunohistochemical staining in normal tissue. It is this fact that makes anti-CD105 a useful antibody for the evaluation of angiogenesis or angiogenic potential[35,36]. In our study, we showed that the staging of EF using fibroblast counts and fibrosis density produced similar results as counting CD105-stained microvessels. Thus, CD105 is a suitable marker for immunohistochemical detection of EF.
Another potential immunohistochemical marker for the detection of fibrosis is osteopontin. While osteopontin is expressed by epithelium, endothelium and smooth muscle cells, it is also expressed by macrophages and T cells with infiltration features[26]. Osteopontin plays a significant role during acute and chronic inflammation when its anti-inflammatory effect can prevent inflammation or alleviate its severity[26]. Osteopontin is essential for cell viability and protects against apoptosis[26]. There are reports of excessive expression of osteopontin in human idiopathic pulmonary fibrosis, interstitial fibrosis in the diabetic kidney and alcoholic liver illness even though full deterioration did not occur in the healing wound and the number of irregular collagen fibrils was less than normal[27,28,31,37,38]; however, other reports claimed that the elimination of tissue debris was a slower than normal process[27]. Pereira et al[28] reported that osteopontin did not show equal distribution in all tissues and that its presence might be directly related to pathophysiology during wound healing and formation of scar tissue. Another study suggested that inhibition of osteopontin synthesis, oscillation or local activity after spinal surgery diminished EF and the frequency of symptoms[28]. In our study, osteopontin levels did not significantly correlate with histopathological EF evaluation methods or immunohistochemically determined CD105 levels. Consequently, osteopontin cannot be considered a reliable marker for the immunohistochemical detection of EF.
To the best of our knowledge, there have not been any studies that investigate the impact of sorafenib on spinal EF. Nevertheless, other research on tumors has shown that the anti-angiogenic effect of VEGF inhibition can reduce neovascularization and make an affirmative contribution to life span[39]. We have reached a similar conclusion in our study. Sorafenib decreased neovascularization in damaged tissue and thus significantly reduced the development of EF as shown using the CD105 antibody, although osteopontin was not a reliable marker.
Secondary operations required because of EF are both challenging and have a high complication rate[1,3,4]. Braverman et al[40] reported a success rate of only 30%-35% in operations performed by them to correct EF and observed adverse results in 10%-20% of cases. Rather than removing or relieving the pressure created by EF, it would be a far better approach to prevent EF from the start[5].
In conclusion, the data obtained from our study showed that when sorafenib was locally applied post-laminectomy, EF was significantly reduced. The staging of fibrosis evaluated by immunohistochemical staining for CD105 was compatible with conventional methods, but staging using osteopontin was not reliable. Neither toxic effects on tissues nor systematic side effects were observed with the use of sorafenib. Therefore, there should be no hindrance to the application of sorafenib for clinical purposes.
Spinal epidural fibrosis (EF) is a natural consequence of surgical trauma arising after laminectomy. In this study, we asked whether sorafenib can prevent the development of EF post-laminectomy using an immunohistochemical approach to quantify EF with CD105 and osteopontin antibodies.
EF is one of most common causes of failed back surgery syndrome, which occurs after laminectomy. Numerous causes and mechanisms have been proposed to explain its development after laminectomy. As treatment approaches for EF are associated with high rates of complications and failed surgery, the main goal is the prevention of EF. Many methods and medicines have been tried in order to prevent the development of EF. Sorafenib is an antineoplastic medicine that has demonstrated preventive effects against fibrosis due to an antiangiogenic mechanism involving inhibition of vascular endothelial growth factor (VEGF).
The goal of this study was to assess VEGF inhibition for the postoperative treatment of fibrosis.
Wistar albino rats (n = 16) were divided into two groups: control (laminectomy only) and sorafenib treatment (laminectomy + topical sorafenib). The animals were euthanatized after six weeks, and EF tissue was examined for histopathological changes after immunohistochemical staining and an EF grade was assigned. SPSS 15.0 for Windows (IBM, Armonk, NY, United States) was used for statistical analysis. Statistical significance was accepted as P < 0.05.
By light microscopy, EF thickness, inflammatory cell density and arachnoid adherences were higher in the control group compared to sorafenib-treated animals. Immunohistochemical staining for CD105 to identify microvessels revealed that EF grade was lower in the treatment group based on vessel count. Staining for osteopontin did not show any statistically significant differences in the extent of EF between groups. Significant differences in fibrosis score, fibroblast density, inflammatory cell density and CD105 immunostaining were observed between the sorafenib and control groups (P < 0.001). All control animals (Group I) received fibrosis scores of grade 3, while scores for sorafenib-treated animals (Group II) did not exceed grade 1 (P < 0.001). Fibroblast density was graded the highest in 100% of Group 1 animals but remained at grade 1 in Group II. Scores for inflammatory cell density were lowest in Group II but highest in Group I, as were scores for microvessel density assessed by CD105 immunostaining (P < 0.001). There was no significant difference in microvessel density scores between groups after osteopontin staining (P = 0.355). By light microscope, it could be seen that EF thickness, inflammatory cell density and arachnoidal adhesions were greater in control animals compared to Group II.
In this study, we examined the efficacy of topical treatment with sorafenib for the prevention of EF in an animal laminectomy model and analyzed immunohistochemical methods for the assessment of microvessel density in fibrotic lesions compared to conventional measures of fibrosis staging. Our results demonstrated that topical sorafenib was effective in reducing EF after laminectomy, likely due to decreased neovascularization resulting from the antiangiogenic effect of sorafenib on VEGF activity. We further show that immunohistochemical assessment of microvessel density using anti-CD105 antibodies provided a new measure of fibrotic development that was compatible with conventional methods of fibrosis staging.
In our study, the local application of sorafenib after laminectomy prevents EF. CD105 is a suitable marker for fibrosis, whereas osteopontin was not found to be reliable. Sorafenib was not observed to have any toxic effects or systemic side effects on normal tissues. Therefore, this application could be tested in clinical trials.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Chui YL, Tajiri K, Wang Y S- Editor: Cui LJ L- Editor: Filipodia E- Editor: Tan WW
| 1. | Gunaldi O, Erdogan S, Guclu DG, Tugcu B, Ofluoglu E, Baydin S, Emel E. “Honey” can prevent epidural fibrosis development after laminectomy: an experimental study. Turk Neurosurg. 2014;24:849-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Ozkan U, Osun A, Samancioglu A, Ercan S, Firat U, Kemaloglu S. The effect of bevacizumab and 5-Fluorouracil combination on epidural fibrosis in a rat laminectomy model. Eur Rev Med Pharmacol Sci. 2014;18:95-100. [PubMed] |
| 3. | Altun I. An Experimental Study of Histopathologic Effects of Hemostatic Agents Used in Spinal Surgery. World Neurosurg. 2016;90:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Larionov SN, Sorokovikov VA, Erdyneyev KC, Lepekhova SA, Goldberg OA. Experimental Model of Intervertebral Disk Mediated Postoperative Epidural Fibrosis. Ann Neurosci. 2016;23:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Henderson R, Weir B, Davis L, Mielke B, Grace M. Attempted experimental modification of the postlaminectomy membrane by local instillation of recombinant tissue-plasminogen activator gel. Spine (Phila Pa 1976). 1993;18:1268-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Zhang C, Kong X, Zhou H, Liu C, Zhao X, Zhou X, Su Y, Sharma HS, Feng S. An Experimental Novel Study: Angelica sinensis Prevents Epidural Fibrosis in Laminectomy Rats via Downregulation of Hydroxyproline, IL-6, and TGF- β 1. Evid Based Complement Alternat Med. 2013;2013:291814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Farrokhi MR, Vasei M, Fareghbal S, Farrokhi N. The effect of methylene blue on peridural fibrosis formation after laminectomy in rats: an experimental novel study. Spine J. 2011;11:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Karatay M, Erdem Y, Koktekir E, Erkoc YS, Caydere M, Bayar MA. The effect of bevacizumab on spinal epidural fibrosis in a postlaminectomy rat model. Turk Neurosurg. 2012;22:753-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Wang S, Li X, Yan L, Nie Q, Dai J, Chen H, Wang J, Sun Y. Tamoxifen inhibits fibroblast proliferation and prevents epidural fibrosis by regulating the AKT pathway in rats. Biochem Biophys Res Commun. 2018;497:937-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Yilmaz A, Karatay M, Yildirim T, Celik H, Sertbas I, Erdem Y, Caydere M, Isik HS, Bayar MA. Prevention of Epidural Fibrosis Using Ranibizumab in a Postlaminectomy Rat Model. Turk Neurosurg. 2017;27:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Kaya M, Yildirim CH, Kosemehmetoglu K, Huseyinoglu U, Erdogan H, Akbasak A, Tasdemiroglu E. Alpha-lipoic acid reduces peridural fibrosis after laminectomy of lumbar vertebrae in rabbits. Acta Neurochir (Wien). 2012;154:1241-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Ma W, Tao L, Wang X, Liu Q, Zhang W, Li Q, He C, Xue D, Zhang J, Liu C. Sorafenib Inhibits Renal Fibrosis Induced by Unilateral Ureteral Obstruction via Inhibition of Macrophage Infiltration. Cell Physiol Biochem. 2016;39:1837-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Qu K, Huang Z, Lin T, Liu S, Chang H, Yan Z, Zhang H, Liu C. New Insight into the Anti-liver Fibrosis Effect of Multitargeted Tyrosine Kinase Inhibitors: From Molecular Target to Clinical Trials. Front Pharmacol. 2016;6:300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Geisler FH. Prevention of peridural fibrosis: current methodologies. Neurol Res. 1999;21 Suppl 1:S9-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Hamby JM, Showalter HD. Small molecule inhibitors of tumor-promoted angiogenesis, including protein tyrosine kinase inhibitors. Pharmacol Ther. 1999;82:169-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59:99-106. [PubMed] |
| 17. | Beyer C, Distler JH. Tyrosine kinase signaling in fibrotic disorders: Translation of basic research to human disease. Biochim Biophys Acta. 2013;1832:897-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Heldin CH. Targeting the PDGF signaling pathway in the treatment of non-malignant diseases. J Neuroimmune Pharmacol. 2014;9:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Prieto M, Rodríguez-Peña A, Arévalo M, Rivas JV, Düwel A, Eleno N, Sánchez RJ, Morales AI, López-Novoa JM, Pérez-Barriocanal F. Effect of the long-term treatment with trandolapril on endoglin expression in rats with experimental renal fibrosis induced by renal mass reduction. Kidney Blood Press Res. 2005;28:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | García-Pozo L, Miquilena-Colina ME, Lozano-Rodríguez T, García-Monzón C. Endoglin: structure, biological functions, and role in fibrogenesis. Rev Esp Enferm Dig. 2008;100:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Gougos A, St Jacques S, Greaves A, O’Connell PJ, d’Apice AJ, Bühring HJ, Bernabeu C, van Mourik JA, Letarte M. Identification of distinct epitopes of endoglin, an RGD-containing glycoprotein of endothelial cells, leukemic cells, and syncytiotrophoblasts. Int Immunol. 1992;4:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Lastres P, Bellon T, Cabañas C, Sanchez-Madrid F, Acevedo A, Gougos A, Letarte M, Bernabeu C. Regulated expression on human macrophages of endoglin, an Arg-Gly-Asp-containing surface antigen. Eur J Immunol. 1992;22:393-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 171] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | St-Jacques S, Cymerman U, Pece N, Letarte M. Molecular characterization and in situ localization of murine endoglin reveal that it is a transforming growth factor-beta binding protein of endothelial and stromal cells. Endocrinology. 1994;134:2645-2657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Letamendía A, Lastres P, Botella LM, Raab U, Langa C, Velasco B, Attisano L, Bernabeu C. Role of endoglin in cellular responses to transforming growth factor-beta. A comparative study with betaglycan. J Biol Chem. 1998;273:33011-33019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 160] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | García-Monzón C, Sánchez-Madrid F, García-Buey L, García-Arroyo A, García-Sánchez A, Moreno-Otero R. Vascular adhesion molecule expression in viral chronic hepatitis: evidence of neoangiogenesis in portal tracts. Gastroenterology. 1995;108:231-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Mazzali M, Kipari T, Ophascharoensuk V, Wesson JA, Johnson R, Hughes J. Osteopontin--a molecule for all seasons. QJM. 2002;95:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 281] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 27. | Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 853] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 28. | Pereira P, Avelino A, Monteiro P, Vaz R, Castro-Lopes JM. New insights from immunohistochemistry for the characterization of epidural scar tissue. Pain Physician. 2014;17:465-474. [PubMed] |
| 29. | He Y, Revel M, Loty B. A quantitative model of post-laminectomy scar formation. Effects of a nonsteroidal anti-inflammatory drug. Spine (Phila Pa 1976). 1995;20:557-563; discussion 579-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 131] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Fayette J, Soria JC, Armand JP. Use of angiogenesis inhibitors in tumour treatment. Eur J Cancer. 2005;41:1109-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Junaid A, Amara FM. Osteopontin: correlation with interstitial fibrosis in human diabetic kidney and PI3-kinase-mediated enhancement of expression by glucose in human proximal tubular epithelial cells. Histopathology. 2004;44:136-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Kasimcan MO, Bakar B, Aktaş S, Alhan A, Yilmaz M. Effectiveness of the biophysical barriers on the peridural fibrosis of a postlaminectomy rat model: an experimental research. Injury. 2011;42:778-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Breuing K, Eriksson E, Liu P, Miller DR. Healing of partial thickness porcine skin wounds in a liquid environment. J Surg Res. 1992;52:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Koukourakis MI, Giatromanolaki A, Thorpe PE, Brekken RA, Sivridis E, Kakolyris S, Georgoulias V, Gatter KC, Harris AL. Vascular endothelial growth factor/KDR activated microvessel density versus CD31 standard microvessel density in non-small cell lung cancer. Cancer Res. 2000;60:3088-3095. [PubMed] |
| 35. | Weidner N. The importance of tumor angiogenesis: the evidence continues to grow. Am J Clin Pathol. 2004;122:675-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Yao Y, Kubota T, Takeuchi H, Sato K. Prognostic significance of microvessel density determined by an anti-CD105/endoglin monoclonal antibody in astrocytic tumors: comparison with an anti-CD31 monoclonal antibody. Neuropathology. 2005;25:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Pardo A, Gibson K, Cisneros J, Richards TJ, Yang Y, Becerril C, Yousem S, Herrera I, Ruiz V, Selman M. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005;2:e251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 385] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 38. | Xiao X, Gang Y, Gu Y, Zhao L, Chu J, Zhou J, Cai X, Zhang H, Xu L, Nie Y. Osteopontin contributes to TGF-β1 mediated hepatic stellate cell activation. Dig Dis Sci. 2012;57:2883-2891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Plastaras JP, Kim SH, Liu YY, Dicker DT, Dorsey JF, McDonough J, Cerniglia G, Rajendran RR, Gupta A, Rustgi AK. Cell cycle dependent and schedule-dependent antitumor effects of sorafenib combined with radiation. Cancer Res. 2007;67:9443-9454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 40. | Braverman DL, Slipman CW, Lenrow DA. Using gabapentin to treat failed back surgery syndrome caused by epidural fibrosis: A report of 2 cases. Arch Phys Med Rehabil. 2001;82:691-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |