Published online Jul 16, 2018. doi: 10.12998/wjcc.v6.i7.150
Peer-review started: February 22, 2018
First decision: March 12, 2018
Revised: March 15, 2018
Accepted: April 22, 2018
Article in press: April 22, 2018
Published online: July 16, 2018
Processing time: 144 Days and 12 Hours
Surgery is the first choice of treatment for patients with non-small-cell lung cancer (NSCLC), but few patients can be treated surgically because of either advanced disease or poor pulmonary function. Other therapies include radiotherapy and chemotherapy, as well as complementary and alternative therapies, usually with disappointing results. Bronchial artery infusion (BAI) is a manageable and effective method for treating advanced NSCLC. Outcome is good by BAI due to its repeatability and low toxicity. Icotinib hydrochloride is a newly developed and highly specific epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor and has been safely and efficiently used to treat advanced NSCLC. We herein report a 73-year-old patient with chronic cough, who was diagnosed with advanced NSCLC with the EGFR mutation of L858R substitution in exon 21, and treated with the combination of oral icotinib and BAI chemotherapy as the first-line therapy, which resulted in a satisfactory clinical outcome. Complete remission of advanced NSCLC can be achieved using the combination of oral icotinib and BAI chemotherapy.
Core tip: Few patients can undergo surgery for treatment of non-small-cell lung cancer because of advanced disease or poor pulmonary function. Combination of bronchial artery infusion of anti-cancer agents and oral targeted drug is safe, tolerable, and effective for patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer (NSCLC). Complete remission of advanced NSCLC can be achieved by this combination therapy.
- Citation: Yang NN, Xiong F, He Q, Guan YS. Achievable complete remission of advanced non-small-cell lung cancer: Case report and review of the literature. World J Clin Cases 2018; 6(7): 150-155
- URL: https://www.wjgnet.com/2307-8960/full/v6/i7/150.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i7.150
Advanced lung cancer is inoperable, and systemic chemotherapy, radiotherapy, or complementary and alternative therapies are usually unsatisfactory. Bronchial artery infusion (BAI) has become an effective treatment for patients with unresectable non-small-cell lung cancer (NSCLC). During this procedure, chemotherapeutic drugs at high concentrations are injected directly into the bronchial arteries which provide blood supply to the tumors, thus the symptoms and adverse effects caused by the anti-cancer drugs are greatly reduced. Treatment with oral targeted drug is very convenient and has been proposed as a novel therapy for patients with advanced NSCLC. As a novel targeted drug and highly specific epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), icotinib hydrochloride (BPI-2009H, ConMana) has been shown to be potent and provide a survival benefit in selected patients with advanced NSCLC[1]. And it has been approved as the first-line therapy in patients with advanced NSCLC with sensitive mutation on February 22, 2011, by China Food and Drug Administration (CFDA)[2]. Oral icotinib combined with BAI chemotherapy can yield a synergetic and complementary effect in patients with advanced NSCLC. We herein describe an elderly patient with advanced NSCLC who was treated with oral icotinib hydrochloride combined with BAI chemotherapy as the first-line therapy, which resulted in a very good clinical outcome.
A 73-year-old man, who had never smoked and no relevant medical and family history, was admitted to our institution on February 24, 2014 with a 2-mo history of cough. After a series of medical examinations including laboratory examinations, Transverse computed tomography (CT) scan, percutaneous lung biopsy and genetic testing, he was diagnosed with advanced NSCLC (left lower lobe, peripheral type, pT4cN0M0, stage IIIA adenocarcinoma, moderate differentiation), and EGFR mutation was found with the L858R substitution in exon 21. He was not a candidate for surgical treatment because of his poor lung function and the grade 3 physical performance evaluated by Eastern Cooperative Oncology Group performance score. The patient denied radiotherapy or systemic chemotherapy, but opted for transarterial infusion chemotherapy and oral targeted drugs.
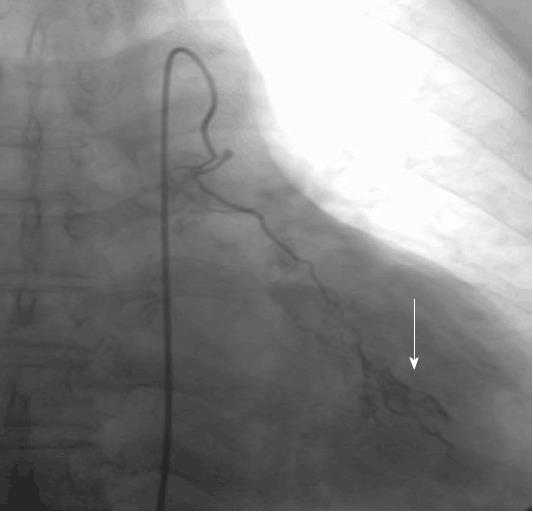
Witten informed consent was obtained from the patient before the BAI. On February 28, 2014 after sufficient preoperative preparation, the patient received left bronchial artery chemical infusion (Figure 1) with 60 mg cisplatin, 40 mg hydroxycamptothecine, and 1000 mg 5-fluorouracil, respectively. On March 4, 2014, the patient started to take icotinib hydrochloride orally (125 mg, every eight hours).
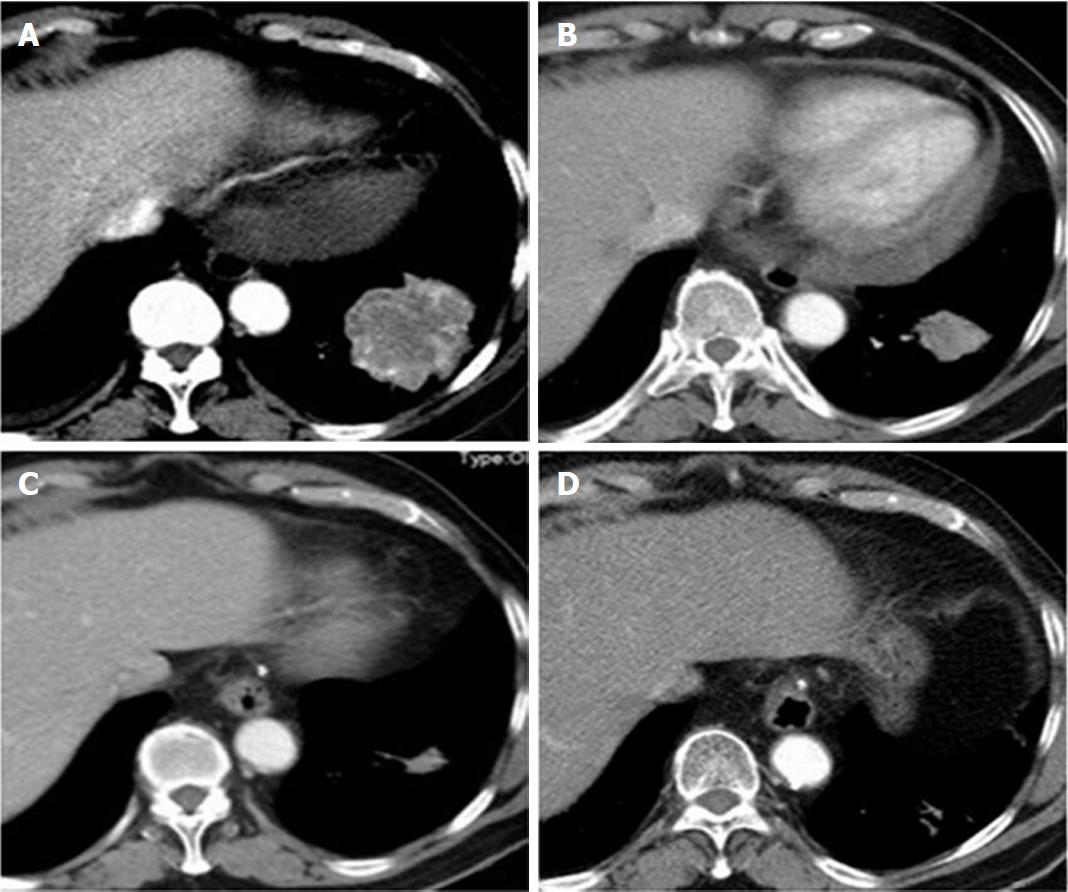
A follow-up CT scan was performed 39 d after the BAI and 34 d after the beginning of oral icotinib hydrochloride. It revealed that the tumor regressed significantly compared with that in the early image (Figure 2A and B). No adverse reaction was observed, and the objective response was evaluated to be partial response according to Response Evaluation Criteria in Solid Tumors 1.1 criteria (RECIST 1.1)[3], and the physical conditions and the quality of life of the patient were markedly improved. His physical performance was scored as grade 1.
Seven months later, another transverse CT scan showed that the tumor further regressed (Figure 2A and C), and the objective response was evaluated to be approximately complete regression (CR) as a biopsy of the lesion showed no tumor cells, but fiber scar tissue. His physical performance was scored as grade 1.
Up till now, the patient has taken oral icotinib hydrochloride for more than 48 mo, and the latest transverse CT scan on December 17, 2017 showed that the tumor tissue almost disappeared (Figure 2C and D). The objective response was evaluated to be approximately CR, and his physical performance was scored as grade 1.
Intra-arterial infusion chemotherapy has been introduced to medical treatment of tumors for more than 50 years[4]. This therapy has the potential to reduce the tumor size and to relieve symptoms, with low toxicity and good repeatability. It is especially suitable for the patients with advanced lung cancer who are intolerable to systemic chemotherapy or radiotherapy[5-8]. Therefore, it is a treatment option for advanced NSCLC patients.
The feeding arteries of locally advanced lung cancer include not only the bronchial arteries, but also various other feeding arteries, which need to be detected precisely by arterial angiography[5]. Precise and extensive angiographic examinations to detect feeding arteries are crucial, and a prerequisite for achieving positive results. A study reported that, among the feeding arteries of lung cancer, bronchial arteries showed the best response to BAI[6]. This method employs direct injection of chemotherapeutics at high concentrations into local lesions of lung cancer and only needs half of the dosage required for systemic chemotherapy[9,10]. The local potency of the anti-cancer drugs administered via BAI to the lesion area is 2-6 times that of the same drugs administered via the intra-venous route[11]. Another study reported that the overall effective rate of BAI was 55.3% in patients with stage III hilar lung cancer[9]. One study demonstrated that the BAI therapy not only reduced the tumor size but also extended patient survival, and improved quality of life of the patients[5].
BAI therapy has the following advantages: allowing doctors to utilize small dosage of anti-cancer agents, but deliver relatively large dosage of the agents into the tumor in situ with minimal systemic side effects to achieve high efficiency of local control, and this therapy is safe and feasible because the side effects are mild[5,8].
However, the efficacy of this therapy for lung cancer has not been sufficiently verified, and BAI is an invasive treatment which may lead to some severe adverse effects, such as spinal paralysis, bronchial ulcers, esophageal ulcers, hemoptysis, pulmonary toxicity and renal injury[12]. We used cisplatin, hydroxycamptothecine and 5-fluorouracil as arterial infusion chemotherapy agents. However, it is necessary to determine the appropriate dosages of the chemotherapeutic drugs for selected patients[5]. During this treatment, just like for systemic chemotherapy, the patients need to be hospitalized repeatedly, which consumes more time on taking care of the patients and increases the economic burden of the patients. These limitations prevent the wide application of BAI as a standard clinical therapy for lung cancer[5,6]. Nevertheless, in our case, the patient was hospitalized only once and received only one procedure of BAI to control rapid growth of the tumor, and the total hospitalization expense was 1728.3 US Dollar, which was markedly lower compared to the expenses for other therapeutic methods.
A current single-center retrospective study which enrolled 40 consecutive patients with advanced NSCLC who underwent transcatheter arterial chemical infusion showed that the total response rate was 32.5%, the disease control rate was 92.5%, and the mean time to tumor progression (TTP) and overall survival (OS) was 9.2 ± 1.4 and 13.1 ± 2.0 mo, respectively[13]. However, the long-term outcome and overall survival are still unclear. The beneficial effect of regional therapy on survival or disease control is usually limited when used alone.
NSCLC with mutations in the EGFR gene is a distinct subgroup of NSCLCs which is particularly sensitive to EGFR-TKIs[14,15]. The most common EGFR mutations in NSCLC were the L858R substitution in exon 21 and the deletions in exon 19. EGFR-TKI is the most effective therapy for patients with advanced EGFR-mutant NSCLC[16].
Icotinib hydrochloride is the first self-developed small molecular drug in China, and was approved by the State Food and Drug Administration of China for the treatment of locally advanced or metastatic NSCLC[1,17]. It was demonstrated that icotinib is inferior to gefitinib in terms of median progression free survival (PFS)[18]. A single-center study evaluated the efficacy of icotinib after its approval as a monotherapy for advanced NSCLC patients with EGFR mutation and patients with wild-type EGFR. The results showed that in the 36 patients with EGFR mutation, the overall response rate (ORR) and disease control rate (DCR) were 58.3% and 88.9%, respectively; while in the 13 patients with wild-type EGFR, the ORR and DCR were 7.7% and 53.8%, respectively[19]. Another study evaluated the efficacy of icotinib as the first-line treatment of pulmonary adenocarcinoma and showed that among a total of 56 patients with lung adenocarcinoma, the ORR and DCR were 46.4% (26/56) and 78.6% (46/56), respectively. In the patients with EGFR mutation, the ORR and DCR were 66.7% (12/18) and 94.4% (17/18), respectively[20].
After long-term treatment with oral targeted drugs, however, nearly all the patients will inevitably develop drug resistance with disease progression after 6-12 mo of treatment[21,22]. Therefore, more in-depth studies on optimizing combination strategies and overcoming drug resistance to icotinib are warranted[22]. One study reported that combination of EGFR-TKI therapy and systemic chemotherapy yielded disappointing results after disease progression using the first-line EGFR-TKI therapy[23]. But an ASPIRATION trial (Asian Pacific trial of Tarceva as first-line therapy in EGFR mutation) showed that, when the first disease progression occurred, continuing with EGFR-TKI therapy might be beneficial in patients with asymptomatic and slow progression[24].
Therefore, we propose the use of arterial infusion chemotherapy in combination with EGFR-TKI therapy as the first-line therapy for disease control in patients with EGFR-mutant advanced NSCLC to achieve better therapeutic effects, extend the progression-free survival (PFS) and OS, and improve quality of life of the patients. A study on EGFR-TKI therapy in combination with arterial infusion chemotherapy reported that the median OS was 28.6 mo (range, 24.1-32.9 mo)[25]. In our case, the patient received arterial infusion chemotherapy combined with oral icotinib therapy as the first-line therapy and no apparent adverse effects were observed during the treatment for more than 48 mo. After the first cycle of icotinib (30 d), objective tumor response was evaluated to be partial remission without any adverse effects such as diarrhoea, acneiform skin rash, paronychia, and so on[26], and physical conditions and the quality of life were markedly improved. The objective tumor response was evaluated to be almost CR, and further puncture biopsy will be obtained to confirm the therapeutic results at an appropriate time. The patient remained in good physical condition and the quality of life. Nevertheless, if possible, percutaneous ablation or minimally invasive thoracoscopic surgery may be performed to eliminate the residual fibrous scar tissue for radical cure.
Although BAI chemotherapy has the advantage of short treatment time and mild toxicity, its wide application is limited because of the requirements for sophisticated physician skills and specialized equipment. These two methods have been separately used in the treatment of NSCLC and resulted in relatively positive clinical efficacy. The combination of the two therapies is considered to generate synergetic and complementary effect in advanced NSCLC. However, the clinical efficacy of BAI chemotherapy combined with targeted therapy for the treatment of advanced NSCLC remains to be further investigated, and results from statistical analysis about the benefit of the treatment based on large-scale clinical trials are needed.
This case report suggests that the combination of oral icotinib hydrochloride and BAI chemotherapy is safe, well-tolerated and effective in Chinese patients suffering from advanced NSCLC with EGFR gene mutations. This strategy can be attempted for complete tumor remission.
A 73-year-old man who was diagnosed with advanced non-small-cell lung cancer (NSCLC), received the combination therapy of bronchial artery infusion chemotherapy and oral icotinib hydrochloride, and the objective response was evaluated to be approximately complete regression.
The patient was admitted to our institution with a 2-mo history of cough.
The differential diagnosis included pulmonary tuberculosis, lobular pneunonia, or benign lung tumors.
Blood test was normal. Genetic testing revealed that epidermal growth factor receptor (EGFR) mutation was found with the L858R substitution in exon 21.
Transverse computed tomography scan showed the tumor mass in the basal segment of the left lower lobe and the greatest dimension of the tumor measured 5.7 cm.
Examination of the pathologic specimen after percutaneous lung biopsy, confirmed a moderate differentiated adenocarcinoma.
The patient received left bronchial artery chemical infusion (60 mg cisplatin, 40 mg hydroxycamptothecine, and 1000 mg 5-fluorouracil, respectively) and oral icotinib hydrochloride (125 mg, every eight hours) was initiated on postoperative day 4.
Other studies on EGFR-TKI therapy in combination with arterial infusion chemotherapy reported that the median OS was 28.6 mo, and in our case, the patient received the treatment for more than 48 mo without apparent adverse effects.
Bronchial artery chemical infusion employs direct injection of chemotherapeutics at high concentrations into local lesions of lung cancer, with the potential to reduce the tumor size and to relieve symptoms, with low toxicity and good repeatability.
This case report suggests that the combination of oral icotinib hydrochloride and BAI chemotherapy is safe, well-tolerated and effective in Chinese patients suffering from advanced NSCLC with EGFR gene mutations.
CARE Checklist (2013) statement: This report follows the guidelines of the CARE Checklist (2013).
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cihan YB, Mehdi I S- Editor: Cui LJ L- Editor: A E- Editor: Tan WW
| 1. | Tan F, Shen X, Wang D, Xie G, Zhang X, Ding L, Hu Y, He W, Wang Y, Wang Y. Icotinib (BPI-2009H), a novel EGFR tyrosine kinase inhibitor, displays potent efficacy in preclinical studies. Lung Cancer. 2012;76:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Shi Y, Sun Y, Yu J, Ding C, Wang Z, Wang C, Wang D, Wang C, Wang Z, Wang M. China experts consensus on the diagnosis and treatment of advanced stage primary lung cancer (2016 version). Asia Pac J Clin Oncol. 2017;13:87-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21640] [Article Influence: 1352.5] [Reference Citation Analysis (1)] |
| 4. | Kahn PC, Paul RE, Rheinlander HF. Selective bronchial arteriography and intra-arterial chemotherapy in carcinoma of the lung. J Thorac Cardiovasc Surg. 1965;50:640-645. [PubMed] |
| 5. | Nakanishi M, Umeda Y, Demura Y, Ameshima S, Chiba Y, Miyamori I, Ishizaki T. Effective use of multi-arterial infusion chemotherapy for advanced non-small cell lung cancer patients: four clinical specified cases. Lung Cancer. 2007;55:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Nakanishi M, Demura Y, Umeda Y, Mizuno S, Ameshima S, Chiba Y, Ishizaki T. Multi-arterial infusion chemotherapy for non-small cell lung carcinoma-significance of detecting feeding arteries and tumor staining. Lung Cancer. 2008;61:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Nakanishi M, Yoshida Y, Natazuka T. Prospective study of transarterial infusion of docetaxel and cisplatin to treat non-small-cell lung cancer in patients contraindicated for standard chemotherapy. Lung Cancer. 2012;77:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Yuan Z, Li WT, Ye XD, Dong S, Peng WJ. Intra-arterial infusion chemotherapy for advanced non-small-cell lung cancer: preliminary experience on the safety, efficacy, and clinical outcomes. J Vasc Interv Radiol. 2013;24:1521-8.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Watanabe Y, Shimizu J, Murakami S, Yoshida M, Tsubota M, Iwa T, Kitagawa M, Mizukami Y, Nonomura A, Matsubara F. Reappraisal of bronchial arterial infusion therapy for advanced lung cancer. Jpn J Surg. 1990;20:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Osaki T, Oyama T, Takenoyama M, Taga S, So T, Yamashita T, Nakata S, Nakanishi R, Yasumoto K. Feasibility of induction chemotherapy using bronchial arterial infusion for locally advanced non-small cell lung cancer: a pilot study. Surg Today. 2002;32:772-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Zhao G, Huang Y, Ye L, Duan L, Zhou Y, Yang K, Ma Q, Lei Y, Song X, Huang M. [Therapeutic efficacy of Traditional Vein Chemotherapy and Bronchial Arterial Infusion Combining with CIKs on III Stage Non-small Cell Lung Cancer.]. Zhongguo Fei Ai Za Zhi. 2009;12:1000-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 12. | Tanaka O, Hashimoto S, Narimatsu Y, Fujiwara H, Kurata T, Okuda S, Yamagami T, Nishimura T, Hiramatsu K, Kuribayashi S. Can selective CT angiography reduce the incidence of severe complications during transcatheter arterial embolization or infusion chemotherapy for thoracic diseases? Diagn Interv Radiol. 2006;12:201-205. [PubMed] |
| 13. | Fu YF, Li Y, Wei N, Xu H. Transcatheter arterial chemical infusion for advanced non-small-cell lung cancer: long-term outcome and predictor of survival. Radiol Med. 2016;121:605-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Kobayashi K, Hagiwara K. Epidermal growth factor receptor (EGFR) mutation and personalized therapy in advanced nonsmall cell lung cancer (NSCLC). Target Oncol. 2013;8:27-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Antonicelli A, Cafarotti S, Indini A, Galli A, Russo A, Cesario A, Lococo FM, Russo P, Mainini AF, Bonifati LG. EGFR-targeted therapy for non-small cell lung cancer: focus on EGFR oncogenic mutation. Int J Med Sci. 2013;10:320-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Paz-Ares L, Soulières D, Melezínek I, Moecks J, Keil L, Mok T, Rosell R, Klughammer B. Clinical outcomes in non-small-cell lung cancer patients with EGFR mutations: pooled analysis. J Cell Mol Med. 2010;14:51-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | Ren GJ, Zhao YY, Zhu YJ, Xiao Y, Xu JS, Shan B, Zhang L. Tumor gene mutations and messenger RNA expression: correlation with clinical response to icotinib hydrochloride in non-small cell lung cancer. Chin Med J (Engl). 2011;124:19-25. [PubMed] |
| 18. | Liu Y, Zhang Y, Feng G, Niu Q, Xu S, Yan Y, Li S, Jing M. Comparison of effectiveness and adverse effects of gefitinib, erlotinib and icotinib among patients with non-small cell lung cancer: A network meta-analysis. Exp Ther Med. 2017;14:4017-4032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Song Z, Yu X, Cai J, Shao L, Lin B, He C, Zhang B, Zhang Y. [Efficacy of icotinib for advanced non-small cell lung cancer patients with EGFR status identified]. Zhongguo Fei Ai Za Zhi. 2013;16:138-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 20. | Yang X, Zhang H, Qin N, Li X, Nong J, Lv J, Wu Y, Zhang Q, Zhang S. [Clinical observation of icotinib hydrochloride in first-line therapy for pulmonary adenocarcinoma]. Zhongguo Fei Ai Za Zhi. 2013;16:364-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 21. | Jackman D, Pao W, Riely GJ, Engelman JA, Kris MG, Jänne PA, Lynch T, Johnson BE, Miller VA. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2010;28:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 657] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 22. | Guan YS, He Q, Li M. Icotinib: activity and clinical application in Chinese patients with lung cancer. Expert Opin Pharmacother. 2014;15:717-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Soria JC, Wu YL, Nakagawa K, Kim SW, Yang JJ, Ahn MJ, Wang J, Yang JC, Lu Y, Atagi S. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015;16:990-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 318] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 24. | Park K, Yu CJ, Kim SW, Lin MC, Sriuranpong V, Tsai CM, Lee JS, Kang JH, Chan KC, Perez-Moreno P. First-Line Erlotinib Therapy Until and Beyond Response Evaluation Criteria in Solid Tumors Progression in Asian Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer: The ASPIRATION Study. JAMA Oncol. 2016;2:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 178] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 25. | Qi H, Jiang S, Yu D, Ni H, Hu Q, Zhang J. Continuing EGFR-TKI treatment in combination with super-selective arterial infusion chemotherapy beyond disease progression for patients with advanced EGFR-mutant non-small cell lung cancer. Med Oncol. 2015;32:256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Hsu WH, Yang JC, Mok TS, Loong HH. Overview of current systemic management of EGFR-mutant NSCLC. Ann Oncol. 2018;29:i3-i9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 266] [Article Influence: 44.3] [Reference Citation Analysis (0)] |