Published online May 16, 2018. doi: 10.12998/wjcc.v6.i5.74
Peer-review started: January 13, 2018
First decision: February 27, 2018
Revised: March 4, 2018
Accepted: March 19, 2018
Article in press: March 20, 2018
Published online: May 16, 2018
Processing time: 123 Days and 3 Hours
To evaluate the safety and efficacy of sorafenib plus transarterial chemoembolization (TACE) treatment for intermediate hepatocellular carcinoma (HCC).
Sixty-seven patients with intermediate-stage [Barcelona Clinic liver cancer stage B (BCLC-B)] HCC who were treated with sorafenib plus TACE or TACE alone between 2009 and 2011 were included in the study. Follow-up was until 2014 or patient death. Two groups were defined in the experiment: The experimental group, treated with sorafenib plus TACE, and the control group, treated with standard TACE alone.
The Kaplan-Meier survival analysis showed that the median overall survival (mOS) of the experimental group was 35.2 mo, while that of the control group was 22.0 mo (P < 0.05). Sorafenib plus TACE showed higher incidence rates of rash, hand-foot syndrome (HFS), and hypertension (P < 0.05) than TACE treatment alone.
Sorafenib plus TACE treatment for BCLC-B HCC significantly prolonged the mOS of patients compared to TACE treatment alone. The most common toxicities with sorafenib were rash (31.6%), HFS (39.5%) and hypertension (31.6%), but there were no intolerable adverse events. The Cox multivariate analysis showed that the survival of patients with BCLC-B HCC depended on the Child-Pugh classification, tumor diameter, and treatment with sorafenib plus TACE compared to TACE alone.
Core tip: Hepatocellular carcinoma (HCC) is a common digestive tract malignancy. Transarterial chemoembolization (TACE) is the standard treatment for intermediate-stage HCC, and it has a limited beneficial effect. To evaluate the safety and efficacy of sorafenib plus TACE treatment for intermediate-stage HCC. Sixty-seven patients with intermediate-stage HCC who were treated with sorafenib plus TACE or TACE alone between 2009 and 2011 were included in the study. This study confirms that sorafenib plus TACE treatment for intermediate-stage HCC significantly prolonged the median overall survival of patients compared to TACE treatment alone. Moreover, this new treatment approach showed tolerable toxicity.
- Citation: Lei XF, Ke Y, Bao TH, Tang HR, Wu XS, Shi ZT, Lin J, Zhang ZX, Gu H, Wang L. Effect and safety of sorafenib in patients with intermediate hepatocellular carcinoma who received transarterial chemoembolization: A retrospective comparative study. World J Clin Cases 2018; 6(5): 74-83
- URL: https://www.wjgnet.com/2307-8960/full/v6/i5/74.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i5.74
Hepatocellular carcinoma (HCC) is the fifth most common cancer among males and is also the second leading cause of cancer-related mortality; there are 9 and 6, respectively, among females around the world[1]. China, the incidence and deaths of HCC account for more than 50% of HCC cases in the world, is still facing the great challenge of disease burden caused by liver cancer[2]. HCC results in over 650000 deaths per year in the world[3]. HCC with occult onset has a high degree of malignancy and develops quickly, and the majority of patients who are diagnosed at a later stage cannot undergo surgical resection[4,5].
These patients who are not suitable for surgical treatment usually use transarterial chemoembolization (TACE), which achieves a limited beneficial effect[6]. The method has a high rate of local tumor control and has been observed to increase the survival of patients with intermediate-stage [Barcelona Clinic liver cancer stage B (BCLC-B)] HCC[7]. However, hypoxia caused by TACE in viable tumor cells leads to the release of angiogenic growth factors, which can induce tumor recurrence or metastasis and a poor outcome for patients[8].
Recently, with the molecular mechanism of HCC pathogenesis studied in-depth and targeted drug research and development, the multi-target signal transduction agent sorafenib has been FDA- and China FDA (CFDA)-approved for the treatment of HCC that cannot be surgically resected and presents distant metastases[9,10]. Sorafenib has been shown to inhibit tumor angiogenesis, tumor growth and metastasis characteristics[11,12].
Sorafenib, a multi-kinase inhibitor, delays tumor progression in patients with HCC by inhibiting tumor cell proliferation and angiogenesis[13-16]. TACE can induce the excessive production of vascular endothelial growth factor (VEGF), and VEGF can promote disease progression or metastasis[17,18]. Since sorafenib can inhibit VEGF growth, TACE combined with sorafenib can reduce the excessive production of VEGF in order to compensate for this effect of TACE, thereby enhancing the therapeutic effect[7]. Therefore, the combination of TACE with sorafenib may provide a benefit for patients with HCC. Many studies have reported that TACE combined with sorafenib significantly prolonged the median overall survival (mOS) time or time to progression (TTP) for patients with unresectable HCC[19-21]. Most of the patients included in these studies had BCLC-B HCC. To date, limited data have focused on the combination of TACE with sorafenib for BCLC-B HCC. Therefore, in this study, a retrospective comparative study to investigate the role of sorafenib plus TACE in BCLC-B HCC was conducted.
Sixty-seven patients with BCLC-B HCC were enrolled in this study. The clinical efficacy and adverse effects of the molecular-targeted drug sorafenib for the treatment of BCLC-B HCC were evaluated retrospectively. The factors influencing the curative effect and prognosis were analyzed. The purpose of this study is to investigate the combination of sorafenib with TACE vs traditional TACE in patients with BCLC-B HCC. This study provides important information for clinicians who are interested in using sorafenib plus TACE to treat BCLC-B HCC.
The retrospective study was approved by the institutional review board at the Second Affiliated Hospital of Kunming Medical University Ethics Committee for Clinical Investigation and was conducted in accordance with the Declaration of Helsinki and good clinical practices.
We retrospectively enrolled 67 patients who had BCLC-B disease at the time of their initial diagnosis. Sixty-seven patients with BCLC-B HCC who were treated with sorafenib plus TACE or TACE alone between 2009 and 2011 were included. Follow-up was until 2014 or patient death. Two groups were defined in the experiment: the experimental group and the control group. The experimental group was treated with sorafenib plus TACE, and the control group was treated with standard TACE. The subjects included in the study met the following criteria: (1) Eligible patients (≥ 18 years old) who were staged according to the BCLC staging classification; (2) the Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0, Child-Pugh A or B, according to the diagnosis using computed tomography (CT)/magnetic resonance imaging (MRI), which was confirmed by B-ultrasound, CT-guided postoperative liver biopsy or enhanced CT/MRI; (3) modified Response Evaluation Criteria in Solid Tumors (mRECIST) evaluation criteria of at least one target lesion; and (4) complete case data. Patients were excluded if (1) They underwent hepatectomy, systemic chemotherapy or radiotherapy; (2) they underwent interferon therapy; (3) they had HIV, secondary primary malignancy or a serious illness; (4) if they had alcoholism; (5) if they had drug addiction; or (6) if they were pregnant or lactating women. Thirty-eight patients were treated with sorafenib combined with TACE and were included in the experimental group. Another 29 patients received TACE alone and were included in the control group.
Sorafenib was administered when the liver function was close to normal, following the first TACE. Patients received a dose of 400 mg sorafenib twice daily[22]. However, the dose was adjusted according to the severity of toxicity. Dose reduction was made according to the product characteristics and international recommendations[23]. Treatment with sorafenib was maintained until clinical and/or radiological progression, until intolerable adverse effects (AEs) occurred, until death, or until patient refusal[24]. All patients treated with sorafenib plus TACE were evaluated for clinical characteristics and toxicity management every 4 wk[22]. TACE was repeated every month if target lesions were detected as a treatment response of partial response (PR) or stable disease (SD), without deterioration of liver biochemistry[25].
TACE used the traditional technology[26]. Iodized oil, an embolic agent, and chemotherapy drugs (100-150 mg oxaliplatin combined with 0.75-1.0 g fluorouracil) were combined into a suspension. The use of iodized oil as a drug carrier allows the treatment to have an affinity for the tumor, allows the introduction of chemotherapy drugs into the cancer tissue, and plays a lasting role in embolization chemotherapy[27].
The follow-up data collection ended in 2014 or patient death. We compiled a detailed record of the patients’ treatments and end points. Patient survival time was monitored from the beginning of TACE to the last follow-up or to a patient’s death. There were 1 and 3 patients lost during the follow-up who were in the experimental treatment group and the control treatment group, respectively. Information extracted from each case included the following: (1) gender; (2) age; (3) Eastern Coorperative Oncology Group performance status (ECOG PS); (4) Child-Pugh classification; (5) serum alpha-fetoprotein (AFP) concentration; (6) serum albumin concentration; (7) serum total bilirubin concentration; (8) lactate dehydrogenase (LDH) concentration; (9) tumor diameter; (10) TACE times; (11) treatment methods; (12) survival status; (13) survival time; (14) efficacy; (15) follow-up time; and (16) AEs.
Response evaluation was performed according to mRECIST[28]. CT or MRI was used for treatment response assessment. The AEs included fatigue, diarrhea, rash, nausea, HFS, hypertension, vomiting, and bone marrow suppression. The results were analyzed retrospectively. Adverse reactions were assessed based on information that was noted in the medical records and graded according to the National Cancer Institute’s Common Toxicity Rating Standard version 4.03 (NCI-CTCAE v4.03)[29].
The main outcomes evaluated included overall survival (OS) and toxicity. Additional outcomes included objective response rate (ORR) and disease control rate (DCR). OS was calculated from treatment to death from any cause. OS is the primary endpoint of the study.
The data are presented as the mean ± SD. The mean values of the outcomes for the two groups were compared using t-tests, and the rates were compared using χ2 tests. Survival analysis was estimated using the Kaplan-Meier survival method. OS as an independent prognostic factor was assessed using the Cox proportional hazards regression model. P < 0.05 was considered statistically significant, and 95% confidence intervals (CIs) were calculated. Data collection, processing and statistical analysis were performed using the SPSS version 22.0 statistical software package.
Sixty-seven patients with BCLC-B disease were included; 38 patients were treated with sorafenib combined with TACE and treated as the experimental group. Another 29 patients received TACE alone and were treated as the control group. Baseline data included gender, age, ECOG PS, Child-Pugh classification, tumor diameter, TACE times, and serum albumin, serum total bilirubin, LDH, and AFP serum concentrations. There were no significant differences in the baseline characteristics between the experimental group and the control group (P > 0.05) (Table 1).
| Characteristics | Sorafenib + TACE (n = 38) | TACE (n = 29) | P value |
| Gender, Male/Female | 24 (63.2%)/14 (36.8%) | 18 (62.1%)/11 (37.9%) | > 0.05 |
| Age (mean ± SD, yr) | 52 ± 5 | 51 ± 6 | > 0.05 |
| ECOG PS 0/1/2/3/4 (%) | 38 (100%)/0 (0%)/0/(0%) 0/(0%) | 29 (100%)/0 (0%)/0/(0%) 0/(0%) | > 0.05 |
| Child-Pugh A/B | 25 (65.8%)/13 (34.2%) | 19 (65.5%)/10 (34.5%) | > 0.05 |
| Tumor diameter (cm) ≥ 6/< 6 | 14 (36.8%)/24 (63.2%) | 10 (34.5%)/19 (65.5%) | > 0.05 |
| Serum albumin (g/L) ≥ 35/< 35 | 20 (52.6%)/18 (47.4%) | 16 (55.2%)/13 (44.8%) | > 0.05 |
| Serum bilirubin (umol/L) ≥ 20/< 20 | 11 (28.9%)/27 (71.1%) | 8 (27.6%)/21 (72.4%) | > 0.05 |
| LDH (U/L) ≥ 245/< 245 | 23 (60.5%)/15 (39.5%) | 16 (55.2%)/13 (44.8%) | > 0.05 |
| AFP (ng/mL) ≥ 200/< 200 | 25 (65.8%)/13 (34.2%) | 19 (65.5%)/10 (34.5%) | > 0.05 |
| Number of TACE ≥ 2/< 2 | 19 (50.0%)/19 (50.0%) | 14 (48.3%)/15 (51.7%) | > 0.05 |
In this study, 2 patients in the experimental group discontinued treatment due to grade 3 AEs at 4 mo and 6 mo, respectively. Two patients stopped treatment at 2 mo and 3 mo, respectively, due to poor financial conditions. Among the 67 patients, 42 were male, and 25 were female. The median age was 57 years (range: 38-71 years). The median follow-up time was 23.0 mo (range: 5.0-40.0 mo). Factors used in the univariate analysis and the number of patients who exhibited each are as follows: ECOG PS 0 (n = 67); Child-Pugh A (n = 44) and Child-Pugh B (n = 23); AFP ≥ 200 ng/ml (n = 44) and AFP < 200 ng/ml (n = 23); LDH ≥ 245 U/L (n = 39) and LDH < 245 U/L (n = 28); serum albumin ≥ 35 g/L (n = 36) and serum albumin < 35 g/L (n = 31); serum total bilirubin ≥ 20 µmol/L (n = 19) and serum total bilirubin < 20 µmol/L (n = 48); tumor diameter ≥ 6 cm (n = 24) and tumor diameter < 6 cm (n = 43); and sorafenib combined with TACE (n = 38) and TACE alone (n = 29). Table 2 shows detailed information for the univariate analysis of all patients for OS. Univariate survival analysis showed that Child-Pugh status, baseline tumor diameter, serum total bilirubin levels, AFP levels, and TACE combined with sorafenib had a significant influence on OS (P < 0.05). However, gender, age, serum albumin levels, LDH levels, and TACE times were not statistically significant (P > 0.05).
| Characteristic | n (%) | mOS (mo) | 95%CI | P value | χ2 |
| Gender | |||||
| Male | 42 (62.7) | 33.4 | 28.03-38.77 | 0.659 | 0.195 |
| Female | 25 (37.3) | 35.2 | 30.40-40.00 | ||
| Age (yr) | |||||
| ≥ 57 | 26 (38.8) | 33.0 | 29.34-36.66 | 0.178 | 1.818 |
| < 57 | 41 (61.2) | 35.2 | 32.58-37.82 | ||
| Child-Pugh | |||||
| A | 44 (65.7) | 35.2 | 31.00-39.40 | 0.000 | 17.805 |
| B | 23 (34.3) | 21.0 | 16.22-25.78 | ||
| Tumor diameter (cm) | |||||
| ≥ 6 | 24 (35.8) | 20.0 | 14.51-25.49 | 0.016 | 5.815 |
| < 6 | 43 (64.2) | 35.2 | 32.63-37.77 | ||
| Serum albumin (g/L) | |||||
| ≥ 35 | 36 (53.7) | 36.0 | 33.34-38.66 | 0.066 | 3.39 |
| < 35 | 31 (46.3) | 23.0 | 18.25-27.75 | ||
| Serum bilirubin (umol/L) | |||||
| ≥ 20 | 19 (28.4) | 24.0 | 18.27-29.73 | 0.006 | 7.612 |
| < 20 | 48 (71.6) | 35.0 | 32.52-37.48 | ||
| LDH (U/L) | |||||
| ≥ 245 | 39 (58.2) | 28.0 | 24.83-31.17 | 0.143 | 2.143 |
| < 245 | 28 (41.8) | 33.0 | 31.19-34.81 | ||
| AFP (ng/mL) | |||||
| ≥ 200 | 44 (65.7) | 28.0 | 20.50-35.50 | 0.011 | 6.448 |
| < 200 | 23 (34.3) | 32.0 | 25.86-38.14 | ||
| Number of TACE | |||||
| ≥ 2 | 33 (49.3) | 29.8 | 23.14-36.46 | 0.079 | 3.809 |
| < 2 | 34 (50.7) | 36.6 | 35.12-38.08 | ||
| Sorafenib + TACE | 38 (56.7) | 35.2 | 30.02-40.38 | 0.000 | 12.645 |
| TACE | 29 (43.3) | 22.0 | 21.23-22.77 |
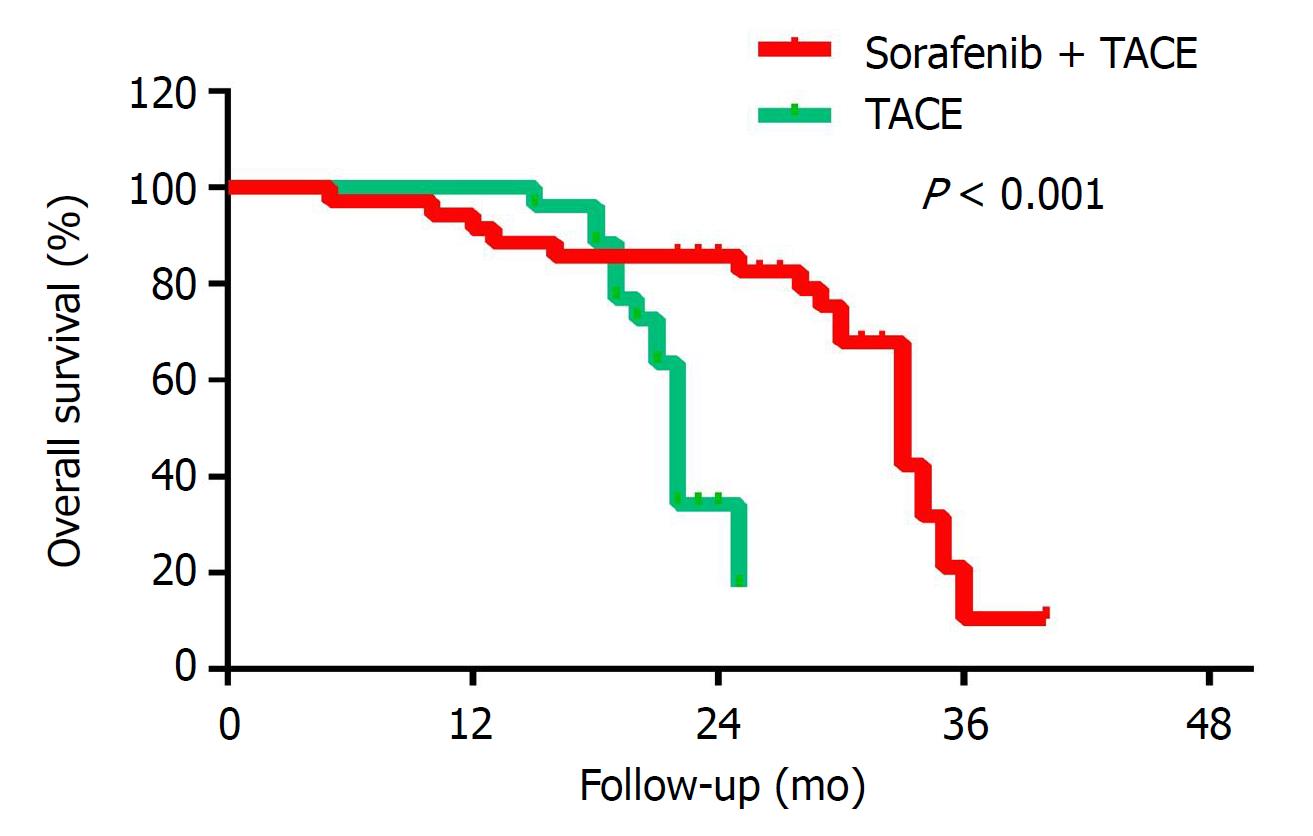
Child-Pugh classification, tumor diameter, serum total bilirubin levels, AFP levels, and combined TACE/sorafenib treatment were subjected to the Cox multivariate regression analysis. The multivariate analysis showed that the Child-Pugh status, tumor diameter and whether the treatment was combined with sorafenib had an independent prognostic value on OS (P = 0.006, 0.018 and 0.0001, respectively), as shown in Table 3. As shown in Figure 1 and Table 4, the Kaplan-Meier survival analysis showed that the mOS of the experimental group was 35.2 mo, while that of control group was 22.0 mo (P < 0.001).
| HR | 95%CI | P value | |
| Child-pugh class | 4.453 | 1.550-12.796 | 0.006 |
| Tumor diameter | 16.551 | 1.625-168.546 | 0.018 |
| AFP | 2.495 | 0.828-7.522 | 0.104 |
| Serum bilirubin | 0.894 | 0.292-2.731 | 0.843 |
| SOR or no SOR | 8.876 | 2.860-27.543 | 0.000 |
| Annual survival rate (%) | mOS (mo) | P value | |||
| 1 yr | 2 yr | 3 yr | |||
| Sorafenib + TACE | 94.7 | 63.2 | 34.6 | 35.2 | < 0.001 |
| TACE | 96.6 | 42 | NA | 22 | |
Response evaluation was performed according to mRECIST criteria for all enrolled patients, as presented in Table 5. Sorafenib combined with TACE group and the TACE alone group were CR (12/38 31.6% vs 4/29 13.8%), PR (11/38 28.9% vs 8/29 27.6%), SD (10/38 26.3% vs 7/29 24.1%), PD (5/38 13.2% vs 10/34 34.5%), ORR (60.5% vs 41.4%) and DCR (86.8% vs 65.5%), respectively. The differences were statistically significant between treatment groups for these outcomes (P < 0.05).
| CR | PR | SD | PD | ORR | DCR | χ2 | P value | |
| Sorafenib + TACE (38) | 12 | 11 | 10 | 5 | 60.5% | 86.8% | 21.586 | 0.000 |
| TACE (29) | 4 | 8 | 7 | 10 | 41.4% | 65.5% |
Table 6 lists the detailed information for toxicity. The AEs included fatigue, diarrhea, rash, nausea, HFS, hypertension, vomiting, and bone marrow suppression. The results were analyzed retrospectively. None of the patients experienced a toxicity grade > 3. The most common grade 3 AEs were HFS (n = 4) and hypertension (n = 3) in the sorafenib plus TACE group. The patients were instructed during the course of treatment to be aware of post-adverse event prophylaxis, symptomatic treatment and sorafenib dose-adjustment remission. With respect to the incidence of rash, HFS and hypertension, the sorafenib plus TACE group showed a higher incidence of these adverse reactions than the control group (P < 0.05). However, the prevalence of these reactions was within the tolerable range.
| Fatigue | Diarrhea | Rash | Nausea | HFS | Hypertensive | Vomiting | Bone marrow suppression | |
| Sorafenib + TACE (38) | 17 | 13 | 12 | 10 | 15 | 12 | 9 | 11 |
| TACE (29) | 10 | 4 | 2 | 3 | 0 | 3 | 3 | 5 |
| χ2 | 0.066 | 3.062 | 6.062 | 4.019 | 14.749 | 4.268 | 3.378 | 1.24 |
| P value | 0.793 | 0.061 | 0.014 | 0.056 | 0.000 | 0.039 | 0.066 | 0.265 |
Sorafenib has made significant progress in clinical practice, and it is an effective treatment for advanced HCC, with good measures of safety and tolerance[30-32]. The development of sorafenib has changed the traditional treatment regimen of HCC and has given patients new hope. So far, sorafenib is the only agent approved by the United States Food and Drug Administration (FDA) for the first-line therapy of patients with advanced HCC[31]. However, whether sorafenib can be used for BCLC-B HCC or with TACE as an adjunctive adjuvant therapy after radical treatment is inconclusive[32].
Data from the SHARP clinical study and the Oriental clinical study have demonstrated that sorafenib can significantly prolong the OS of patients with advanced HCC and that the safety is also better than other treatments[32-34]. However, the survival of patients treated with sorafenib monotherapy compared with the placebo group did not have such obvious benefits[35]. The efficacy of sorafenib monotherapy for the treatment of HCC is still limited. In addition, some patients received sorafenib monotherapy after the tumor was still significantly progressing[36,37]. The efficacy of sorafenib combined with TACE for the treatment of advanced HCC is being clinically recognized[38-40]. TACE is the preferred treatment for BCLC-B HCC and is suitable for patients who cannot undergo surgical resection or if the tumor could be resected but the patient cannot tolerate surgery after further examination[41]. However, TACE usually does not result in complete necrotic lesions. TACE often induces tumor angiogenesis and stimulates tumor growth or metastasis; therefore, the disease control is limited[42,43]. First, TACE causes a hypoxic tumor microenvironment, and in the hypoxic state of tumor angiogenesis, residual tumor proliferation can occur, stimulating tumor recurrence or metastasis. Second, the effects of TACE have a limited range and time, and the procedure may need to be repeated. Third, TACE can induce an excessive production of VEGF, that can promote disease progression or metastasis[44,45]. Therefore, sorafenib can inhibit tumor cell proliferation and angiogenesis and delay the progress of disease in patients with HCC[11]. Moreover, sorafenib can inhibit VEGF growth. Thus, some scholars have studied the use of TACE plus sorafenib to reduce the excessive production of VEGF to compensate for this effect of TACE and enhance the therapeutic effect in recent years[46,47]. However, whether sorafenib can be combined with TACE to improve the efficacy of BCLC-B HCC was not clear and needed further study.
In this retrospective study, 67 BCLC-B HCC patients were enrolled. The patient’s baseline characteristics were comparable between the two groups (Table 1). The mOS of sorafenib combined with TACE was 35.2 (95%CI: 30.02-40.38) mo, while that of TACE alone was 22.0 (95%CI: 21.23-22.77) mo. These data show that TACE combined with sorafenib can prolong the mOS of BCLC-B HCC patients. Compared with the control group, the experimental group had a significantly longer mOS (P < 0.001). For the experimental group, the 1-, 2-, and 3-year survival rates were 94.7%, 63.2% and 34.6%, respectively. For the control group, the 1-, 2-, and 3-year survival rates were 96.6%, 42.0% and 0, respectively (Table 4, Figure 1). Our major finding was that sorafenib plus TACE treatment for BCLC-B HCC can significantly prolong the mOS of patients. The reason may be that sorafenib acts as a multi-kinase inhibitor and inhibits tumor cell proliferation and angiogenesis, thereby delaying tumor progression in BCLC-B HCC. Sorafenib combined with TACE can inhibit VEGF growth to compensate for the excessive production of VEGF following TACE and thus enhance the therapeutic effect.
The survival curves of this study showed that in the first 20 mo, the experimental group has a shorter survival time than the control group (Figure 1). The rationale for this observation is that the Kaplan-Meier survival analysis is a comparison of survival rates by the curve, rather than at a certain point in time. Some studies[48,49] show that sorafenib targeting is effective at inhibiting tumor angiogenesis and that the prolonged use of sorafenib is reflected gradually so after a period of treatment in the sorafenib plus TACE group. Disease progression is often due to the heterogeneity of the tumor or the emergence of resistance in treatment. Patients with high tumor heterogeneity tend to develop PD early in treatment, whereas resistant patients often appear during treatment. Short-term sorafenib use may increase tumor invasion and metastasis, which are common problems related to VEGF inhibition. The early outcome of the experimental group was slightly worse than the control group, but the later outcome of the experimental group was significantly better than the control group.
The results of this study also showed that the Child-Pugh grade, tumor diameter and the combination treatment with sorafenib were three independent factors that affect the mOS. According to Child-Pugh grading criteria, patients with Child-Pugh A are without hepatic encephalopathy, ascites, esophageal and gastric variceal bleeding, or other serious complications. Moreover, with a tumor diameter smaller, there is a smaller tumor burden. Therefore, treatment efficacy and compliance is improved.
The objective response rate (ORR) and disease control rate (DCR) in the experimental group were 60.5% and 86.8%, respectively. The objective response rate (ORR) and disease control rate (DCR) the control group was 41.4% and 65.5%, respectively. The χ2 test showed that the χ2 value was 21.586, and the difference between the treatment groups was statistically significant (P < 0.05; Table 5). The results indicated that the molecular-targeted drug sorafenib for the treatment of BCLC-B HCC had a significant effect.
No patients experienced a toxicity grade > 3. The toxicity profile of sorafenib in our study is similar to what has been reported previously[50-52].The most common grade 3 AEs were HFS (n = 4) and hypertension (n = 3) in the sorafenib plus TACE group. The patients were instructed during the course of treatment to be aware of post-adverse event prophylaxis, symptomatic treatment and sorafenib dose-adjustment remission. The incidences of rash, HFS and hypertension were 12/38 (31.6%), 15/38 (39.5%), and 12/38 (31.6%), respectively, consistent with the results reported in the study. However, the prevalence of these reactions was within the tolerable range. The majority of AEs were alleviated with supportive symptomatic treatment and dosage adjustment.
The presence of sufloxacin-induced adverse reactions and the high cost of sorafenib limit the clinical applications. Therefore, the prevention and treatment of adverse reactions is also the key to ensure treatment compliance in patients, and active measures to slow or eliminate the adverse reactions during treatment and to maximize the quality of life will achieve the best therapeutic effect.
However, considering the design of this study is a small-scale retrospective comparative analysis, there is a need for well-designed multi-center, randomized, controlled trials to further explore the factors that affect the prognosis of survival. Additional areas that need to be studied include whether TACE affects the patient’s liver function and if this will affect sorafenib treatment and repeated TACE can induce systemic therapy (i.e., sorafenib molecular-targeting therapy) resistance, which in turn may increase tumor recurrence and metastasis. In order to improve the survival and quality of life of patients with HCC, sorafenib combined with the timing of TACE is also important. In addition, clinical work can explore the optimal combination of sorafenib and TACE, especially the best time for the TACE procedure to reduce the adverse reactions and increase patient compliance.
In conclusion, our results confirm that sorafenib plus TACE treatment for BCLC-B HCC significantly prolonged the mOS of patients compared to TACE treatment alone. Moreover, this new treatment approach showed tolerable toxicity. This study provides important information for clinicians who are interested in using sorafenib plus TACE therapies to treat BCLC-B HCC.
Transcatheter arterial chemoembolization (TACE) is the standard treatment for mid-term [Barcelona Clinical Liver Cancer Stage B (BCLC-B)] hepatocellular carcinoma (HCC). However, it limited beneficial effect. In recent years, several reports described the outcome Sorafenib combined with TACE for hepatocellular carcinoma; however the results are inconsistent. Moreover, most of these studies were conducted in developed countries. For developing countries, the data was few.
The aging population is growing at a remarkable rate all over the world. HCC incidence and age have a certain relationship. The incidence of HCC is increasing year by year, threatening people’s health. HCC with occult onset has a high degree of malignancy and spread quickly, and the majority of patients who are diagnosed at a later stage can not undergo surgical resection. These patients who are not suitable for surgical treatment usually use TACE, which achieves a limited beneficial effect. Therefore, we conducted the study, retrospective study to evaluate the safety and efficacy of sorafenib plus TACE treatment for BCLC-B HCC in Chinese patients.
The aim of this study is to evaluate the safety and efficacy of sorafenib plus TACE treatment for BCLC-B HCC.
A retrospective comparative study collected data was conducted at the Second Affiliated Hospital of Kunming Medical University. Sixty-seven patients with BCLC-B HCC who were treated with sorafenib plus TACE or TACE alone between 2009 and 2011 were included in the study. Follow-up was until 2014. Two groups were defined in the experiment: the experimental group, treated with sorafenib plus TACE, and the control group, treated with standard TACE alone. Compared to the safety and effectiveness of the two groups.
The analysis showed that the median overall survival (mOS) of the experimental group was 35.2 mo, while that of the control group was 22.0 mo (P < 0.05). Meanwhile, sorafenib plus TACE showed higher incidence rates of rash, hand-foot syndrome (HFS), and hypertension (P < 0.05) than TACE treatment alone. The most common toxicities with sorafenib were rash (31.6%), HFS (39.5%) and hypertension (31.6%), but there were no intolerable adverse events.
TACE is the preferred treatment for BCLC-B HCC and is suitable for patients who can not undergo surgical resection or who can be resected but who can not tolerate surgery after further examination. However, TACE usually does not result in complete necrotic lesions. TACE generally induces tumor angiogenesis and stimulates tumor growth or metastasis; therefore, disease control is limited. Sorafenib can inhibit tumor cell proliferation and angiogenesis and delay the progression of the disease in HCC patients. Moreover, sorafenib can inhibit the growth of VEGF. Some scholars have studied the use of TACE plus sorafenib to reduce the excessive production of VEGF to compensate for this effect of TACE and improve the therapeutic effect. Therefore, we conducted this work. Sorafenib plus TACE treatment for BCLC-B HCC significantly prolonged the mOS of patients compared to TACE treatment alone. The most common toxicities with sorafenib were rash (31.6%), HFS (39.5%) and hypertension (31.6%), but there were no intolerable adverse events. The results of this study also showed that the Child-Pugh grade, tumor diameter and the combination treatment with sorafenib were three independent factors that affect the mOS. This study confirms that sorafenib plus TACE treatment for BCLC-B HCC significantly prolonged the mOS of patients compared to TACE treatment alone. Moreover, this new treatment approach showed tolerable toxicity. However, whether Sorafenib can be used in combination with TACE to improve the efficacy of BCLC-B HCC requires a prospective and large sample study.
Due to small sample sizes and retrospective studies, it can be difficult to draw reliable conclusions. In conclusion, our results confirm that sorafenib plus TACE treatment for BCLC-B HCC significantly prolonged the mOS of patients compared to TACE treatment alone. Moreover, this new treatment approach showed tolerable toxicity. This study provides important information for clinicians who are interested in using sorafenib plus TACE therapies to treat BCLC-B HCC.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aghakhani A, Gad EH, Zafrakas M S- Editor: Wang XJ L- Editor: A E- Editor: Wang CH
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21360] [Article Influence: 2136.0] [Reference Citation Analysis (3)] |
| 2. | Sun Y, Wang Y, Li M, Cheng K, Zhao X, Zheng Y, Liu Y, Lei S, Wang L. Long-term trends of liver cancer mortality by gender in urban and rural areas in China: an age-period-cohort analysis. BMJ Open. 2018;8:e020490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Ye JZ, Chen JZ, Li ZH, Bai T, Chen J, Zhu SL, Li LQ, Wu FX. Efficacy of postoperative adjuvant transcatheter arterial chemoembolization in hepatocellular carcinoma patients with microvascular invasion. World J Gastroenterol. 2017;23:7415-7424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Gomaa AI, Waked I. Recent advances in multidisciplinary management of hepatocellular carcinoma. World J Hepatol. 2015;7:673-687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Harding JJ, Abou-Alfa GK. Systemic therapy for hepatocellular carcinoma. China Clinical Oncol. 2013;2:37. [RCA] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6568] [Article Influence: 469.1] [Reference Citation Analysis (1)] |
| 7. | Qu XD, Chen CS, Wang JH, Yan ZP, Chen JM, Gong GQ, Liu QX, Luo JJ, Liu LX, Liu R. The efficacy of TACE combined sorafenib in advanced stages hepatocellullar carcinoma. BMC Cancer. 2012;12:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Arizumi T, Ueshima K, Minami T, Kono M, Chishina H, Takita M, Kitai S, Inoue T, Yada N, Hagiwara S. Effectiveness of Sorafenib in Patients with Transcatheter Arterial Chemoembolization (TACE) Refractory and Intermediate-Stage Hepatocellular Carcinoma. Liver Cancer. 2015;4:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 9. | Inghilesi AL, Gallori D, Antonuzzo L, Forte P, Tomcikova D, Arena U, Colagrande S, Pradella S, Fani B, Gianni E. Predictors of survival in patients with established cirrhosis and hepatocellular carcinoma treated with sorafenib. World J Gastroenterol. 2014;20:786-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Nojiri K, Sugimoto K, Shiraki K, Tameda M, Inagaki Y, Ogura S, Kasai C, Kusagawa S, Yoneda M, Yamamoto N. Sorafenib and TRAIL have synergistic effect on hepatocellular carcinoma. Int J Oncol. 2013;42:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Nakano M, Tanaka M, Kuromatsu R, Nagamatsu H, Tajiri N, Satani M, Niizeki T, Aino H, Okamura S, Iwamoto H. Sorafenib for the treatment of advanced hepatocellular carcinoma with extrahepatic metastasis: a prospective multicenter cohort study. Cancer Med. 2015;4:1836-1843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | van Malenstein H, Maleux G, Vandecaveye V, Heye S, Laleman W, van Pelt J, Vaninbroukx J, Nevens F, Verslype C. A randomized phase II study of drug-eluting beads versus transarterial chemoembolization for unresectable hepatocellular carcinoma. Onkologie. 2011;34:368-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Ang C, O’Reilly EM, Abou-Alfa GK. Targeted agents and systemic therapy in hepatocellular carcinoma. Recent Results Cancer Res. 2013;190:225-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Xie B, Wang DH, Spechler SJ. Sorafenib for treatment of hepatocellular carcinoma: a systematic review. Dig Dis Sci. 2012;57:1122-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Dhir M, Melin AA, Douaiher J, Lin C, Zhen WK, Hussain SM, Geschwind JF, Doyle MB, Abou-Alfa GK, Are C. A Review and Update of Treatment Options and Controversies in the Management of Hepatocellular Carcinoma. Ann Surg. 2016;263:1112-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 16. | Abou-Alfa GK. Approaching the era of personalised therapy for liver cancer? Lancet Oncol. 2013;14:7-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 84] [Reference Citation Analysis (0)] |
| 17. | Yoo JJ, Lee JH, Lee SH, Lee M, Lee DH, Cho Y, Lee YB, Yu SJ, Kim HC, Kim YJ. Comparison of the effects of transarterial chemoembolization for advanced hepatocellular carcinoma between patients with and without extrahepatic metastases. PLoS One. 2014;9:e113926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Liu C, Sun L, Xu J, Zhao Y. Clinical efficacy of postoperative adjuvant transcatheter arterial chemoembolization on hepatocellular carcinoma. World J Surg Oncol. 2016;14:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Zhang ZH, Liu QX, Zhang W, Ma JQ, Wang JH, Luo JJ, Liu LX, Yan ZP. Combined endovascular brachytherapy, sorafenib, and transarterial chemobolization therapy for hepatocellular carcinoma patients with portal vein tumor thrombus. World J Gastroenterol. 2017;23:7735-7745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Wang W, Bai W, Wang E, Zhao Y, Liu L, Yang M, Cai H, Xia D, Zhang L, Niu J. mRECIST response combined with sorafenib-related adverse events is superior to either criterion alone in predicting survival in HCC patients treated with TACE plus sorafenib. Int J Cancer. 2017;140:390-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Cui HZ, Dai GH, Shi Y, Chen L. Sorafenib combined with TACE in advanced primary hepatocellular carcinoma. Hepatogastroenterology. 2013;60:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69:223-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 379] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 23. | Marrero JA, Kudo M, Venook AP, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JH, de Guevara LL. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J Hepatol. 2016;65:1140-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 295] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 24. | Al-Rajabi R, Patel S, Ketchum NS, Jaime NA, Lu TW, Pollock BH, Mahalingam D. Comparative dosing and efficacy of sorafenib in hepatocellular cancer patients with varying liver dysfunction. J Gastrointest Oncol. 2015;6:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 25. | Gadaleta CD, Ranieri G. Trans-arterial chemoembolization as a therapy for liver tumours: New clinical developments and suggestions for combination with angiogenesis inhibitors. Crit Rev Oncol Hematol. 2011;80:40-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Guan YS, He Q, Wang MQ. Transcatheter arterial chemoembolization: history for more than 30 years. ISRN Gastroenterol. 2012;2012:480650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Zhao Y, Wang WJ, Guan S, Li HL, Xu RC, Wu JB, Liu JS, Li HP, Bai W, Yin ZX. Sorafenib combined with transarterial chemoembolization for the treatment of advanced hepatocellular carcinoma: a large-scale multicenter study of 222 patients. Ann Oncol. 2013;24:1786-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 28. | Moschouris H, Malagari K, Papadaki MG, Kornezos I, Stamatiou K, Anagnostopoulos A, Chatzimichael K, Kelekis N. mRECIST criteria and contrast-enhanced US for the assessment of the response of hepatocellular carcinoma to transarterial chemoembolization. Diagn Interv Radiol. 2014;20:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3292] [Article Influence: 219.5] [Reference Citation Analysis (36)] |
| 30. | Federico A, Orditura M, Cotticelli G, DE Sio I, Romano M, Gravina AG, Dallio M, Fabozzi A, Ciardiello F, Loguercio C. Safety and efficacy of sorafenib in patients with advanced hepatocellular carcinoma and Child-Pugh A or B cirrhosis. Oncol Lett. 2015;9:1628-1632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10247] [Article Influence: 602.8] [Reference Citation Analysis (2)] |
| 32. | El-Serag HB, Margaret M, Alkek AB. Current Status of Sorafenib Use for Treatment of Hepatocellular Carcinoma. Gastroenterol Hepatol (NY). 2017;13:623-625. [PubMed] |
| 33. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 84] [Reference Citation Analysis (0)] |
| 34. | Arizumi T, Ueshima K, Chishina H, Kono M, Takita M, Kitai S, Inoue T, Yada N, Hagiwara S, Minami Y. Duration of stable disease is associated with overall survival in patients with advanced hepatocellular carcinoma treated with sorafenib. Dig Dis. 2014;32:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | van Malenstein H, Dekervel J, Verslype C, Van Cutsem E, Windmolders P, Nevens F, van Pelt J. Long-term exposure to sorafenib of liver cancer cells induces resistance with epithelial-to-mesenchymal transition, increased invasion and risk of rebound growth. Cancer Lett. 2013;329:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 36. | Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1371] [Cited by in RCA: 1412] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 37. | Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1901] [Cited by in RCA: 1900] [Article Influence: 118.8] [Reference Citation Analysis (0)] |
| 38. | Cabrera R, Pannu DS, Caridi J, Firpi RJ, Soldevila-Pico C, Morelli G, Clark V, Suman A, George TJ Jr, Nelson DR. The combination of sorafenib with transarterial chemoembolisation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2011;34:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 39. | Sansonno D, Lauletta G, Russi S, Conteduca V, Sansonno L, Dammacco F. Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: a randomized clinical trial. Oncologist. 2012;17:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 40. | Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, Shi M. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 41. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1986] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 42. | Huang M, Wang L, Chen J, Bai M, Zhou C, Liu S, Lin Q. Regulation of COX-2 expression and epithelial-to-mesenchymal transition by hypoxia-inducible factor-1α is associated with poor prognosis in hepatocellular carcinoma patients post TACE surgery. Int J Oncol. 2016;48:2144-2154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 43. | Kim HC, Lee JH, Chung JW, Kang B, Yoon JH, Kim YJ, Lee HS, Jae HJ, Park JH. Transarterial chemoembolization with additional cisplatin infusion for hepatocellular carcinoma invading the hepatic vein. J Vasc Interv Radiol. 2013;24:274-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 395] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 45. | Shim JH, Park JW, Kim JH, An M, Kong SY, Nam BH, Choi JI, Kim HB, Lee WJ, Kim CM. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99:2037-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 46. | Choi GH, Shim JH, Kim MJ, Ryu MH, Ryoo BY, Kang YK, Shin YM, Kim KM, Lim YS, Lee HC. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology. 2013;269:603-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 47. | Park JW, Koh YH, Kim HB, Kim HY, An S, Choi JI, Woo SM, Nam BH. Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatol. 2012;56:1336-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 48. | Zhang X, Wang K, Wang M, Yang G, Ye X, Wu M, Cheng S. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE for hepatocellular carcinoma with portal vein tumor thrombus: a systematic review and meta-analysis. Oncotarget. 2017;8:29416-29427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 49. | Buczak K, Ori A, Kirkpatrick JM, Holzer K, Dauch D, Roessler S, Endris V, Lasitschka F, Parca L, Schmidt A. Spatial Tissue Proteomics Quantifies Inter- and Intratumor Heterogeneity in Hepatocellular Carcinoma (HCC). Mol Cell Proteomics. 2018;17:810-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 50. | Berk V, Kaplan MA, Tonyali O, Buyukberber S, Balakan O, Ozkan M, Demirci U, Ozturk T, Bilici A, Tastekin D. Efficiency and side effects of sorafenib therapy for advanced hepatocellular carcinoma: a retrospective study by the anatolian society of medical oncology. Asian Pac J Cancer Prev. 2013;14:7367-7369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Abou-Alfa GK. Sorafenib use in hepatocellular carcinoma: more questions than answers. Hepatology. 2014;60:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 458] [Article Influence: 32.7] [Reference Citation Analysis (0)] |