Published online Apr 16, 2018. doi: 10.12998/wjcc.v6.i4.44
Peer-review started: January 9, 2018
First decision: January 29, 2018
Revised: February 6, 2018
Accepted: March 7, 2018
Article in press: March 7, 2018
Published online: April 16, 2018
Processing time: 97 Days and 14.8 Hours
To investigate the prevalence and causes of cholestasis in patients with inflammatory bowel diseases in the Swiss Inflammatory Bowel Diseases Cohort.
A retrospective cohort study was performed of all the patients in the Swiss Inflammatory bowel disease Cohort. Total bile acid was measured for all patients and cholestasis was defined as a concentration > 8 μmol/L. The characteristics of patients with or without cholestasis were compared. Bile acid profiles were then determined for 80 patients with high total bile acid and 80 matched patients with low total bile acid. Bile acid profiles were compared for smokers vs nonsmokers, ileal vs colonic disease, and inflammatory vs non inflammatory diseases.
Ninety-six patients had more than 8 μmol/L total bile acid, giving a prevalence of 7.15%. Patients with an obvious cause of cholestasis, such as primary sclerosing cholangitis, were then excluded, leaving 1190 participants with total bile acid < 8 μmol/L and 80 with total bile acid > 8 μmol/L. In multivariate analysis, calcium supplementation was significantly associated with cholestasis (odds ratio, 2.36, 95%CI: 1.00-5.21, P = 0.040) whereas current smoking significantly reduced the risk of cholestasis (odds ratio, 0.42, 95%CI: 0.17-0.91, P = 0.041). Levels of all conjugated bile acids were higher in the cholestasis group than in the control group. When we compared patients with ileal vs colonic disease, the former had higher levels of primary, secondary, and tertiary bile acids whereas patients with colonic disease had higher levels of conjugated bile acids.
Prevalence of cholestasis is high. Smoking appears to reduce cholestasis. Conjugated bile acids are higher in cholestasis and in colonic disease whereas unconjugated in ileal disease.
Core tip: Inflammatory bowel diseases (IBDs) are often associated with cholestasis. This study shows that the prevalence of cholestasis in IBD patients is high at 7%. Current smoking seems to be a protective factor against cholestasis, maybe reflecting a different gut function than in nonsmokers. Ileal disease was more often associated with elevated non conjugated bile acids levels. Colonic disease was characterized by higher conjugated bile acids levels.
- Citation: Girardin M, Hadengue A, Frossard JL, the Swiss IBD Cohort Study Group. High prevalence of cholestasis, with increased conjugated bile acids in inflammatory bowel diseases patients. World J Clin Cases 2018; 6(4): 44-53
- URL: https://www.wjgnet.com/2307-8960/full/v6/i4/44.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i4.44
Inflammatory bowel diseases (IBDs) primarily affect the gut, but about 15% of patients also present extra-intestinal manifestations involving the joints, eyes, skin or liver[1]. Flare-ups of the disease are accompanied by an increase in inflammatory markers (such as C-reactive protein) and cholestasis parameters. In some cases, cholestasis may even present as an isolated increase in blood alkaline phosphatase levels found during routine laboratory follow-up. Clinically, cholestasis is characterized by the presence of jaundice, pruritus or signs of malabsorption, whereas biologically, cholestasis is defined as an increased activity of alkaline phosphatase, gamma-glutamyl transpeptidase, conjugated bilirubin or an increased total bile acid level in the blood.
The causes of cholestasis in IBD, either Crohn’s disease (CD) or ulcerative colitis (UC), are multiple. These can range from drug-induced liver toxicity (Azathioprine, nodular regenerative hyperplasia) to specific liver diseases complicating IBD (primary sclerosing cholangitis (PSC), gallstone disease) or para-inflammatory phenomena (TNF-α induced cholestasis)[2].
The proportion of patients with PSC reported in IBD series is about 5% for UC patients and 3.6% for CD patients[3]. The diagnosis of PSC in IBD patients is of the greatest importance because of the high risk of developing colon carcinoma[4] and cholangio-cellular carcinoma. Treatment with ursodesoxycholic acid or 5-ASA have been claimed to decrease the incidence of colon cancer[5].
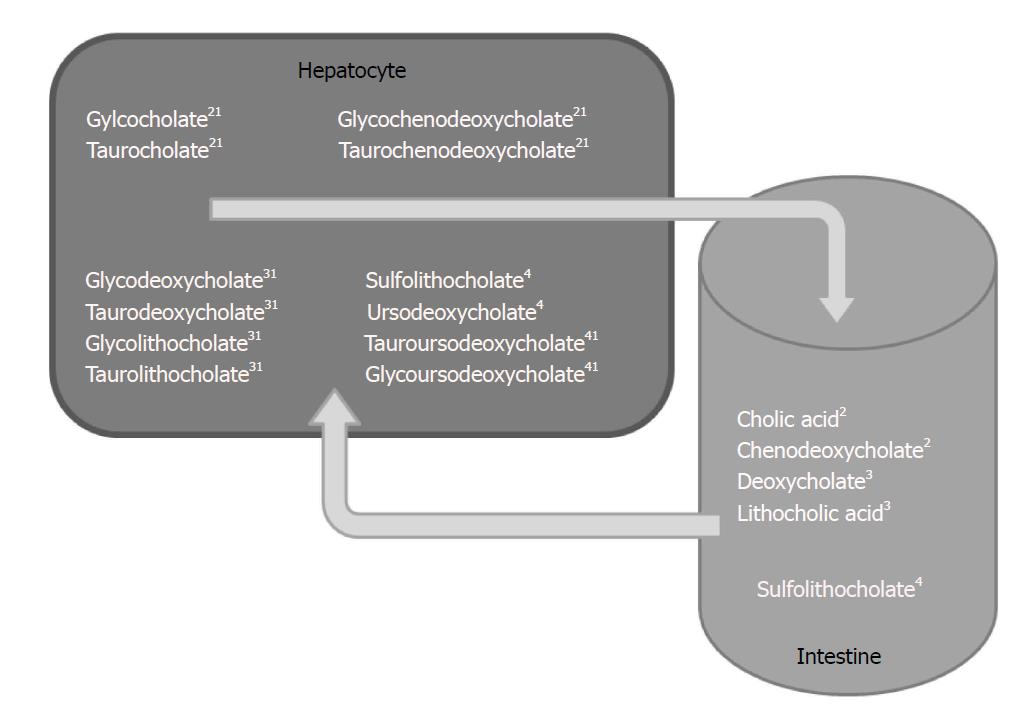
There are so far no available data on the potential impact of cholestasis on IBD itself. This is an important topic however because cholestasis plays an important role in the malabsorption of liposoluble vitamins, drugs and other liposoluble substances, through the associated decrease in intestinal bile acid concentration and reduced micelle formation. Briefly, after being produced by the liver, primary bile acids – cholic acid and chenodeoxycholate –are glyco- or tauro-conjugated and excreted in the bile into the intestine. At this point, the bile acids are deconjugated by bacteria and transformed into secondary bile acids-deoxycholate and lithocholic acid. Primary and secondary bile acids are then reabsorbed into the entero-hepatic cycle and either glyco- or tauro-conjugated, or transformed into tertiary bile acids-sulfolithocholate and ursodeoxycholate. These pathways could all potentially be altered by IBD. In addition, bile acids are thought to play a role in dysplasia[6], intra-cellular signalling[7] as well as in interactions with the intestinal flora[8].
Since the exact prevalence of cholestasis in IBD patients remains unknown and because cholestasis may have a profound impact on the course of the disease, a fundamental understanding of its aetiologies and clinical relevance are essential.
The primary aim of this study was thus to establish the prevalence of clinical and biological cholestasis in the Swiss IBD Cohort (SIBDC). The secondary aims were to determine the causes of cholestasis and their prevalence as well as to identify bile acid subtypes in confirmed cases of cholestasis.
The SIBDC consists of more than 2800 patients with confirmed IBD, included since 2006 from all over Switzerland. Clinical data on the disease type, endoscopy, evolution, treatments as well as complications are available as along with serum samples. Data are collected at inclusion and then yearly, during a meeting with the study coordinator. The exclusion criteria of this study were age younger than 16 years and unavailability of serum samples. IBD activity was described using the Crohn’s disease activity index (CDAI) for CD and the Modified Truelove and Witts Index (MTWI) for UC, whereas the site of inflammation was described using the Montreal classification[9]. Cholestasis was defined as a total bile acid (TBA) level higher than 8 μmol/L[10,11]. In order to measure the prevalence of cholestasis, a TBA assay was performed on all serum specimens using a highly sensitive and specific ELISA test (Labor Eberhard, Dortmund, Germany). Thereafter, a serum bile acid profile was established for all patients with a TBA above 8 μmol/L, and for an equal number of patients adjusted for age and type of disease with a TBA less than 8 μmol/L. These latter patients were randomly selected among those in the SIBDC. This further analysis was performed using ultra-high performance liquid chromatography with tandem mass spectrometry. Serum samples were mixed with a methanolic standard solution. After several centrifugations and dilutions with methanol/water 1:1, the final 20 µL of the supernatant was used for high performance liquid chromatography on a C18 column. The final analyses were performed using a QUATTRO Micro tandem mass spectrometer (Screening-Labor, Hannover, Germany)[12]. For a better understanding of the bile acid profiles, Figure 1 shows a diagram that summarizes bile acid metabolism.
Categorical and continuous variables were described as percentages, means and standard deviations. Groups were compared using Fisher’s exact test for categorical data and the Student t-test for continuous variables. Multivariate analysis was performed using the software R statistics. All variables with P < 0.1 in univariate analysis were included in multivariate analysis to account for all possible confounders. All significance tests were two-sided, and P < 0.05 were considered significant. The statistical methods of this study were reviewed by Mr Fournier Nicolas from the SIBDC.
The study was approved by the ethical review board of the SIBDC and of Geneva University in September 2012. Written, informed consent was obtained from each patient included in the study at inclusion in the SIBDC. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee.
A total of 1342 patients were included in this study, of which 96 had TBA levels > 8 μmol/L, with a mean level of 21.8 ± 18 μmol/L, and 1246 had TBA levels < 8 μmol/L. The prevalence of cholestasis was thus 7.15%, as defined by elevated bile acids in the SIBDC.
The characteristics of the patients are listed in Table 1. Age, type of disease and activity indexes were similar in the two groups. There were more males in the cholestasis group (63% vs 51%, P = 0.027) and nonsmokers (83% vs 72%, P = 0.023). Patients in the cholestasis group had lower serum albumin levels (38 vs 39.8 g/L, P = 0.016) and higher serum alkaline phosphatase levels (108.1 vs 69.7 U/L, P = 10-12); a higher proportion received calcium (39% vs 29%, P = 0.038), and vitamin E supplements (3% vs 1%, P = 0.016), and were treated with tacrolimus (3% vs 1%, P = 0.0093) and ursodesoxycholic acid (10% vs 1%, P = 2.8 x 10-8). The proportion of patients with PSC was much higher in the cholestasis group (5% vs 1%, P = 0.00007). Multivariate analysis performed at this point showed that PSC (P = 0.012) and tacrolimus treatment (P = 0.017) were the only factors significantly associated with cholestasis.
| TBA > 8 | TBA < 8 | P value | |
| (n = 96) | (n = 1246) | ||
| Age (mean ± SD, yr) | 44 ± 1.6 | 41.5 ± 0.4 | 0.118 |
| Male | 60 (63) | 639 (51) | 0.027 |
| Female | 35 (36) | 603 (48) | |
| CD | 47 (49) | 716 (57) | 0.267 |
| UC | 45 (47) | 498 (40) | |
| IC | 3 (3) | 26 (2) | |
| Disease duration (mean ± SD, yr) | 11 ± 1.2 | 9.8 ± 0.3 | 0.446 |
| CDAI (mean ± SD) | 92.8 ± 8.7 | 82.1 ± 2.7 | 0.316 |
| MTWI (mean ± SD) | 3.6 ± 0.6 | 4.1 ± 0.2 | 0.436 |
| Smokers | 16 (17) | 339 (27) | 0.023 |
| Non smokers | 80 (83) | 903 (72) | |
| Extra intestinal manifestations | |||
| Arthritis | 21 (22) | 362 (2) | 0.133 |
| Uveitis | 4 (4) | 68 (5) | 0.588 |
| Pyoderma gangrenosum | 1 (1) | 12 (1) | 0.938 |
| Erythema nodosum | 4 (4) | 42 (3) | 0.679 |
| Oral soars | 5 (5) | 70 (6) | 0.867 |
| Spondylarthritis | 3 (3) | 52 (4) | 0.617 |
| Primary sclerosing cholangitis | 5 (5) | 10 (1) | 0.000 |
| Colorectal cancer | 0 (0) | 5 (0) | 0.533 |
| Colonic dysplasia | 1 (1) | 5 (0) | 0.364 |
| Lymphoma | 0 (0) | 0 (0) | Ns |
| Osteoporosis | 17 (18) | 184 (15) | 0.436 |
| Anemia | 11 (11) | 229 (18) | 0.088 |
| Thrombosis | 5 (5) | 20 (2) | 0.011 |
| Gallstone | 5 (5) | 33 (3) | 0.145 |
| Urinary stones | 2 (2) | 38 (3) | 0.591 |
| Malabsorption | 5 (5) | 38 (3) | 0.247 |
| Lab values | |||
| TBA (mean ± SD, μmol/L) | 21.9 ± 5.5 | 3.1 ± 0.1 | 1.36 × 10-30 |
| CRP (mean ± SD, mg/L) | 9.8 ± 1.6 | 11.7 ± 0.7 | 0.460 |
| Ferritine (mean ± SD, μg/L) | 158.5 ± 39.9 | 239.2 ± 35.9 | 0.516 |
| B12 vitamin (mean ± SD, pmol/L) | 604.1 ± 155.9 | 446.8 ± 27.1 | 0.130 |
| Alkaline phosphatase (mean ± SD, U/L) | 108.1 ± 10.8 | 69.7 ± 1.2 | 10.00 × 10-13 |
| Albumin (mean ± SD, g/L) | 38.0 ± 0.8 | 39.8 ± 0.2 | 0.016 |
| Medical therapies | |||
| Oral 5-ASA | 39 (41) | 415 (33) | 0.147 |
| Topical 5-ASA | 9 (9) | 114 (9) | 0.855 |
| Sulfasalazine | 0 (0) | 28 (2) | Ns |
| Metronidazole | 1 (1) | 31 (2) | 0.723 |
| Ciprofloxacine | 4 (4) | 28 (2) | 0.281 |
| Clarithromycine | 0 (0) | 1 (0) | Ns |
| Cyclosporine | 0 (0) | 12 (1) | Ns |
| Tacrolimus | 3 (3) | 8 (1) | 0.009 |
| Azathioprine | 38 (40) | 407 (33) | 0.177 |
| 6-Mercaptopurine | 5 (5) | 67 (5) | Ns |
| Remicade | 9 (9) | 183 (15) | 0.174 |
| Humira | 1 (1) | 51 (4) | 0.172 |
| Cimzia | 0 (0) | 19 (2) | Ns |
| Methotrexate | 5 (5) | 79 (6) | 0.827 |
| Budesonide | 9 (9) | 107 (9) | 0.708 |
| Systemic prednisone | 20 (21) | 170 (14) | 0.051 |
| Topical prednisone | 2 (2) | 28 (2) | 0.716 |
| Ursodesoxycholic acid | 10 (10) | 7 (1) | 2.84 × 10-8 |
| Cholestiramine | 2 (2) | 24 (2) | 0.708 |
| Supplements | |||
| Iron | 12 (13) | 188 (15) | 0.492 |
| Vitamin B12 | 13 (14) | 191 (15) | 0.638 |
| Folate | 8 (8) | 125 (10) | 0.591 |
| Multivitamin | 2 (2) | 76 (6) | 0.105 |
| Magnesium | 6 (6) | 54 (4) | 0.381 |
| Calcium | 37 (39) | 356 (29) | 0.038 |
| Vitamin D | 28 (29) | 270 (22) | 0.088 |
| Vitamin E | 3 (3) | 9 (1) | 0.016 |
| Potassium | 1 (1) | 16 (1) | Ns |
| Lactase | 0 (0) | 3 (0) | Ns |
| Pancreatic enzymes | 0 (0) | 6 (0) | Ns |
| Fishoil | 4 (4) | 37 (3) | 0.511 |
| Enteric nutrition | 0 (0) | 6 (0) | Ns |
| Parenteric nutrition | 1 (1) | 3 (0) | 0.165 |
| Probiotics | 3 (3) | 16 (1) | 0.141 |
Sixteen patients had very high TBA levels, between 21 and 483 μmol/L, much higher than those of the other patients. A detailed chart review of these particular patients was performed. The apparent causes for such severe cholestasis were PSC for 2 patients, important clinical and laboratory inflammatory conditions for 2 patients (disease flare with high C reactive protein levels), a complex fistula phenotype for 3 patients, and treatment with azathioprine, methotrexate or tacrolimus for 6 patients. In 25% of these patients the underlying cause of cholestasis remained unexplained after complete investigation.
In order to better define the factors associated with the emergence of cholestasis, we decided to exclude all patients with an obvious cause of cholestasis (PSC, treatment with calcineurin inhibitors, and transplanted patients, who are often treated with these drugs). We therefore removed from the final analysis patients with PSC (n = 5), patients who had undergone organ transplantation (n = 3), patients treated with drugs known to cause cholestasis, namely cyclosporine (n = 12) and tacrolimus (n = 11), and finally patients who had received drugs that can substantially modify the bile acid metabolism (Supplementary Table 1), namely ursodesoxycholic acid (n = 16) and cholestyramine (n = 25) drugs. This yielded a cohort of 1190 patients with a TBA level < 8 μmol/L (93.7%) and 80 patients with a TBA > 8 μmol/L (6.3%). All data considered together, we found that alkaline phosphatase levels, albumin level, supplementation with vitamin D, azathioprine use, current smoking status and previous deep venous thrombosis were significantly associated with cholestasis. There was also a close to significant association with calcium supplementation (P = 0.076). Table 2 shows all the comparison between the groups in univariate analysis.
| TBA > 8 | TBA < 8 | P value | |
| (n = 80) | (n = 1190) | ||
| TBA (mean ± SD, μmol/L) | 14.66 ± 1.24 | 3.07 ± 0.06 | 6.15 × 10-144 |
| Alkaline phosphatase (mean ± SD, U/L) | 81 ± 5.81 | 69.3 ± 1.25 | 0.021 |
| Albumin (mean ± SD, g/L) | 38.0 ± 0.92 | 39.9 ± 0.21 | 0.017 |
| Vitamin D | 25 (31) | 254 (21) | 0.038 |
| Vitamin E | 2 (3) | 9 (1) | 0.103 |
| Calcium | 30 (38) | 336 (28) | 0.076 |
| Prednisone | 16 (20) | 160 (13) | 0.100 |
| Azathioprine | 37 (46) | 383 (32) | 0.009 |
| Smokers | 12 (15) | 330 (28) | 0.012 |
| Thrombosis | 4 (5) | 17 (1) | 0.015 |
A multivariate analysis of factors influencing the presence of cholestasis in IBD was then performed (Table 3). We found that supplementation with calcium was significantly associated with cholestasis [OR: 2.36 (1.00-5.21), P = 0.040], albeit with a borderline confidence interval, while current smoking status was inversely associated with cholestasis [OR: 0.42 (0.17-0.91), P = 0.041].
| OR | CI | P value | |
| Gender | 1.48 | 0.83-2.67 | 0.188 |
| Alkaline phosphatase | 1.00 | 0.99-1.01 | 0.282 |
| Albumin | 0.96 | 0.92-1.01 | 0.108 |
| Current smoking status | 0.42 | 0.17-0.91 | 0.041 |
| Azathioprine treatment | 1.57 | 0.873-2.79 | 0.128 |
| Prednisone treatment | 1.15 | 0.53-2.36 | 0.707 |
| Vitamin D supplement | 0.72 | 0.29-1.79 | 0.471 |
| Vitamin E supplement | 4.36 | 0.56-24.46 | 0.110 |
| Calcium supplement | 2.36 | 1.00-5.21 | 0.040 |
| Thrombosis | 1.38 | 0.19-5.89 | 0.701 |
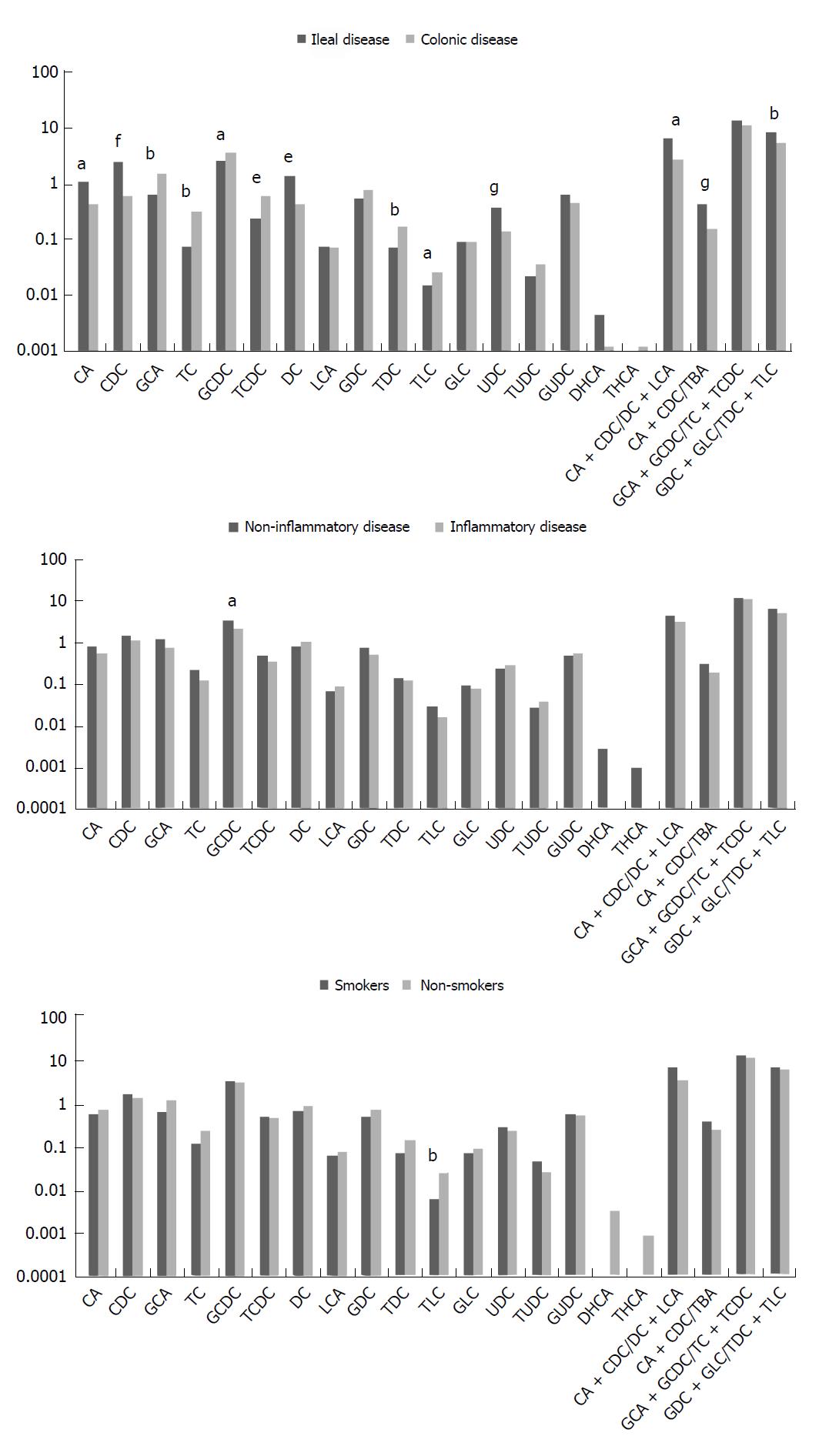
Bile acid subtype profiles were then determined for the group of 80 patients with cholestasis (TBA > 8 μmol/L) and for 80 patients without cholestasis, matched with the former group for age and type of disease. Results of the bile acid profile are fully presented in Table 4. Note that the bile acids are classified as primary, secondary or tertiary bile acids. Table 4 also shows the levels of tauro- and glyco- conjugated bile acids and the primary to secondary and primary to total bile acids ratios, to better characterize the different types present. The levels of 13 of these subtypes were significantly higher in the cholestasis group than in the control group, 3 of which markedly so; specifically, for glycochenodeoxycholate, the levels were 4.72 μmol/L vs 1.42 μmol/L (P = 1.54 × 10-16), for glycodeoxycholate 1.01 μmol/L vs 0.3 μmol/L; P = 1.39 × 10-9, and for tauroursodeoxycholate, 0.2 μmol/L vs 0.05 μmol/L; P = 8.78 × 10-7. Interestingly, these three are respectively primary, secondary and tertiary conjugated bile acids. Overall, we found that the conjugated bile acids were all more elevated in the cholestasis group than in the control group.
| TBA > 8 (80) | TBA < 8 (80) | P value | |
| TBA | 14.64 (1.24) | 3.31 (0.22) | 1.07 × 10-15 |
| Primary | |||
| CA | 1.09 (0.29) | 0.29 (0.04) | 0.008 |
| Cholic acid | |||
| CDC chenodeoxycholate | 2.06 (0.41) | 0.68 (0.13) | 0.002 |
| Secondary | |||
| DC | 1.35 (0.27) | 0.28 (0.04) | 0.000 |
| deoxycholate | |||
| LCA | 0.07 (0.01) | 0.07 (0.01) | 0.9 |
| lithocholic acid | |||
| Tertiary | |||
| UDC | 0.34 (0.05) | 0.14 (0.03) | 0.000 |
| ursodeoxycholate | |||
| Primary conjugated | |||
| TC | 0.37 (0.08) | 0.04 (0.01) | 0.000 |
| taurocholate | |||
| GCA | 1.78 (0.28) | 0.38 (0.05) | 1.83 × 10-6 |
| gylcocholate acid | |||
| TCDC | 0.74 (0.11) | 0.18 (0.02) | 1.58 × 10-6 |
| taurochenodeoxycholate | |||
| GCDC | 4.72 (0.33) | 1.42 (0.12) | 1.54 × 10-16 |
| glycochenodeoxycholate | |||
| Secondary conjugated | |||
| GDC | 1.01 (0.10) | 0.30 (0.04) | 1.38 × 10-9 |
| glycodeoxycholate | |||
| TDC | 0.20 (0.03) | 0.05 (0.01) | 8.78 × 10-7 |
| taurodeoxycholate | |||
| TLC | 0.04 (0.01) | 0.01 (0.00) | 8.46 × 10-5 |
| taurolithocholate | |||
| GLC | 0.09 (0.01) | 0.09 (0.01) | 0.646 |
| glycolithocholate | |||
| Tertiary conjugated | |||
| TUDC | 0.06 (0.01) | 0.001 (0.00) | 1.69 × 10-8 |
| tauroursodeoxycholate | |||
| GUDC | 0.84 (0.12) | 0.19 (0.03) | 4.58 × 10-7 |
| glycoursodeoxycholate | |||
| Hydroxydes | |||
| DHCA | 0.004 (0.002) | 0.001 (0.001) | 0.291 |
| dihydroxycholestanoic acid | |||
| THCA | 0.001 (0.001) | 0.000 (0.000) | 0.275 |
| trihydroxycholestanoic acid | |||
| Ratio | |||
| Primary/secondary | 3.48 (0.92) | 4.42 (1.34) | 0.566 |
| Primary/TBA | 0.23 (0.05) | 0.31 (0.04) | 0.211 |
| Conjugated glycine/taurine | |||
| Primary | 11.64 (1.18) | 10.70 (0.89) | 0.537 |
| Secondary | 6.40 (0.61) | 5.15 (0.66) | 0.209 |
Based on these results, we investigated the potential relationship between high TBA levels and three dissimilar factors that can interfere with the metabolism of bile acids, namely the gut microbiota, inflammatory activity, and smoking status. We compared patients with ileal disease to those with colonic disease, patients with high inflammation activity to those without inflammation and active smokers to those who did not smoke. The corresponding univariate analyses are shown in supplementary Table 2.
Patients with ileal disease were more likely to receive vitamin D and B12 supplementation than those with colonic disease were (35% vs 20%, P = 0.025 and 26% vs 5%, P = 0.0001, respectively). They were also more likely to be treated with certolizumab pegol and budesonide (3% vs 0%, P = 0.041, and 18% vs 7%, P = 0.027, respectively), and less likely to be treated with oral or topical 5ASA (53% vs 15%, P = 5.8 × 10-7, and11% vs 0%, P = 0.004, respectively). There was a marked difference in the proportion of smokers in the two groups, with, 90% of patients with colonic disease being nonsmokers vs just 66% in the ileal disease group (P = 0.0001).
Patients with active IBD had a higher UC activity index (8.3 ± 1.2 vs 3.3 ± 0.5, P = 0.0001) while the CD activity index was not different between the two groups. Patients with active IBD were more likely to have anemia (34% vs 11%, P = 0.0007), had lower albumin levels (35.6 ± 1.3 vs 40.2 ± 0.6, P = 0.001) and were more often treated with metronidazole (5% vs 0%, P = 0.014).
Patients who were active smokers were more likely to suffer from CD (81% vs 48%, P = 0.003), and pulmonary embolism (6% vs 0%, P = 0.038) but were less likely to receive systemic steroids (9% vs 27%, P = 0.036).
The differences in bile acid subtypes are illustrated in Figure 2. Interestingly, there are several significant differences in terms of the site of bowel inflammation (ileal vs colonic). Patients in the ileal disease group were indeed found to have more primary, secondary and tertiary bile acids, specifically higher chenodeoxycholate levels (2.41 μmol/L vs 0.59 μmol/L, P = 3.3 × 10-5), primary to TBA ratios (0.43 vs 0.15, P = 7.8 × 10-6), deoxycholate levels (1.34 μmol/L vs 0.41 μmol/L, P = 0.001) and ursodeoxycholate levels (0.37 μmol/L vs 0.13 μmol/L, P = 1.3 × 10-5). On the contrary, patients in the colonic disease group had higher concentration of conjugated bile acids namely glychocolate (1.42 μmol/L vs 0.6 μmol/L, P = 0.006), taurocholate (0.3 μmol/L vs 0.07 μmol/L, P = 0.008), taurochenodeoxycholate (0.62 μmol/L vs 0.24 μmol/L, P = 0.001) and taurodeoxycholate (0.17 μmol/L vs 0.06 μmol/L, P = 0.0008), all of which are either primary or secondary conjugated bile acids.
Low inflammatory activity was associated with a higher glychochenodeoxycholate level (3.38 μmol/L vs 2.15 μmol/L, P = 0.02), a primary conjugated bile acid, whereas nonsmokers had higher taurolithocholate level (0.03 μmol/L vs 0.006 μmol/L, P = 0.007), a secondary conjugated bile acid.
In this study, cholestasis was observed in 7.15% of patients in the SIBDC. As expected, the patients with the most severe cholestasis were those with PSC and those treated with a drug known to induce cholestasis.
We were surprised to find a low prevalence of PSC in our cohort (1.1%) compared with the values previously reported in the literature that range from 3.6% for CD to 5% for UC[3]. This might influence the prevalence of cholestasis in our cohort as PSC is one of the most important causes of cholestasis in IBD. One explanation could be that there are more patients with CD than with UC in our SIBDC cohort. We assumed that each case of PSC included in the central registry had been fully verified; however, we cannot exclude the possibility that some cases of PSC may have been missed.
To highlight factors more rarely associated with cholestasis, we chose to compare patients with and without cholestasis after having excluded those with the most common causes. Patients with the highest TBA levels (for example those with PSC or treatment with tacrolimus) were thereby removed from the analysis, making it less powerful. This decision was made to allow a more detailed analysis of the causes of moderate cholestasis, which would otherwise have remained unexplained.
Although some of the causes of cholestasis were expected in certain patients, notably those with higher alkaline phosphatase activity, lower albumin level and increased vitamin D supplementation because of disturbed lipid absorption, some of the other causes identified were more surprising. In particular, we found that patients with cholestasis were more likely to have been exposed to thiopurines than those in the control group. This class of drug frequently causes liver adverse events, including transient elevation of transaminases, or more rarely, nodular regenerative hyperplasia changes[13]. Thiopurine-induced cholestasis has seldom been described[14].
Another interesting point was that active smoking was protective against the development of cholestasis. Because smoking is a well-known risk factor for disease activity in CD, we would anticipate on the contrary that any increased inflammatory activity would lead to more pronounced cholestasis in smokers.
We found that nonsmokers had different bile acid patterns from smokers, with higher taurolithocholate levels, a secondary conjugated bile acid, indicating that this bile acid is better reabsorbed and then conjugated by the liver and suggesting that nonsmokers’ intestines are probably more functional. Since stopping smoking has been shown to have an impact on gut microbiota[15], we speculate that smoking may affect the way in which the microbiota transform bile acids in the intestine.
Calcium supplementation was associated in multivariate analysis with the presence of cholestasis, although this association was only just statistically significant. It is true that calcium supplementation may be a surrogate marker for inflammatory flares of the disease because it is often prescribed in conjunction with prednisone. It is noteworthy that calcium supplementation has also recently been shown to modify the composition of stool and the content of bile acids[16].
Looking at the bile acid subtypes that characterized cholestasis in these patients, we found that patients with cholestasis had higher levels of all the conjugated bile acids. Looking in detail at each subtype, we found that patients with and without cholestasis differed mainly in terms of their levels of glycochenodeoxycholate, glycodeoxycholate and tauroursodeoxycholate, which are conjugated bile acids, respectively primary, secondary and tertiary. This suggests that the liver plays a role in pathogenesis. Similarly, Gnewuch et al[17] showed that UC patients with hepatobiliary manifestations had increased primary and secondary conjugated bile acid levels. Denk et al[18] showed in a rat model of cholestasis that the best anti-cholestatic effect was obtained with conjugated ursodeoxycholate, making hepatic conjugation an essential pathway against cholestasis.
The bile acid subtype profiles of patients with ileal disease were very different from those of patients with colonic disease, with all the primary, secondary and tertiary bile acids being found in higher concentrations in the former. This data strongly supports the role of an altered microbiota and absorption in ileal disease. Bile acids are deconjugated and transformed into secondary bile acids, but are less well absorbed in this form. Conversely, we found that primary and secondary conjugated bile acids were significantly reduced in patients with ileal involvement, most probably because of gut malabsorption, a condition already reported in patients with ileal resection by Gnewuch et al[17] Similarly, we identified an increased glycine to taurine conjugation ratio in patients with ileal involvement, which has also been reported previously in patients with ileal diseases[19].
Patients with high inflammation activity had lower levels of glycochenodeoxycholate, a primary conjugated bile acid, than those with a low or no inflammation activity did, most probably because of disturbed intestinal absorption due to inflammation. Both primary and secondary bile acids have to be reabsorbed in order to be conjugated once more in the liver. This data is consistent with previous findings that demonstrated the presence of bile acid dysmetabolism in the stools of IBD patients and lower levels of secondary bile acids in their sera[20].
The main strengths of our study are the number of patients included and the richness of the data in the SIBDC, which is the largest IBD cohort in Switzerland. The main weakness of our findings is the absence of any correlation with gut microbiota, which seems to play a significant role in bile acid metabolism in these patients.
In conclusion, our study reports the prevalence of cholestasis in IBD patients for the first time, with 7% of the cohort being affected. Current smoking activity seems to be a protective factor against cholestasis. However, this may only reflect the effect of smoking on the intestinal inflammatory disease. Regarding bile acid subtype patterns, conjugated bile acids were more elevated in patients with cholestasis, suggesting an intrahepatic process. Interestingly, ileal disease was more often associated with elevated primary, secondary and tertiary bile acids, suggesting bacterial involvement, whereas a colonic disease was characterized by higher levels of conjugated bile acids.
Inflammatory bowel diseases (IBDs) are often associated with cholestasis, which can be caused by inflammation, drugs, or extra-intestinal manifestations. There is a lack of data in the literature on the prevalence and characteristics of cholestasis.
Measuring cholestasis and its prevalence and describing its characteristics and the main associated factors should improve our understanding of its causes and help to prevent or avoid the development of cholestasis in IBD.
The main objective of this study was to establish the prevalence of clinical and biological cholestasis. The secondary objective was then to clarify the causes and characteristics of cholestasis by studying the corresponding bile acid subtypes.
A total of 1342 patients from the Swiss Inflammatory Bowel Disease Cohort were included and cholestasis was measured via their total bile acid levels. Patients with the main well-known causes of cholestasis (such as primary sclerosing cholangitis for example) were then excluded to study less obvious causes. The concentrations of different bile acid subtypes were measured in two groups of patients, either with or without cholestasis. The bile acid profiles of patients in the two groups were compared for different clinical situations, notably ileal vs colonic disease and for smokers vs nonsmokers.
The prevalence of cholestasis was found to be high, at 7.15%. Multivariate analysis showed that calcium supplementation was significantly associated with cholestasis whereas current smoking significantly reduced the risk of cholestasis. Regarding bile acid subtypes, patients in the cholestasis group had higher levels of all conjugated bile acids than those in the control group did. Comparing patients with ileal disease with those with colonic disease, the former had higher levels of unconjugated bile acids while the latter had higher levels of conjugated bile acids. These results highlight the probable role played by gut bacteria and the liver in the different types of cholestasis. What exactly these roles are should now be studied in more detail.
This study found that the prevalence of cholestasis is high in patients with IBD. Different subtypes of bile acid were associated with cholestasis, colonic disease, and ileal disease, indicating probable interactions between the gut microbiota, the liver and the different types of IBD. As expected, ileal or colonic involvement, smoking status and inflammation activity all modified the type of cholestasis presented by patients. A prospective analysis of cholestasis and its characteristics should be performed to improve our understanding of this condition and develop prevention strategies.
Cholestasis is frequent in IBD. The characteristics of cholestasis vary with the type of IBD and inflammatory activity. Cholestasis should now be studied prospectively to better understand how the gut microbiota and the liver are involved.
Swiss IBD Cohort Study Group: Claudia Anderegg; Peter Bauerfeind; Christoph Beglinger; Stefan Begré; Dominique Belli; José M. Bengoa; Luc Biedermann; Beat Bigler; Janek Binek; Mirjam Blattmann; Stephan Boehm; Jan Borovicka; Christian P. Braegger; Nora Brunner; Patrick Bühr; Bernard Burnand; Emanuel Burri; Sophie Buyse; Matthias Cremer; Dominique H. Criblez; Philippe de Saussure; Lukas Degen; Joakim Delarive; Christopher Doerig; Barbara Dora; Gian Dorta; Mara Egger; Tobias Ehmann; Ali El-Wafa; Matthias Engelmann; Jessica Ezri; Christian Felley; Markus Fliegner; Nicolas Fournier; Montserrat Fraga ; Pascal Frei; Remus Frei; Michael Fried; Florian Froehlich; Christian Funk; Raoul Ivano Furlano; Suzanne Gallot-Lavallée; Martin Geyer; Marc Girardin; Delphine Golay; Tanja Grandinetti; Beat Gysi; Horst Haack; Johannes Haarer; Beat Helbling; Peter Hengstler; Denise Herzog; Cyrill Hess; Klaas Heyland; Thomas Hinterleitner; Philippe Hiroz; Claudia Hirschi; Petr Hruz; Rika Iwata; Res Jost; Pascal Juillerat; Vera Kessler Brondolo; Christina Knellwolf; Christoph Knoblauch; Henrik Köhler; Rebekka Koller; Claudia Krieger-Grübel; Gerd Kullak-Ublick; Patrizia Künzler; Markus Landolt; Rupprecht Lange; Frank Serge Lehmann; Andrew Macpherson; Philippe Maerten; Michel H. Maillard; Christine Manser; Michael Manz; Urs Marbet; George Marx; Christoph Matter; Valérie McLin; Rémy Meier; Martina Mendanova; Christa Meyenberger; Pierre Michetti; Benjamin Misselwitz; Darius Moradpour; Bernhard Morell; Patrick Mosler; Christian Mottet; Christoph Müller; Pascal Müller; Beat Müllhaupt; Claudia Münger-Beyeler; Leilla Musso; Andreas Nagy; Michaela Neagu; Cristina Nichita; Jan Niess; Natacha Noël; Andreas Nydegger; Nicole Obialo; Carl Oneta; Cassandra Oropesa; Ueli Peter; Daniel Peternac; Laetitia Marie Petit; Franziska Piccoli-Gfeller; Julia Beatrice Pilz; Valérie Pittet; Nadia Raschle; Ronald Rentsch; Sophie Restellini; Jean-Pierre Richterich; Sylvia Rihs; Marc Alain Ritz; Jocelyn Roduit; Daniela Rogler; Gerhard Rogler; Jean-Benoît Rossel; Markus Sagmeister; Gaby Saner; Bernhard Sauter; Mikael Sawatzki; Michela Schäppi; Michael Scharl; Martin Schelling; Susanne Schibli; Hugo Schlauri; Sybille Schmid Uebelhart; Jean-François Schnegg; Alain Schoepfer; Frank Seibold; Mariam Seirafi; Gian-Marco Semadeni; David Semela; Arne Senning; Marc Sidler; Christiane Sokollik; Johannes Spalinger; Holger Spangenberger; Philippe Stadler; Michael Steuerwald; Alex Straumann; Bigna Straumann-Funk; Michael Sulz; Joël Thorens; Sarah Tiedemann; Radu Tutuian; Stephan Vavricka; Francesco Viani; Jürg Vögtlin; Roland Von Känel; Alain Vonlaufen; Dominique Vouillamoz; Rachel Vulliamy; Jürg Wermuth; Helene Werner; Paul Wiesel; Reiner Wiest; Tina Wylie; Jonas Zeitz; Dorothee Zimmermann.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Switzerland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Abdel-Salam OM, Suzuki H, Zhang XQ S- Editor: Cui LJ L- Editor: A E- Editor: Wang CH
| 1. | Lakatos PL, Lakatos L, Kiss LS, Peyrin-Biroulet L, Schoepfer A, Vavricka S. Treatment of extraintestinal manifestations in inflammatory bowel disease. Digestion. 2012;86 Suppl 1:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Trauner M, Fickert P, Stauber RE. Inflammation-induced cholestasis. J Gastroenterol Hepatol. 1999;14:946-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 160] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Saich R, Chapman R. Primary sclerosing cholangitis, autoimmune hepatitis and overlap syndromes in inflammatory bowel disease. World J Gastroenterol. 2008;14:331-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 4. | Brentnall TA, Haggitt RC, Rabinovitch PS, Kimmey MB, Bronner MP, Levine DS, Kowdley KV, Stevens AC, Crispin DA, Emond M. Risk and natural history of colonic neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis. Gastroenterology. 1996;110:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 205] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Pardi DS, Loftus EV Jr, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 355] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 6. | De Gottardi A, Dumonceau JM, Bruttin F, Vonlaufen A, Morard I, Spahr L, Rubbia-Brandt L, Frossard JL, Dinjens WN, Rabinovitch PS. Expression of the bile acid receptor FXR in Barrett’s esophagus and enhancement of apoptosis by guggulsterone in vitro. Mol Cancer. 2006;5:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Keitel V, Kubitz R, Häussinger D. Endocrine and paracrine role of bile acids. World J Gastroenterol. 2008;14:5620-5629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Kurdi P, van Veen HW, Tanaka H, Mierau I, Konings WN, Tannock GW, Tomita F, Yokota A. Cholic acid is accumulated spontaneously, driven by membrane deltapH, in many lactobacilli. J Bacteriol. 2000;182:6525-6528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [PubMed] |
| 10. | Skrede S, Solberg HE, Blomhoff JP, Gjone E. Bile acids measured in serum during fasting as a test for liver disease. Clin Chem. 1978;24:1095-1099. [PubMed] |
| 11. | Mashige F, Tanaka N, Maki A, Kamei S, Yamanaka M. Direct spectrophotometry of total bile acids in serum. Clin Chem. 1981;27:1352-1356. [PubMed] |
| 12. | Janzen N, Sander S, Terhardt M, Das AM, Sass JO, Kraetzner R, Rosewich H, Peter M, Sander J. Rapid quantification of conjugated and unconjugated bile acids and C27 precursors in dried blood spots and small volumes of serum. J Lipid Res. 2010;51:1591-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Musumba CO. Review article: the association between nodular regenerative hyperplasia, inflammatory bowel disease and thiopurine therapy. Aliment Pharmacol Ther. 2013;38:1025-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Ben Salem C, Ben Salah L, Belajouza C, Bouraoui K. Azathioprine-induced severe cholestatic hepatitis in patient carrying TPMT*3C polymorphism. Pharm World Sci. 2010;32:701-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Biedermann L, Brülisauer K, Zeitz J, Frei P, Scharl M, Vavricka SR, Fried M, Loessner MJ, Rogler G, Schuppler M. Smoking cessation alters intestinal microbiota: insights from quantitative investigations on human fecal samples using FISH. Inflamm Bowel Dis. 2014;20:1496-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | Lupton JR, Steinbach G, Chang WC, O’Brien BC, Wiese S, Stoltzfus CL, Glober GA, Wargovich MJ, McPherson RS, Winn RJ. Calcium supplementation modifies the relative amounts of bile acids in bile and affects key aspects of human colon physiology. J Nutr. 1996;126:1421-1428. [PubMed] |
| 17. | Gnewuch C, Liebisch G, Langmann T, Dieplinger B, Mueller T, Haltmayer M, Dieplinger H, Zahn A, Stremmel W, Rogler G. Serum bile acid profiling reflects enterohepatic detoxification state and intestinal barrier function in inflammatory bowel disease. World J Gastroenterol. 2009;15:3134-3141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Denk GU, Maitz S, Wimmer R, Rust C, Invernizzi P, Ferdinandusse S, Kulik W, Fuchsbichler A, Fickert P, Trauner M. Conjugation is essential for the anticholestatic effect of NorUrsodeoxycholic acid in taurolithocholic acid-induced cholestasis in rat liver. Hepatology. 2010;52:1758-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Garbutt JT, Heaton KW, Lack L, Tyor MP. Increased ratio of glycine- to taurine-conjugated bile salts in patients with ileal disorders. Gastroenterology. 1969;56:711-720. [PubMed] |
| 20. | Duboc H, Rajca S, Rainteau D, Benarous D, Maubert MA, Quervain E, Thomas G, Barbu V, Humbert L, Despras G. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 649] [Article Influence: 54.1] [Reference Citation Analysis (0)] |