Published online Feb 16, 2018. doi: 10.12998/wjcc.v6.i2.11
Peer-review started: November 4, 2017
First decision: November 30, 2017
Revised: December 15, 2017
Accepted: January 16, 2018
Article in press: January 16, 2018
Published online: February 16, 2018
Processing time: 96 Days and 2.3 Hours
We present a case of a healthy 72-year-old man with herpes simplex hepatitis (HSVH) development soon after ordinary surgery for biliary stones. A sudden onset of hepatitis associated with high fever and leukopenia emerged on postoperative day 5, followed by a rapid and lethal course (died on day 9), despite an acyclovir therapy on day 8. Postmortem liver biopsy revealed positive immunostaining for herpes simplex virus (HSV) type-1. The serum tests (available after the death) were negative for anti-HSV immunogloblulins, but positive for HSV DNA. A review of 15 cases of postsurgical HSVH along with 42 cases of non-surgical HSH showed that (1): A wide spectrum of surgical procedures was involved; and (2): High mortality (87%) associated with lower rates of ante-mortem diagnosis (20%) and acyclovir treatment (20%). Due to the difficulty in diagnosis and lethal nature, an early clinical suspension and prompt empirical anti-viral intervention are imperative for postsurgical hepatitis with undetermined etiology, characterized by fever and leucopenia.
Core tip: Fatal fulminant herpetic hepatitis developed in a 72-year-old healthy man following ordinary surgery for biliary stones. A. sudden onset of hepatitis associated with high fever and leukopenia emerged on postoperative day 5, followed by a rapid and lethal course (died on day 9), despite an acyclovir treatment on day 8. A literature review showed that (1): A wide spectrum of surgical procedures is involved; and (2): High mortality (87%) associated with low rates of ante-mortem diagnosis (20%) and acyclovir treatment (20%). An early clinical suspension and prompt empirical anti-viral intervention are imperative for postsurgical hepatitis with undetermined etiology, characterized by fever and leucopenia.
- Citation: Yokoi Y, Kaneko T, Sawayanagi T, Takano Y, Watahiki Y. Fatal fulminant herpes simplex hepatitis following surgery in an adult. World J Clin Cases 2018; 6(2): 11-19
- URL: https://www.wjgnet.com/2307-8960/full/v6/i2/11.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i2.11
Acute hepatitis represents a rare complication of herpes simplex virus (HSV) infection in adults and usually occurs in association with compromised cellular immunity[1]. However, the HSV hepatitis (HSVH) can occur in apparently healthy patients as well, and often cause fulminant hepatic failure with high mortality more than 70%[2-32]. Although the exact pathogenesis of HSVH in immunocompetent patients is unknown, several mechanisms are postulated including a large HSV inoculums at the time of initial infection that overcomes immunological defenses, an occult impairment in cellular immunity, reactivation of a latent virus in association with reinfection by a second strain of the HSV, and heterogeneity of the virus as a result of many atypical strains[12]. Because of a wide clinical spectrum and rarity, the HSVH needs further investigation for its etiology and pathogenesis. We here presented a previously healthy and immunocompetent man who ran lethal course due to HSVH following surgical therapy.
A 72-year-old Japanese male was admitted to the hospital with a 1-wk history of fever and epigastric pain. The patient’s past history was negative for chronic disease, excessive use of alcohol, and corticosteroid treatment. He was in healthy condition with body mass index of 20, but had smoking habit for 50 years. An endoscopic biliary stent and percutaneous trans-hepatic gallbladder drainage (PTGBD) were performed under the diagnosis of biliary and gallbladder stones, respectively. His symptom was rapidly resolved. Eschelichia coli was grown from the bile culture.
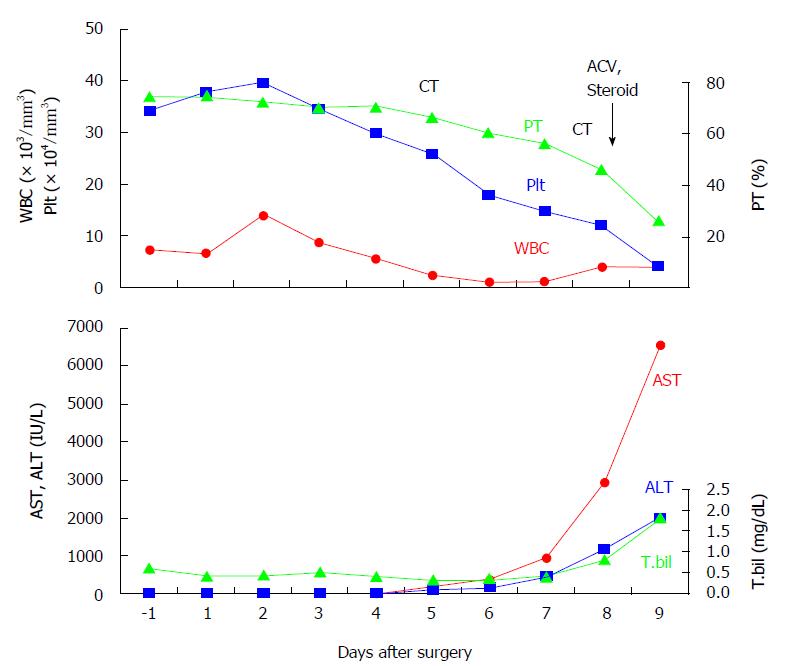
After quitting smoking for 1 mo at home, he underwent open cholecystectomy and choledocholithotomy followed by two day course of antibiotics. Neither the patient nor his family had recent history of flu-like symptoms. The laboratory data before surgery showed mild anemia (hemoglobin 11.1 g/dL) and undernutrition (albumin 3.4 g/dL), but did not provide evidences of organ dysfunction and systemic inflammation or infection. His body mass index was 20.3, and cell counts of leukocytes, lymphocytes, and platelets were unremarkable. No mucocutaneous lesions were noticed anywhere including PTGBD site. The clinical course after surgery was summarized in Figure 1. On day 5 following uneventful postoperative course, he developed diarrhea and became febrile with sudden onset of leucopenia (2600/mm3) and elevation of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Hepatitis B virus (HBV) surface antigen and hepatitis C virus (HCV) antibody were negative. Bacterial cultures including blood and urine were unrevealing. A contrast-enhanced CT (CECT) denied the hepatic circulatory disproportions, biliary congestion, and abdominal abscess formation. No drugs had been treated since two day course of postoperative antibiotic coverage. On day 8, the patient showed a rapid elevation of liver aminotransferase without alteration of cholestasis (AST: 2946 IU/L, ALT: 1190 IU/L, total bilirubin: 1.0 mg/dL) as well as coagulation disorder (INR: 2.0, D-dimer: 155.4 mg/dL, platelets: 8.3/mm3) (Figure 1). After having excluded a pharmacological etiology on the basis of a detailed examination of the patient’s medical records, the viral and bacterial screening was repeated and empiric therapy with acyclovir (ACV) intravenous injection (10 mg/kg, every eight hours) was initiated. Methylprednisone (1 g/d), thrombomodulin, and transfusion of fresh frozen plasma and platelets were also treated. Late on this day, the patient developed a tremor and impaired consciousness, possibly caused by encephalopathy. A repeated CECT showed heterogeous contrast distribution and a mottled liver without any abscess formation again. The patient developed hypotension and deep coma, and succumbed on day 9.
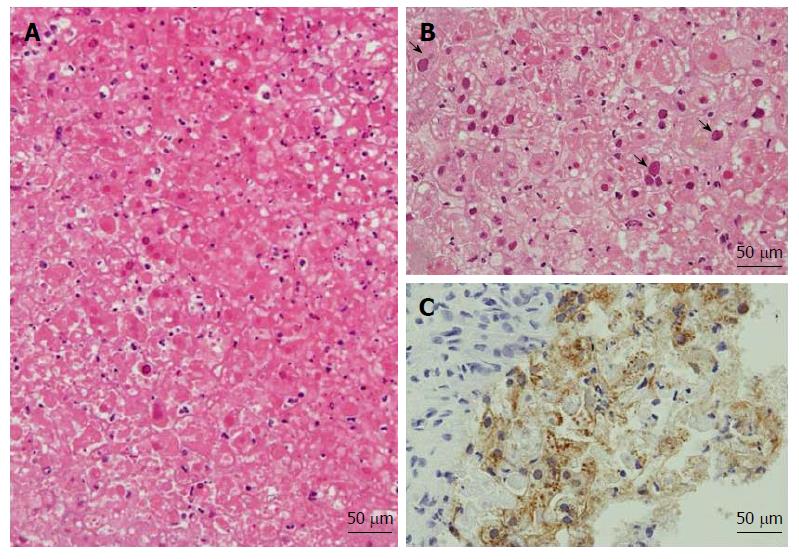
Postmortem liver biopsy revealed diffuse necrosis and loss of normal architecture with characteristic findings of intranuclear inclusion (Figure 2A and B). Immnostaining was positive for HSV-1 (Figure 2C), but not for HSV-2 and cytomegalovirus (CMV). Molecular analysis by PCR of liver tissues was negative for CMV infection.
The results of serological tests that were available after the patient’s death were as follows; negative for Hepatitis A IgM, Hepatitis B core IgM, Epstein Barr Virus IgM, and CMV IgG, HSV IgM and IgG, but positive for CMV IgM. Serum PCR was positive for HSV DNA, but HCV- and Hepatitis E Virus-RNA were undetectable.
A MEDLINE search using “fulminant hepatitis”, “herpes”, and “adult” as key words, and cross references from the reports found resulted in fourteen cases that seemed to be postsurgical HSVH (Table 1). These cases and the present case are summarized in Table 1. The median age was 58 years (15-93 years) with no gender differences. The nationality was United States (n = 8) and Japan (n = 2), followed by Canada, France, and Italy (each one). The predisposing factors associated with HSV infection included cancer (n = 5), Hodginkin’s disease (n = 1), and immunosuppressive treatment (n = 4). The operation was variable, pancreato-biliary (n = 5), neurologic- (n = 4) with postoperative glucocorticoids (n = 3), gynecologic- (n = 2), urological- (n = 1), oral- (n = 1), cardiovascular-surgery with glucocorticoids (n = 1), splenectomy (n = 1), and thymectomy (n = 1). The involved diseases were benign nature (n = 10) and malignancy (n = 5). The postulated transmission was per-esophageal (n = 1), genital tract (n = 2), and unknown (n = 12). As clinical manifestation, high fever was uniform (100%) and digestive disorders including nausea, vomiting and diarrhea (n = 6), with rare presentation of herpetiform lesions (n = 3, 27.3%). As clinical course, fever was the first presentation on median time of day 4.5 (day 1-14), followed by gastrointestinal symptoms on day 7 (day 4-9), and liver dysfunction on days 8.5 (day 1-20). Liver test showed predominance of AST (median of 10340 U/L) over ALT (median of 5116 U/L) with slight elevation of total bilirubin levels (median of 2.1 mg/dL) The HSV serotypes 1 and 2 were in 5 and 3 patients, respectively. Serological studies, IgM and IgG for HSV were positive in two (40%) and 1 (20%) in 5 patients. Ante-mortem diagnosis was achieved in 3 (20%) of 15 patients. ACV was treated in 3 (20%) patients, and liver transplantation was performed in two (13.3%) patients. Thirteen patients were died, accounting for mortality of 86.7%.
| No. authors (year), (reference) | Age (yr), sex/ races or nationality | Surgical procedures (diseases)/transmission pathway | Manifestation (days after surgery )/herpetiform lesions | Laboratory data Leucocytes (/mm3), Platelets (× 104/mm3), AST/ALT (U/L), TB (mg/dL). Coagulopathy, encephalopathy | HSV serology (IgM/ IgG), HSV type, HSV PCR | Ante-mortem diagnosis/ methods | Treatment/outcome (survival time after surgery, time after onset of liver dysfunction) |
| 1.Douglas (1977)[33] | 17, M/ United States | Splenectomy, liver biopsy, Ln excision (remission of Hodgekin’s disease)/ND | Fever (day 3), vomiting and diarrhea (day 4), liver dysfunction (day 5)/ none | ND, ND, 8700/ND, ND | ND/ND, ND, ND | No/ autopsy | Supportive/died (11 d, 6 d) |
| 2. Marrie (1982)[34], | 28, M/ white, Canada | Drainage, steroids (Brain stem injury)/ ND | Fever(12 HD)/ tongue, palate (20HD, 6 pod) | ND, ND, 9000/7000, ND. Encepahopathy | ND/ND, HSV-1, ND | No/liver biopsy | Spontaneously improved |
| 3. Williams (1985)[35] | 57, M/ United States | Aorto-coronary bypass, PSL (postcardiotomy syndrome)/esophagitis | Nausea, weakness, epigastric discomfort, liver dysfunction (day 8)/ none | ND, ND, 10050/4905, ND. Coagulopathy | ND/ND, HSV, ND | No/ autopsy | Supportive/died (10 d, 2 d) |
| 4. Fisher (1985)[36] | 62, M/ United States | Transperitoneal ureteroithotomy and cholecystectomy (ureteral obstruction and cholelithiasis)/ ND | Fever (day 3), vomiting (day 6), diarrhea (day 9), liver dysfunction (day 9)/ none | 5600, 8.1, > 500/ND, 1.2, Coagulopathy, Encephalopathy | ND/ND, HSV-1, ND | No/ autopsy | Supportive/died (14 d, 5 d) |
| 5-7, Goodman (1986)[8] | 81, F/ United States | Gastrectomy (stomach cancer)/ ND | Fever (day 8)/ ND | ND | ND/ND, ND, ND | No/ autopsy | Supportive/died (ND, 6 d) |
| 93, F/ United States | Biliary bypass (bile duct cancer)/ ND | ND/ ND | ND | ND/ND, ND, ND | No/ autopsy | Supportive/died (ND, 16 d) | |
| 59, M/ United States | PD (bile duct cancer ) stormy course /ND | ND/ ND | ND | ND/ND, ND, ND | No/ autopsy | Supportive/died (ND, 30 d)2 | |
| 8. Katz (1994)[37] | 76, M/ United States | Hemimandibulectomy, radical neck, and tracheotomy (plasmacytoma)/ND | Fever (day 6), diarrhea (day 9), liver dysfunction (day 11)/ none | 10100, ND, 7460/2970, ND. Coagulopathy, Encephalopathy | ND/ND, ND, ND | No/ autopsy | Supportive/died (15 d, 4 d) |
| 9. Kaufman (1997)[38] | 66, M/ United States | Tumor excision, intraoperative PSL (menigioma)/ ND | Fever (day 14), liver dysfunction (day 20), lethargic (day 20)/ mo | 4800, 2, 10340/ND, 1.9. Coagulopathy, Encephalopathy | ND/ND, HSV-1, ND | No/autopsy | Supportive/died (21 d, 1 d) |
| 10. Price (2001)[39] | Nulliparous/ United States | Laparoscopy, hysteroscopy (tubal infertility)/genital tract | Fever, nausea, abd pain, vaginal burning (day 3) /none | 900, ND, ND/ND, ND. Coagulopathy | -/-, HSV-1, ND | No/autopsy | Supportive/died (9 days, 6 d) |
| 11. Kohno (2001)[40] | 58, M/ Japanese | Craniotomy (hypertensive cerebral hemorrhage)/ ND | Liver dysfunction (day 7)/none | 11100, 9.3, 10956/5327, 3.5. Coagulopathy, Encephalopathy | ND/ND, HSV-2, ND | No/autopsy | Supportive/died (16 d, 9 d) |
| 12. Ichai (2005)[20] | 15 F/ France | Craniotomy (brain tumor) , PSL/ ND | Fever (day 9), Liver dysfunction (day 12)/ none | 9000, ND, 5000/ 4500, ND | +/+, HSV-2, PCR positive | Yes/serology, explanted liver culture | LT/ died (CR l yr) |
| 13. Biancofiore (2007)[41] | 25, M/ Italy | Thymectomy (myasthenia gravis)/ND | Fever (day 2), liver dysfunction (day 10)/ | 7820, 3.8, 15000/6818, 2.1. Encephalopathy | -/-, HSV-1, PCR positive | Yes/PCR (urine, blood, cerebrospinal fluid) | ACV (day 10) , LT (d 12)/died (12 d, 9 d after LT) |
| 14. Chaudhary (2017)[42] | 48 F/ Hispanic, United States | Repair of vaginal cuff (Vaginal cuff dehiscence/ trans-vaginal | liver dysfunction (day 1)/ vaginal ulcers | ND, ND, 20692/63, 8.5. Encephalopathy | +/-, HSV-2, PCR (serum, cerebrospinal fuid) | Yes/ vaginal ulcers, serology, PCR | ACV (day 4), foscarnet (day 10)/ survived |
| 15. Present case | 72, M/ Japanese | cholecystectomy and choledocholithotomy (biliary stone)/ ND | Fever (day 5), diarrhea (day 5), liver dysfunction (day 5)/ none | 1200, 4.3, 6557/2039, 2.0. Coagulopathy, Encephalopathy | -/-, HSV-1, ND | No/ autopsyr | ACV (day 8)/died (9 d, 4 d) |
| Summary | 58 yr (15-93)/Male (n = 8), United States (n = 10), Japan (n = 2), Canada (n = 1), France (n = 1), Italy (n = 1) | Malignancy (n = 5), benign (n = 10), Immunosuppressive status or treatment (n = 5)/ surgical wound (n = 2), trans-esophagus (n = 1) | Fever: (n = 15), day 4.5 (day 1-14), Digestive symptoms (n = 6): day 7 (day 4-9), Liver dysfunction (n = 15) day 8.5 (day 1-20)/ Herpetiform lesions: present (3/11 cases) | Leukocytes: 3.905 (900-10100), Plts: 4.3 (2-8.1), AST/ALT: 10.340 (5000-20692)/5116 (2970-7000), TB: 2.1 (2-8.5). Coagulopathy (n = 8), Encephalopathy (n = 7) | IgM +/- (2/3 cases)/ IgG +/- (1/4 cases), HSV: type-1(n = 5), type-2 (n = 3), PCR (n = 3) | Yes/no: 3/12 cases, Autopsy (n = 12), biopsy (n = 1), PCR (n = 3), serology (n = 1), | Supportive (n = 10), ACV (n = 3), LT (n = 2)/ Outcome: survived (n = 2), died (n = 13), Survival time1 after surgery: 13 (6-30) d, Survival time after liver dysfunction: 5.5 (1-9) d |
To further elucidate the clinico-laboratory feature of postsurgical HSVH, we compared with that of non-surgical and immunocompetent patients (non-surgical- HSVH)[2-32] (n = 42) (Table 2). As statistical analysis, differences were compared using Fisher’s exact test or χ2 test, and the Mann-Whitney U test for categorical variables and continuous measures, respectively. P value < 0.05 was considered statistically significant.
| Postsurgical patients (n = 15) | Non-surgical patients (n = 42) | P value | |
| Gender (M/F) (n) | 8/7 | 18/24 | 0.454 |
| Age (yr) | 58 (15-93) (n = 15) | 44 (15-87) (n = 43) | 0.060 |
| Clinical manifestation | |||
| (present/absent or ND) | |||
| Fever (n) | 14/1 | 42/0 | 0.263 |
| Nausea, vomiting, diarrhea (n) | 6/9 (17.5%) | 10/32 (23.8%) | 0.312 |
| Herpetic lesion present/none (n) | 3/8 (27.3%) | 20/19 (51.2%) | 0.308 |
| Transmission route | |||
| Identified or suspected (n) | 3 (20%) | 10 (23.8%) | 0.535 |
| Surgical wounds (Trans-genital tracts) (n = 2) | Sexually (n = 6) | ||
| Tran-esophagus (n = 1) | Percutaneous (n = 1) | ||
| Trans-esophagus (n = 2) | |||
| Trans-rectum (n = 1) | |||
| Leukocyte count (/mm3) | 3905 (900-10100) (n = 14) | 2600 (1000-7300) (n = 39) | 0.152 |
| AST (U/L) | 10,340 (5000-20692) (n = 10) | 5664 (92-18937) (n = 39) | 0.006 |
| ALT | 5116 (2970-7000) (n = 8) | 3248 (141-13980) (n = 29) | 0.048 |
| Total bilirubin (mg/dL) | 2.1 (2-8.5) (n = 6) | 4.4 (0.1-35) (n = 25) | 0.154 |
| Serology | |||
| positive/negative (n) | 2/3 (40%) | 14/12 (53.8%) | 0.654 |
| PCR analyzed (yes/no) (n) | 3/12 (20%) | 5/39 (8.4%) | 0.407 |
| HSV type (1/2/1 and 2) (n) | 5/3/0 | 14/14/3 | 1.0 |
| Ante-mortem diagnosis | |||
| yes/no (n) | 3/12 (20%) | 24/18 (57.1%) | 0.017 |
| ACV treatment | |||
| Yes/ no (n) | 3/12 (20%) | 21 / 21 (50%) | 0.041 |
| Liver transplantation | |||
| Yes/no (n) | 2/13 | 6/38 | 1.0 |
| Outcome | |||
| Survived/ died (overall) (n) | 2/13 (13.3%) | 12/30 (28.6%) | 0.312 |
| Survival time12 (d) | 13 (6-30) (n = 12) | 10 (5-29) (n = 26) | 0.729 |
| Survival time2 after liver dysfunction emerged (d) | 5.5 (1-9) (n = 11) | 5 (1-16)) (n = 18) | 0.821 |
Although both patients were comparable in clinical manifestation of fever and digestive symptoms, herpetic lesion was found in smaller numbers in postsurgical HSVH (27.3% vs 51.2%). Viral transmission pathway was identified in three (20%) of the postsurgical HSH patients including surgical wound (n = 2) and trans-esophageal (n = 1), the rate comparable that of non-surgical- HSVH patients (23.8%). Although reduction of leukocytes counts and mild elevation of total bilirubin levels were comparable, the levels of serum transaminase were significantly higher in postsurgical HSVH (AST: 10340 vs 5664 U/L, ALT: 5116 vs 3248 U/L, respectively). For laboratory diagnosis, the rates of positive serological study and performance of PCR were 40% and 53.8%, and 20% and 8.4%, respectively. Ante-mortem diagnosis was made in significantly (P = 0.017) smaller numbers of postsurgical HSVH patients (20% vs 57.1%). Similarly, in S-HSVH patients, ACV was treated in significantly (P = 0.041) lower numbers of patients (20% vs 47.7%), and survival rates were lower as compared with in counterpart (13.3% vs 28.6%). The surviving days after symptom or surgery occurred, and those after detection of liver dysfunction were comparable between the patients (13 d vs 10 d, 5.5 d vs 5 d, respectively).
Treatment with anti-viral agent, ACV, dramatically decreases mortality and hospital stay of HSV hepatitis[23]. We reanalyzed the case reports since 1986 (ACV era), when ACV was first used in our collected papers[9] (Table 3). Although the rate of ante-mortem diagnosis before 1985 was achieved in less than half of patients in both groups, the rate since 1986 was significantly (P = 0.040) lower in postsurgical group than in non-surgical group (27.3% vs 63.4%). The increase of the rate in ACV era was greater in non-surgical group (63.3% from 33.3%) than in postsurgical group (27.3% from 0%), but not statistically significant. Likewise, in non-surgical HSVH patients, the rates of ACV use and survival during ACV era were significantly increased as compared with those before 1985 [63.4% vs 0%, (P = 0.013), and 36.4% vs 0%, (P = 0.0321), respectively]. In contrast, in postsurgical HSVH patients, those rates were marginally increased or conversely decreased during ACV era (27.3% vs 0%, and 9.2% vs 25%, respectively). The rates of ACV treatment and survival during ACV era was lower in postsurgical HSVH as compared with those in non-surgical HSVH [27.3% vs 63.4%, (P = 0.040), and 9.2% vs 36.4%, (P = 0.086), respectively]. Finally, the timing of ACV treatment after detection of liver dysfunction was comparable (8 d vs 10 d), and the survival rates of the patients treated with ACV were not statistically different in both HSVH patients [25% vs 57% (P = 0.322)].
| Postsurgical patients (n = 15) | Non-surgical patients (n = 42) | P value (Postsurgical vs Non-surgical) | |
| Ante-mortem diagnosis | |||
| Yes/ no (before 1985) (n) | 0/4 (0%) | 3/6 (33.3%) | 0.497 |
| Yes/ no (since 1986) (n) | |||
| 3/8 (27.3%) | 21/12 (63.4%) | 0.040 | |
| P values | |||
| (before1985 vs since 1986) | |||
| 0.363 | 0.166 | ||
| ACV treatment | |||
| Yes/ no (before 1985) (n) | 0/4 (0%) | 0/9 (0%) | 1.0 |
| Yes/ no (since 1986) (n ) | |||
| 3/8 (27.3%) | 21/12 (63.4%) | 0.040 | |
| P values | |||
| (before1985 vs since 1986) | |||
| 0.363 | 0.013 | ||
| Outcome | |||
| Survived/ died (before 1985) (n) | 1/3 (25%) | 0/9 (0%) | 0.308 |
| Survived/ died (since 1986) (n) | |||
| 1/10 (9.2%) | 12/21 (36.4%) | 0.086 | |
| P values | |||
| (before1985 vs since 1986) | |||
| 0.476 | 0.0321 | ||
| Timing of ACV treatment1 | 8 (4-10) (n = 3) | 10 (6-15) (n = 16) | 0.365 |
| Outcome after ACV treatment | |||
| Survived/ died | 1/3 (25%) | 12/9 (57%) | 0.322 |
We presented a lethal case of HSVH developing soon after surgery. Clinical manifestation included high fever without herpetic mucocutaneous lesions, rapid and relentless elevation of serum aminotransaminase values (AST > ALT) associated with a relative normal serum bilirubin concentration (anicteric hepatitis), leucopenia, negative serology for HSV, coagulopathy, and encephalopathy. Although serum IgM level for CMV was increased after the surgery, the pathological role of CMV co-infection seemed to be minimal, because no viral was detected by immunostaining and PCR analysis in the liver.
Development of fulminant HSVH following surgery is extremely rare. In a review of fifteen cases (Table 1), several features were pointed out. (1) A wide spectrum of diseases (benign and malignant) and surgery (pancreatobiliary-, neurologic-, gynecologic-, urologic-, and digestive-surgery) involved; (2) cancer and postoperative steroid use are risk factor; (3) fever is the first and uniform presentation on median time of postoperative day 4.5, followed by gastrointestinal symptoms on day 7, liver dysfunction and leucopenia on day 8.5, and death resulting on day 13; (4) rare presentation of herpetiform lesions (27.3%); (5) vigorous deterioration of anicteric hepatitis; (6) low rates of positive IgM (40%) and IgG (20%); (7) extremely high mortality (87%) associated with low rates of ante-mortem diagnosis (20%) and acyclovir treatment (20%). These findings suggest that postsurgical HSVH can develop in a wide surgical spectrum, but preferably occurs in latently immunocompromised hosts. There were no definite criteria for diagnosis. Nevertheless, sudden onset and rapid deterioration of the lethal hepatitis needs prompt diagnosis and anti-viral treatment for survival.
Although our patient was mildly anemic and undernourished, he seemed to be non-immunocompromised, because he was healthy enough to undergo ordinary surgery for biliary stones and showed normal humoral response against CMV infection. However, there is a concern that immune-modulation by an excessive inflammation and viral entry by surgical manipulation may enhance to HSV infection and present with unique clinical course in postsurgical HSVH patients. The postsurgical HSVH as compared with non-surgical HSVH occurring in apparently healthy patients (Table 2) showed that (1) More vigorous liver necrosis as suggested by higher values of serum aminotransferases, although comparable magnitudes of disease rapidity and high mortality; (2) surgical wound as transmission pathway in only a few cases (20%). These findings do not likely support immunological disturbance and viral entry conferred by surgery as the major factors for postsurgical HSVH development. Alternatively, large HSV inoculums at the time of initial infection and/or viral heterogeneity (hepato-virulent strain) may be possible mechanisms[12].
It is of interest that establishment of ante-mortem diagnosis, ACV application and survival were significantly improved in non-surgical HSVH during ACV era (since 1986), whereas the improvement was limited in postsurgical HSVH (Table 3). Since survival benefit was comparably conferred by ACV treatment, a delay or failure in diagnosis as well as anti-viral therapy may result in worse prognosis of postsurgical HSVH. Several factors specific for postsurgical condition including hemodynamic changes, hepatotoxic drugs, anesthesia[33,36,37], and septic infection make complicated in differential diagnosis of postoperative hepatitis. CT image is helpful for excluding circulatory or abscess changes, but it does not lead to direct diagnosis. HSV serological examination has also limitations and herpetic lesions are frequently lacked as shown in this patient[20,26]. For diagnosis of HSV hepatitis, liver biopsy is gold standard, but coexisting coagulatory disorder often hampers its practice. Alternatively, PCR detection of the viral genome is recommended[20], but the use of sophisticated equipment is not always available in local hospitals. Nevertheless, due to a wide clinical spectrum, we can encounter the disease in non-specialized facilities, as presented here. Due to the difficulty and delay in diagnosis, rapid and lethal process of the disease nature, and low risk-benefit ratio, we propose empiric ACV therapy for postsurgical patients presenting with fulminant hepatitis of undetermined etiology.
In conclusion, postsurgical HSVH develops in a wide surgical spectrum with predisposing factors of malignancy and postoperative immunosuppression. Because of the difficulty in definite diagnosis and lethal nature of the disease, a high index of suspicion along with empiric antiviral intervention is imperative for undetermined fulminant hepatitis characterized by fever and leucopenia.
A healthy 72-year-old man developed fatal herpes simplex hepatitis soon after ordinary surgery for biliary stones.
A sudden onset of hepatitis associated with high fever and leukopenia emerged on postoperative day 5, followed by a rapid and lethal course (died on day 9).
Several factors specific for postsurgical condition including hemodynamic changes, hepatotoxic drugs, anesthesia, and septic infection make complicated in differential diagnosis of postoperative hepatitis.
A rapid elevation of liver aminotransferases (aspartate aminotransferase > alanine aminotransferase) without alteration of cholestasis was followed by coagulation disorder and encephalopathy.
A contrast-enhanced computed tomography showed heterogeous contrast distribution and a mottled liver without any abscess formation.
Postmortem liver biopsy revealed diffuse necrosis and loss of normal architecture with characteristic findings of intranuclear inclusions.
Empiric therapy with acyclovir, intravenous injection (10 mg/kg, every eight hours) was initiated on postoperative day 8.
Fatal herpetic hepatitis occurs in non-surgical healthy adults as well, whereas the disease following surgery is rarer and is more difficult for precise diagnosis.
Development of herpetic hepatitis is fatal complication following surgery, because of the difficulty for precise diagnosis and rapid deterioration of the disease.
Due to the difficulty in diagnosis and lethal nature, an early clinical suspension and prompt empirical anti-viral intervention are imperative for postsurgical hepatitis with undetermined etiology, characterized by fever and leucopenia.
The authors would like to appreciate Dr. Makoto Kuroda, Department of Pathology, Fujita Health University, for his definite diagnosis and valuable advice. We also appreciate Drs. Satoshi Baba and Isao Kosugi, Department of Pathology, Hamamatsu University School of Medicine, for their excellent immunostaining for CMV virus in the liver tissues.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Rajcani B S- Editor: Cui LJ L- Editor: A E- Editor: Li RF
| 1. | Bliss SJ, Moseley RH, Del Valle J, Saint S. Clinical problem-solving. A window of opportunity. N Engl J Med. 2003;349:1848-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Francis TI, Osuntokun BO, Kemp GE. Fulminant hepatitis due to herpes hominis in an adult human. Am J Gastroenterol. 1972;57:329-332. [PubMed] |
| 3. | Joseph TJ, Vogt PJ. Disseminated herpes with hepatoadrenal necrosis in an adult. Am J Med. 1974;56:735-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Eron L, Kosinski K, Hirsch MS. Hepatitis in an adult caused by Herpes simplex virus type I. Gastroenterology. 1976;71:500-504. [PubMed] |
| 5. | Connor RW, Lorts G, Gilbert DN. Lethal herpes simplex virus type 1 hepatitis in a normal adult. Gastroenterology. 1979;76:590-594. [PubMed] |
| 6. | Whorton CM, Thomas DM, Denham SW. Fatal systemic herpes simplex virus type 2 infection in a healthy young woman. South Med J. 1983;76:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 7. | Rubin MH, Ward DM, Painter CJ. Fulminant hepatic failure caused by genital herpes in a healthy person. JAMA. 1985;253:1299-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Goodman ZD, Ishak KG, Sesterhenn IA. Herpes simplex hepatitis in apparently immunocompetent adults. Am J Clin Pathol. 1986;85:694-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Baxter RP, Phillips LE, Faro S, Hoffman L. Hepatitis due to herpes simplex virus in a nonpregnant patient: treatment with acyclovir. Sex Transm Dis. 1986;13:174-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Andre PM, Camus C, Ruffault A, Cartier F. Herpes hepatitis and high levels of circulating interferon. J Infect Dis. 1987;156:529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | McMinn PC, Lim IS, McKenzie PE, van Deth AG, Simmons A. Disseminated herpes simplex virus infection in an apparently immunocompetent woman. Med J Aust. 1989;151:588-590; 592; 594. [PubMed] |
| 12. | Miyazaki Y, Akizuki S, Sakaoka H, Yamamoto S, Terao H. Disseminated infection of herpes simplex virus with fulminant hepatitis in a healthy adult. A case report. APMIS. 1991;99:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Tomita T, Garcia F, Mowry M. Herpes simplex hepatitis before and after acyclovir treatment. Immunohistochemical and in situ hybridization study. Arch Pathol Lab Med. 1992;116:173-177. [PubMed] |
| 14. | Lasserre M, Huguet C, Terno O. Acute severe herpes simplex hepatitis with virus-associated hemophagocytic syndrome in an immunocompetent adult. J Hepatol. 1993;18:256-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Farr RW, Short S, Weissman D. Fulminant hepatitis during herpes simplex virus infection in apparently immunocompetent adults: report of two cases and review of the literature. Clin Infect Dis. 1997;24:1191-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Velasco M, Llamas E, Guijarro-Rojas M, Ruiz-Yagüe M. Fulminant herpes hepatitis in a healthy adult: a treatable disorder? J Clin Gastroenterol. 1999;28:386-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Peters DJ, Greene WH, Ruggiero F, McGarrity TJ. Herpes simplex-induced fulminant hepatitis in adults: a call for empiric therapy. Dig Dis Sci. 2000;45:2399-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Fahy RJ, Crouser E, Pacht ER. Herpes simplex type 2 causing fulminant hepatic failure. South Med J. 2000;93:1212-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Pinna AD, Rakela J, Demetris AJ, Fung JJ. Five cases of fulminant hepatitis due to herpes simplex virus in adults. Dig Dis Sci. 2002;47:750-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Ichai P, Roque Afonso AM, Sebagh M, Gonzalez ME, Codés L, Azoulay D, Saliba F, Karam V, Dussaix E, Guettier C. Herpes simplex virus-associated acute liver failure: a difficult diagnosis with a poor prognosis. Liver Transpl. 2005;11:1550-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Czartoski T, Liu C, Koelle DM, Schmechel S, Kalus A, Wald A. Fulminant, acyclovir-resistant, herpes simplex virus type 2 hepatitis in an immunocompetent woman. J Clin Microbiol. 2006;44:1584-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Abbo L, Alcaide ML, Pano JR, Robinson PG, Campo RE. Fulminant hepatitis from herpes simplex virus type 2 in an immunocompetent adult. Transpl Infect Dis. 2007;9:323-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Norvell JP, Blei AT, Jovanovic BD, Levitsky J. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13:1428-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Levitsky J, Duddempudi AT, Lakeman FD, Whitley RJ, Luby JP, Lee WM, Fontana RJ, Blei AT, Ison MG; US Acute Liver Failure Study Group. Detection and diagnosis of herpes simplex virus infection in adults with acute liver failure. Liver Transpl. 2008;14:1498-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Lakhan SE, Harle L. Fatal fulminant herpes simplex hepatitis secondary to tongue piercing in an immunocompetent adult: a case report. J Med Case Rep. 2008;2:356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Beersma MF, Verjans GM, Metselaar HJ, Osterhaus AD, Berrington WR, van Doornum GJ. Quantification of viral DNA and liver enzymes in plasma improves early diagnosis and management of herpes simplex virus hepatitis. J Viral Hepat. 2011;18:e160-e166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Poley RA, Snowdon JF, Howes DW. Herpes Simplex Virus Hepatitis in an Immunocompetent Adult: A Fatal Outcome due to Liver Failure. Case Rep Crit Care. 2011;2011:138341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Wind L, van Herwaarden M, Sebens F, Gerding M. Severe hepatitis with coagulopathy due to HSV-1 in an immunocompetent man. Neth J Med. 2012;70:227-229. [PubMed] |
| 29. | Takeshita A, Tsuda Y, Fukunishi S, Asai A, Fukuda A, Hayashi M, Hirose Y. Two healthy females with fulminant hepatic failure caused by herpes simplex virus infection. Pathol Int. 2014;64:48-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Schuermans W, Mulliez S, Padalko E, Reynders M, Boel A, Van Vaerenbergh K, De Beenhouwer H. Let’s not forget herpes simplex virus in case of fulminant hepatic failure. Acta Gastroenterol Belg. 2014;77:359-361. [PubMed] |
| 31. | Czakó L, Dobra M, Terzin V, Tiszlavicz L, Wittmann T. Sepsis and hepatitis together with herpes simplex esophagitis in an immunocompetent adult. Dig Endosc. 2013;25:197-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Rimawi BH, Meserve J, Rimawi RH, Min Z, Gnann JW Jr. Disseminated Herpes Simplex Virus with Fulminant Hepatitis. Case Reports Hepatol. 2015;2015:463825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Douglas HJ, Eger EI 2nd, Biava CG, Renzi C. Hepatic necrosis associated with viral infection after enflurane anesthesia. N Engl J Med. 1977;296:553-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Marrie TJ, McDonald AT, Conen PE, Boudreau SF. Herpes simplex-hepatitus use of immunoperoxidase to demonstrate the viral antigen in hepatocytes. Gastroenterology. 1982;82:71-76. [PubMed] |
| 35. | Williams TL, Morgan JR, Denzler TB. Fulminant herpes simplex hepatitis following coronary bypass and postcardiotomy syndrome. Am Heart J. 1985;110:679-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Fisher NA, Iwata RT, Eger EI 2nd, Smuckler EA. Hepatic necrosis associated with herpes virus after isoflurane anesthesia. Anesth Analg. 1985;64:1131-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Katz J, Magee J, Baker B, Eger EI 2nd. Hepatic necrosis associated with herpesvirus after anesthesia with desflurane and nitrous oxide. Anesth Analg. 1994;78:1173-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Kaufman B, Gandhi SA, Louie E, Rizzi R, Illei P. Herpes simplex virus hepatitis: case report and review. Clin Infect Dis. 1997;24:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 125] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Price TM, Harris JB. Fulminant hepatic failure due to herpes simplex after hysteroscopy. Obstet Gynecol. 2001;98:954-956. [PubMed] |
| 40. | Kohno M, Hoshino U, Kobayashi J, Shiota G. A case of fulminant hepatitis caused by herpes simplex virus type 2 after the operation for hypertensive intracerebral hemorrhage. Kanzo. 2001;42:217-222. [DOI] [Full Text] |
| 41. | Biancofiore G, Bisà M, Bindi LM, Urbani L, Tascini C, Menichetti F, Filipponi F. Liver transplantation due to Herpes Simplex virus-related sepsis causing massive hepatic necrosis after thoracoscopic thymectomy. Minerva Anestesiol. 2007;73:319-322. [PubMed] |
| 42. | Chaudhary D, Ahmed S, Liu N, Marsano-Obando L. Acute Liver Failure from Herpes Simplex Virus in an Immunocompetent Patient Due to Direct Inoculation of the Peritoneum. ACG Case Rep J. 2017;4:e23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |