Published online Dec 26, 2018. doi: 10.12998/wjcc.v6.i16.1175
Peer-review started: October 19, 2018
First decision: November 12, 2018
Revised: November 11, 2018
Accepted: November 14, 2018
Article in press: November 15, 2018
Published online: December 26, 2018
Processing time: 66 Days and 18.7 Hours
Appendicitis, the inflammation of the appendix, is the most common abdominal surgical emergency requiring expedient surgical intervention. Extended-spectrum beta-lactamases (ESBLs) are bacterial enzymes that catalyse the degradation of the beta-lactam ring of penicillins and cephalosporins (but without carbapenemase activity), leading to resistance of these bacteria to beta-lactam antibiotics. Recent increases in incidence of ESBL-producing bacteria have caused alarm worldwide. Proportion estimates of ESBL-Enterobacteriaceae hover around 46% in China, 42% in East Africa, 12% in Germany, and 8% in the United States.
The impact of ESBL-producing bacteria on appendiceal abscesses and consequent pelvic abscesses are yet to be examined in depth. A literature review using the search words “appendiceal abscesses” and “ESBL Escherichia coli (E. coli)” revealed very few cases involving ESBL E. coli in any capacity in the context of appendiceal abscesses. This report describes the clinical aspects of a patient with appendicitis who developed a postoperative pelvic abscess infected with ESBL-producing E. coli. In this report, we discuss the risk factors for contracting ESBL E. coli infection in appendicitis and post-appendectomy pelvis abscesses. We also discuss our management approach for post-appendectomy ESBL E. coli pelvic abscesses, including drainage, pathogen identification, and pathogen characterisation. When ESBL E. coli is confirmed, carbapenem antibiotics should be promptly administered, as was done efficaciously with this patient. Our report is the first one in a developed country involving ESBL E. coli related surgical complications in association with a routine laparoscopic appendectomy.
Our report is the first involving ESBL E. coli and appendiceal abscesses, and that too consequent to laparoscopic appendectomy.
Core tip: This report describes the clinical aspects of a patient with appendicitis who developed a postoperative pelvic abscess infected with extended-spectrum beta-lactamase (ESBL) producing Escherichia coli (E. coli). Our report is the first reliable report involving ESBL E. coli and appendiceal abscesses. This report is also the first one in a developed country involving ESBL E. coli related surgical complications in association with a routine laparoscopic appendectomy.
- Citation: Tse A, Cheluvappa R, Selvendran S. Post-appendectomy pelvic abscess with extended-spectrum beta-lactamase producing Escherichia coli: A case report and review of literature. World J Clin Cases 2018; 6(16): 1175-1181
- URL: https://www.wjgnet.com/2307-8960/full/v6/i16/1175.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i16.1175
Extended-spectrum beta-lactamases (ESBLs) are bacterial enzymes that lead to the degradation of the beta-lactam ring of penicillins, cephalosporins, and monobactams (but not carbapenems) leading to these beta-lactam antibiotics being ineffective in treating infections with bacteria producing ESBLs[1]. Beta-lactamase inhibitors, such as clavulanic acid, inhibit ESBLs. However, there is sparse evidence available for good clinical outcomes with beta-lactamase inhibitor combinations[2]. The bacterial ESBLs are commonly encoded by transferable multi-antibiotic resistance plasmids, often including resistance to aminoglycosides[2]. Therefore, the antibiotic choices are limited to carbapenems (drug-group of choice), fluoroquinolones, tigecycline and polymyxins[2]. The substantial increases in recent worldwide prevalence of ESBL-producing bacteria have alarmed public health officials and infectious diseases personnel[1]. Bacteria producing ESBLs have been isolated with significant rates in Asia, Latin America and the Middle East[3]. Recent global increases in faecal colonisation by ESBL bacteria have been noted[4]. South and South East Asia are considered to be major regions for ESBL related infections and colonisation[5-7]. A Pakistani meta-analysis estimated the proportion of ESBL Enterobacteriaceae colonisation (nosocomial and community) to be 40%[8]. Proportion estimates of ESBL Enterobacteriaceae hover around 46% in China[9], 42% in East Africa[10], 10% to 15% in Germany[11], and 4% to 12% in the United States[12,13]. These data have significant clinical and public health import. However, the specific impact of ESBL bacteria on appendiceal abscesses and consequent pelvic abscesses are yet to be determined.
The human vermiform appendix has abundant lymphoid tissue and is exposed constantly to gastrointestinal bacteria and viruses of various hues. Inflammation of the appendix is termed “appendicitis’’. The causality of acute appendicitis has been shrouded in mystery, although the most popular theory posits luminal obstruction of the appendix (by faecoliths, lymphoid hyperplasia, or malignancy) incarcerating gut secretions, leading to increased intraluminal pressure and mucosal ischemia, resulting in gut bacterial infection[14]. The incidence of appendicitis is approximately 7%, making it the most common abdominal pathology requiring emergency surgery[15]. The peak incidence of appendicitis without perforation is in the 2nd and 3rd decades of life[16]. Mortality due to acute appendicitis is around 0.3%[17]. The mortality increases to 1.7% if perforation is present[17]. The approximate annual incidence of acute appendicitis in Australia is 177 per 100000[18]. Appendectomy for appendicitis is one of the most common emergency surgeries performed in public hospitals in Australia[19]. A well-known complication of appendectomies is the development of postoperative intra-abdominal abscesses. It occurs in 3% to 25% of appendectomies[20,21], with higher occurrences after perforated or gangrenous appendicitis[22-25].
Our patient was a 16-year-old girl from a Pakistani background who presented with acute appendicitis but went on to develop a pelvic abscess with ESBL E. coli after laparoscopic appendectomy. We monitored and treated this patient at various stages. Data was collated from progress notes, procedure summaries, pathology reports, radiology films or reports, surgical procedure-entries, and clinical files pertaining to this patient. We carried out a systematic literature search on Pubmed and Google Scholar using different combinations of the words: appendiceal, appendicitis, appendectomy, appendicectomy, abscess, ESBL, extended spectrum beta lactamase, or E. coli. There have only been 2 previous reports involving ESBL E. coli in any context of appendiceal abscesses. One of the 2 reports was 6-years-old, and the other, 9-years-old[26,27]. Unfortunately, those 2 reports appeared to be from publications not affiliated to reputable publishing houses[26,27]. Moreover, those 2 reports are not indexed in PubMed[26,27].
A 16-year-old girl of Pakistani extraction presented with a 7-d-history of right iliac fossa pain with anorexia, diarrhoea, and a body temperature of 38.7 °C. Her outpatient ultrasound scan did not show the appendix, although the possibility of the presence of a right ovarian cyst was suspected. Laboratory examination showed elevated levels of inflammatory markers: white cell count (WCC) 21.61 x 109/L, and C reactive protein (CRP) 69 mg/L. Based on these findings, a clinical diagnosis of appendicitis was made and the patient was commenced on 1 g Ceftriaxone daily and 500 mg of Metronidazole twice-daily. The patient underwent emergency surgery on the same night of arrival. On laparoscopy, a large phlegmon was identified, encompassing a perforated appendix and a faecolith. An appendectomy was performed with irrigation of the right para-colic gutter and pelvis with approximately 500 mL of normal saline, which we thought was adequate. The appendiceal stump area chosen for closure with Polydioxanone Endoloop® was free from visible inflammation. A 15 French Blake drain was placed in the pelvis extending to the right iliac fossa at the site of the phlegmon.
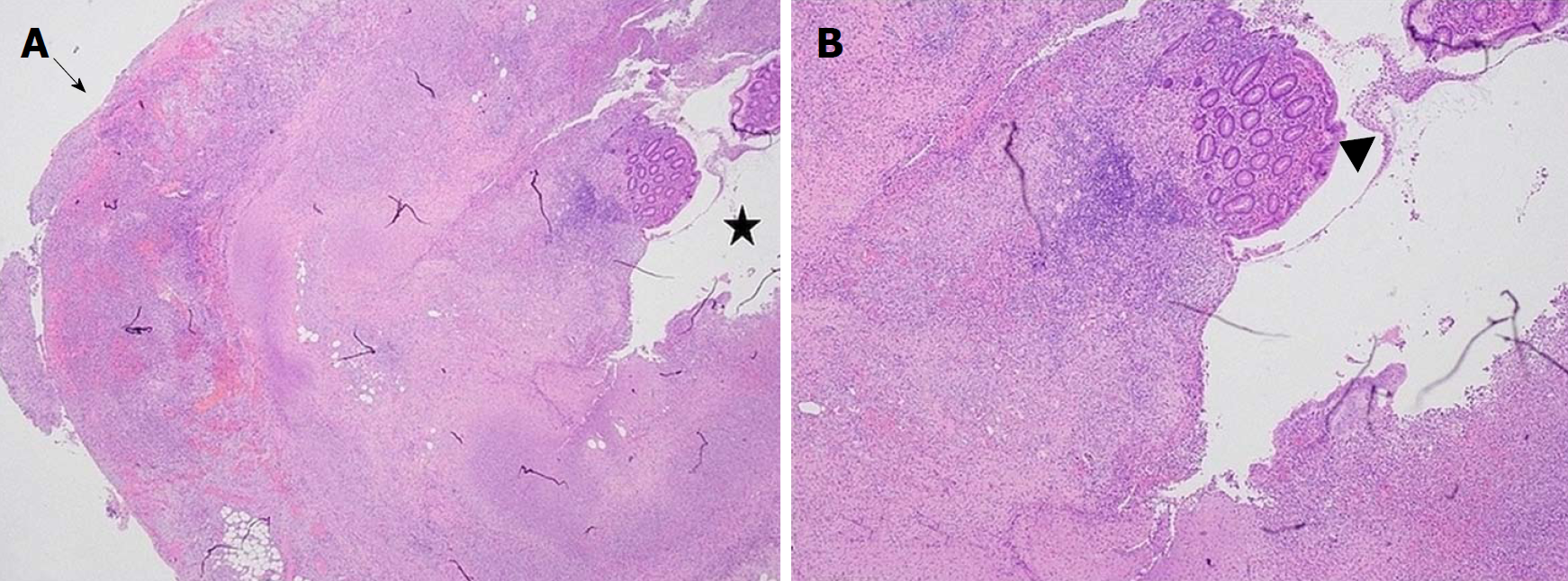
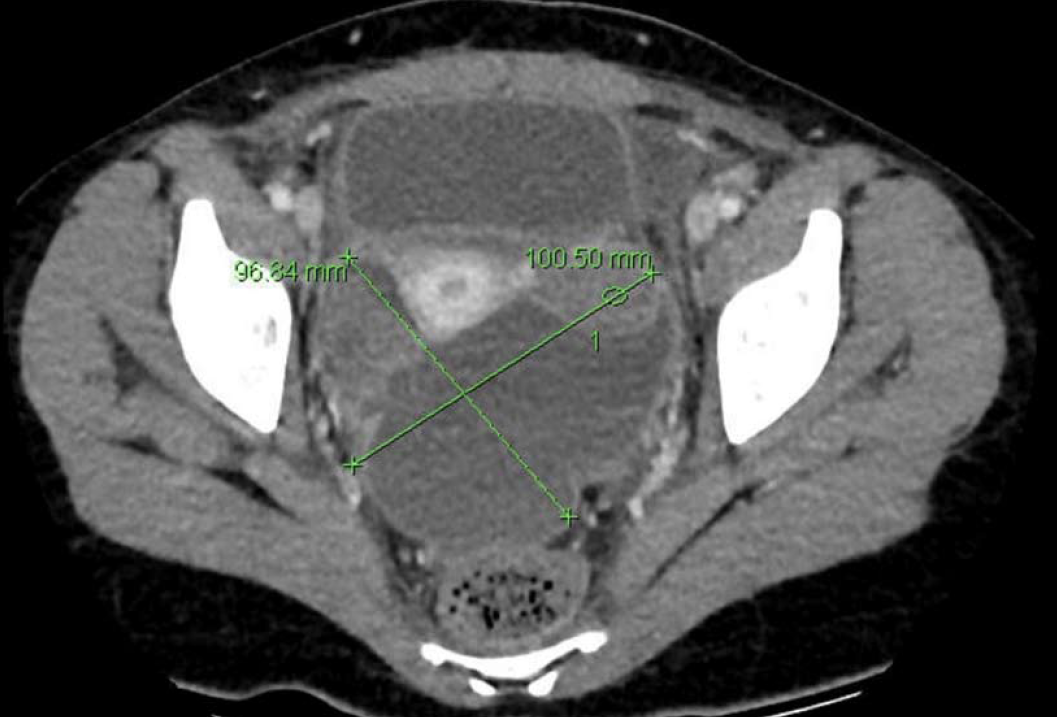
Subsequent histopathological examination reported acute suppurative and necrotic appendix (Figure 1). The 15 French Blake drain was removed on post-operative day 2 with minimal drainage. However, on postoperative day 3, the patient developed persistent pyrexia of 38.2 °C, in conjunction with increased haematological inflammatory markers (WCC 28.78 x 109/L, CRP 100 mg/L). A computed tomography (CT) scan performed on day 4 showed a large pelvic collection 10.1 cm x 9.6 cm (Figures 2 and 3).
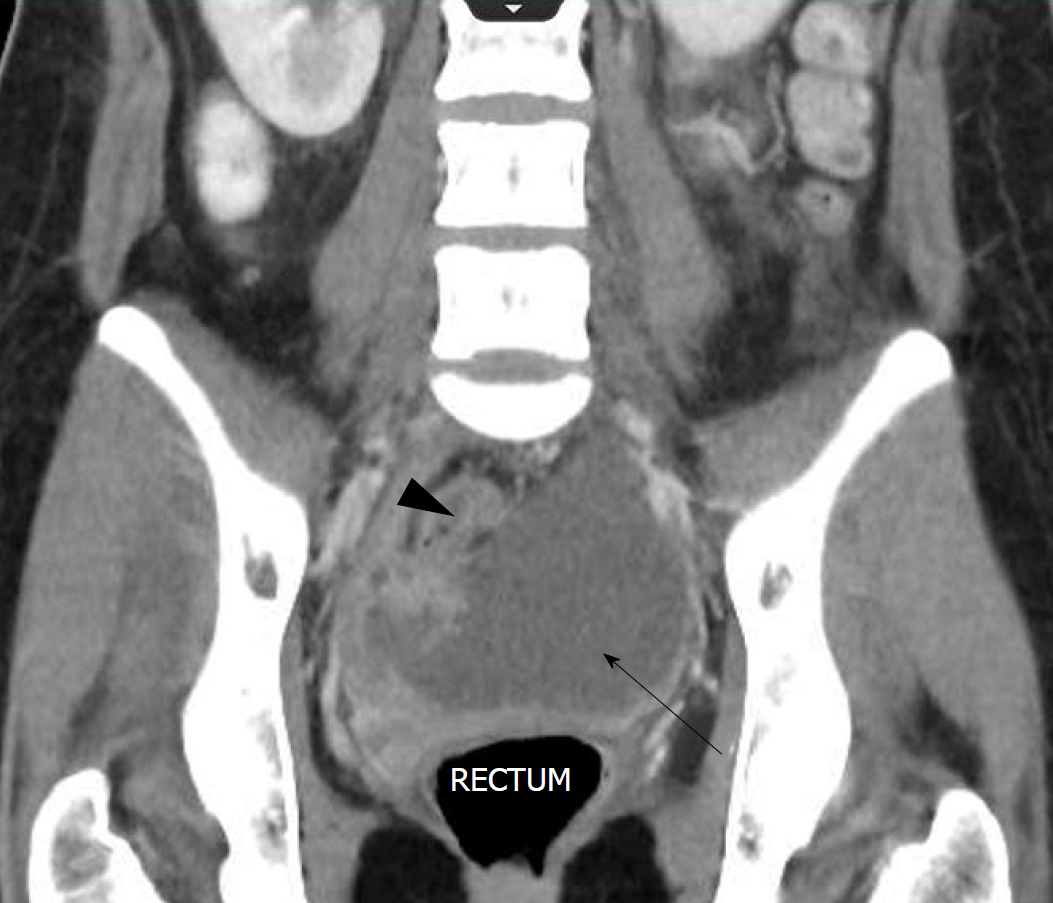
The CT report stated that there was “a peripheral enhancing collection in the pelvis on the right tracking inferiorly and along anterior pelvic side wall, crossing the midline, and being continuous with a large low density collection containing high density material in the pouch of Douglas”.
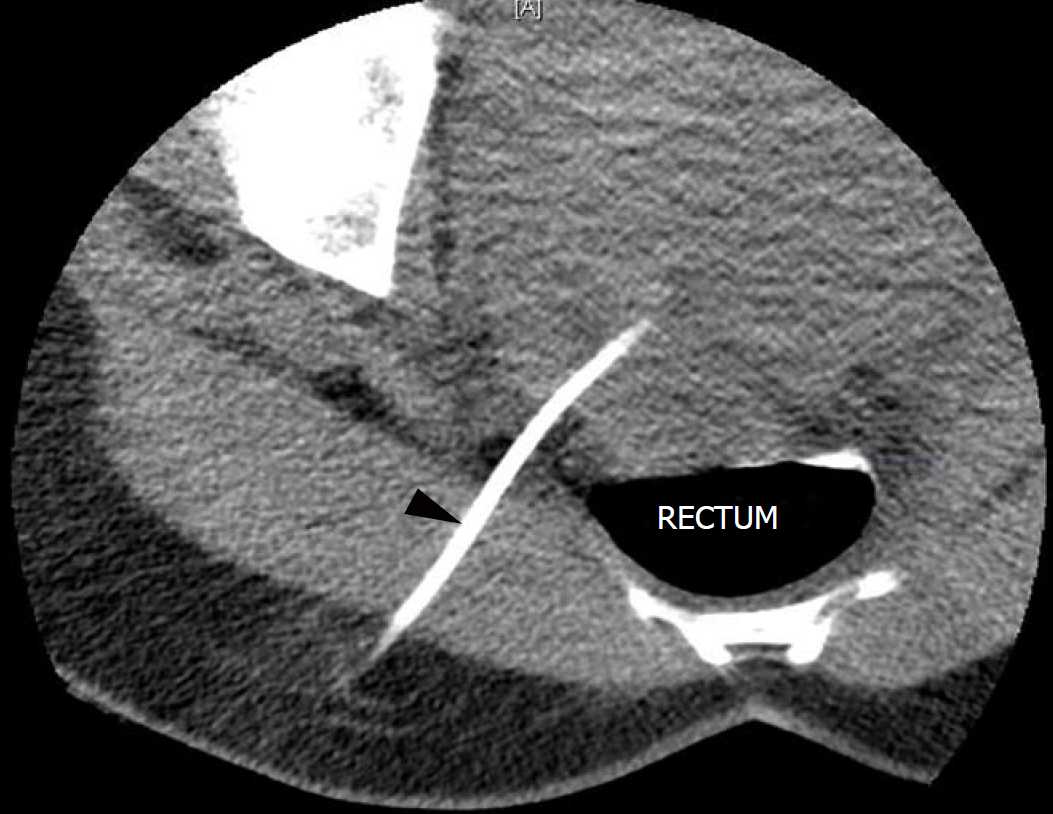
The pelvic collection in the pouch of Douglas was drained percutaneously under CT-guidance, and an 8 French Pigtail drain was left in situ in the rectouterine pouch of Douglas (Figure 4).
The tissue culture and percutaneously drained collection culture grew ESBL E. coli, susceptible only to Gentamicin and Meropenem. Our patient’s antibiotic regimen was then changed from Ceftriaxone and Metronidazole to Meropenem. Meropenem was given for 5 d during the patient’s hospital stay. On postoperative day 9, the percutaneous 8 French Pigtail drain was removed under aseptic conditions and the patient was discharged.
Under the instructions of the Infectious Diseases Specialist, a further 4 d of Irtepenum were given to the patient to be taken as an outpatient in ambulatory care.
Follow up ultrasounds were done at discharge and 2 wk after surgery. The ultrasonograms showed residual volumes of 53 mL at discharge; and 66 mL, 2 wk after surgery. However, the patient was clinically stable, and quite involved in her day-to-day activities. A follow up ultrasound done 2 mo after surgery showed complete resolution of the pelvic collection. The patient was then discharged from our surgical services.
Bacteria producing ESBLs were first described in Germany in 1983[28]. The ESBL enzymes hydrolyse the oxymino group on the beta-lactam cores of beta-lactam antibiotics[29]. The ESBL enzymes do not seem to have the ability to degrade carbapenems and cephamycins, which are structurally similar to cephalosporins, albeit with increased anaerobic coverage[29]. The ESBLs are often encoded by large plasmids which also encode resistance to other antibiotics simultaneously, most commonly quinolones and aminoglycosides[2]. These transferrable bacterial plasmids confer multi-drug resistance to bacteria which host them, thereby limiting the repertoire of efficacious antibacterial options[30].
Nosocomial infections with ESBL E. coli are common worldwide[1]. The incidence of community-acquired infections with ESBL E. coli are also increasing worldwide[31]. The gastrointestinal tract is obviously the main reservoir for ESBL producing Enterobactericeae, including the notorious 2, E. coli and Klebsiella[5-7]. Colonisation is a strong risk factor for subsequent infection[5-7]. Travel to South Asia has emerged as a major risk factor for colonisation[5-7]. The unscrupulous misuse of antibiotics for traveller’s diarrhoea in South Asia has only served to promote travel to South Asia as one of the most important risk factors for colonisation with ESBL Enterobacteriaceae[32,33]. Traditionally, colonisation has been iatrogenically associated with exposure to healthcare, as in the case of nosocomial infections during hospital stay[34]. More recently, community acquired ESBL bacterial infections have increased in incidence and prevalence, especially in the community setting of antimicrobial, immunosuppressive, and/or corticosteroid use[34].
The prevalence of ESBL-producing E.coli in acute appendicitis has been reported to be from 3.5% to 16.6%[35-37]. One study held ESBL E. coli accountable for 74.5% of isolated resistant bacteria isolated from acute appendicitis[38]. ESBL-producing E. coli can have a significant impact on the management of acute appendicitis. However there is scanty evidence describing the impact of ESBL-producing Enterobacteriaceae on appendiceal abscesses.
In this report, our patient developed a large postoperative pelvic abscess despite being on broad-spectrum antibiotics and a pelvic drain. The abscess was promptly identified, percutaneously drained and sent for culture and sensitivity. When the microbiology report identified ESBL-producing E. coli in the fluid drained, the patient was promptly treated with carbapenem under the guidance of the Infectious Diseases Specialists. The patient recovered and returned to her daily lifestyle quickly.
The choice of Polydioxanone Endoloop® or Endostapler did not seem to be influential in the pathogenesis of intraabdominal abscess. A retrospective study of 708 patients displayed a higher incidence (OR = 1.36) of developing intraabdominal abscess whilst using Endoloop®, when compared to Endostapler[39]. Conversely, a retrospective study involving 242 patients showed higher incidence of intraabdominal abscess when Endostapler was used in cases of perforated appendicitis, in contrast to when Endoloop® was used (OR = 7.09)[40]. However, a larger, better-designed, technically-superior, prospective study involving 1369 patients showed no difference in incidence of intraabdominal abscess between Endoloop® use versus Endostapler use (OR = 0.96)[41]. Interestingly, using multivariable analysis, this prospective study also showed that complicated appendicitis was the only independent risk factor for an intraabdominal abscess (OR = 6.26)[41]. Another retrospective study showed no difference[42].
Although carbapenems like imipenem, meropenem, doripenem, and ertapenem are also antibiotics containing beta-lactam rings, they are the first-line option for treating ESBL bacteria. Ertapenem may be preferred for community-acquired infections due to its once-daily dosing regimen[43]. However, it is indeed noteworthy that perioperative appendicitis patients colonised with ESBL bacteria do not necessarily require cultures or specific antibiotics; unless there is a complication like abscess, perforation, or peritonitis[44].
Bacteria producing ESBLs are not widely considered in surgical prophylaxis or postoperative complications unless the patient is previously known to be colonised. This is the first case report on ESBL related surgical complications in a routine laparoscopic appendectomy published in a developed Western country[45]. There are currently 2 published case reports, one of which was 6-years-old and the other 9-years-old, describing patients with perforated appendicitis with an abscess due to ESBL-producing E. coli[26,27]. We had mentioned in the Methods section earlier that these 2 reports were from journals neither indexed in Pubmed nor from reputable publishing groups[26,27].
Our report is the first reliable report involving ESBL E. coli and appendiceal abscesses. This report is also the first one in a developed country involving ESBL E. coli related surgical complications in association with a routine laparoscopic appendectomy. In a major multicultural city like Sydney (our location), ESBL bacterial complications need to be carefully considered in perioperative infections, recalcitrant or otherwise. Moreover, surgical antimicrobial prophylaxis and guidelines may need to be reviewed, as ESBL bacterial infections become widespread.
CARE Checklist (2013) statement: Guidelines of the CARE Checklist (2013) have been adopted while writing this manuscript.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Erginel B, Hawser S, Okumura K, Otowa Y S- Editor: Ma YJ L- Editor: A E- Editor: Wu YXJ
| 1. | Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev. 2005;18:657-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2039] [Cited by in RCA: 2241] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 2. | Falagas ME, Karageorgopoulos DE. Extended-spectrum beta-lactamase-producing organisms. J Hosp Infect. 2009;73:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 248] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 3. | Morrissey I, Hackel M, Badal R, Bouchillon S, Hawser S, Biedenbach D. A Review of Ten Years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011. Pharmaceuticals (Basel). 2013;6:1335-1346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 187] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 4. | Karanika S, Karantanos T, Arvanitis M, Grigoras C, Mylonakis E. Fecal Colonization With Extended-spectrum Beta-lactamase-Producing Enterobacteriaceae and Risk Factors Among Healthy Individuals: A Systematic Review and Metaanalysis. Clin Infect Dis. 2016;63:310-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 326] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 5. | Tängdén T, Cars O, Melhus A, Löwdin E. Foreign travel is a major risk factor for colonization with Escherichia coli producing CTX-M-type extended-spectrum beta-lactamases: a prospective study with Swedish volunteers. Antimicrob Agents Chemother. 2010;54:3564-3568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 284] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 6. | Strysko JP, Mony V, Cleveland J, Siddiqui H, Homel P, Gagliardo C. International travel is a risk factor for extended-spectrum β-lactamase-producing Enterobacteriaceae acquisition in children: A case-case-control study in an urban U.S. hospital. Travel Med Infect Dis. 2016;14:568-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Arcilla MS, van Hattem JM, Haverkate MR, Bootsma MCJ, van Genderen PJJ, Goorhuis A, Grobusch MP, Lashof AMO, Molhoek N, Schultsz C. Import and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): a prospective, multicentre cohort study. Lancet Infect Dis. 2017;17:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 325] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 8. | Abrar S, Hussain S, Khan RA, Ul Ain N, Haider H, Riaz S. Prevalence of extended-spectrum-β-lactamase-producing Enterobacteriaceae: first systematic meta-analysis report from Pakistan. Antimicrob Resist Infect Control. 2018;7:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | Zhang J, Zheng B, Zhao L, Wei Z, Ji J, Li L, Xiao Y. Nationwide high prevalence of CTX-M and an increase of CTX-M-55 in Escherichia coli isolated from patients with community-onset infections in Chinese county hospitals. BMC Infect Dis. 2014;14:659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Sonda T, Kumburu H, van Zwetselaar M, Alifrangis M, Lund O, Kibiki G, Aarestrup FM. Meta-analysis of proportion estimates of Extended-Spectrum-Beta-Lactamase-producing Enterobacteriaceae in East Africa hospitals. Antimicrob Resist Infect Control. 2016;5:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Leistner R, Schröder C, Geffers C, Breier AC, Gastmeier P, Behnke M. Regional distribution of nosocomial infections due to ESBL-positive Enterobacteriaceae in Germany: data from the German National Reference Center for the Surveillance of Nosocomial Infections (KISS). Clin Microbiol Infect. 2015;21:255.e1-255.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Flamm RK, Sader HS, Farrell DJ, Jones RN. Ceftaroline potency among 9 US Census regions: report from the 2010 AWARE Program. Clin Infect Dis. 2012;55 Suppl 3:S194-S205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Castanheira M, Farrell SE, Krause KM, Jones RN, Sader HS. Contemporary diversity of β-lactamases among Enterobacteriaceae in the nine U.S. census regions and ceftazidime-avibactam activity tested against isolates producing the most prevalent β-lactamase groups. Antimicrob Agents Chemother. 2014;58:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Larner AJ. The aetiology of appendicitis. Br J Hosp Med. 1988;39:540-542. [PubMed] |
| 15. | Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132:910-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 205] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Marudanayagam R, Williams GT, Rees BI. Review of the pathological results of 2660 appendicectomy specimens. J Gastroenterol. 2006;41:745-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Velanovich V, Satava R. Balancing the normal appendectomy rate with the perforated appendicitis rate: implications for quality assurance. Am Surg. 1992;58:264-269. [PubMed] |
| 18. | Organisation for Economic Co-operation and Development. Health at a glance 2015: health care activities. Paris: OECD-Organisation for Economic Co-operation and Development 2016; Available from: https://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance-2015_health_glance-2015-en. |
| 19. | Australia Institute of Health and Welfare. Admitted patient care 2016-2017: Australian hospital statistics. 2018;. |
| 20. | Emil S, Elkady S, Shbat L, Youssef F, Baird R, Laberge JM, Puligandla P, Shaw K. Determinants of postoperative abscess occurrence and percutaneous drainage in children with perforated appendicitis. Pediatr Surg Int. 2014;30:1265-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Fike FB, Mortellaro VE, Juang D, Sharp SW, Ostlie DJ, St Peter SD. The impact of postoperative abscess formation in perforated appendicitis. J Surg Res. 2011;170:24-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Asarias JR, Schlussel AT, Cafasso DE, Carlson TL, Kasprenski MC, Washington EN, Lustik MB, Yamamura MS, Matayoshi EZ, Zagorski SM. Incidence of postoperative intraabdominal abscesses in open versus laparoscopic appendectomies. Surg Endosc. 2011;25:2678-2683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Kouwenhoven EA, Repelaer van Driel OJ, van Erp WF. Fear for the intraabdominal abscess after laparoscopic appendectomy: not realistic. Surg Endosc. 2005;19:923-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Reid RI, Dobbs BR, Frizelle FA. Risk factors for post-appendicectomy intra-abdominal abscess. Aust N Z J Surg. 1999;69:373-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Guy S, Wysocki P. Risk factors for intra-abdominal abscess post laparoscopic appendicectomy for gangrenous or perforated appendicitis: A retrospective cohort study. Int J Surg Open. 2018;10:47-54. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Miyagi H, Okada T, Honda S, Minato M, Taketomi A. Appendical Perforation by Infection with Extended-Spectrum Beta-Lactamase (ESBL)-Producing Escherichia coli: Case Report. Surg Sci. 2012;3:53-55. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Nakata M, Miyauchi Y, Sonoda M. A Case of Appendicitis with Postoperative Infections Due to ESBL- Producing E. coli. Japanese J Pediatr Surg. 2009;41:762-766. |
| 28. | Knothe H, Shah P, Krcmery V, Antal M, Mitsuhashi S. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection. 1983;11:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 533] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 29. | Stapley EO, Birnbaum J, Miller AK, Wallick H, Hendlin D, Woodruff HB. Cefoxitin and cephamycins: microbiological studies. Rev Infect Dis. 1979;1:73-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Hague R. What is the threat from extended spectrum β-lactamase-producing organisms in children? Arch Dis Child. 2011;96:325-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Doi Y, Park YS, Rivera JI, Adams-Haduch JM, Hingwe A, Sordillo EM, Lewis JS 2nd, Howard WJ, Johnson LE, Polsky B, Jorgensen JH, Richter SS, Shutt KA, Paterson DL. Community-associated extended-spectrum β-lactamase-producing Escherichia coli infection in the United States. Clin Infect Dis. 2013;56:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 246] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 32. | Reuland EA, Sonder GJ, Stolte I, Al Naiemi N, Koek A, Linde GB, van de Laar TJ, Vandenbroucke-Grauls CM, van Dam AP. Travel to Asia and traveller’s diarrhoea with antibiotic treatment are independent risk factors for acquiring ciprofloxacin-resistant and extended spectrum β-lactamase-producing Enterobacteriaceae-a prospective cohort study. Clin Microbiol Infect. 2016;22:731.e1-731.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Kantele A, Lääveri T, Mero S, Vilkman K, Pakkanen SH, Ollgren J, Antikainen J, Kirveskari J. Antimicrobials increase travelers’ risk of colonization by extended-spectrum betalactamase-producing Enterobacteriaceae. Clin Infect Dis. 2015;60:837-846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 228] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 34. | Lee JA, Kang CI, Joo EJ, Ha YE, Kang SJ, Park SY, Chung DR, Peck KR, Ko KS, Lee NY. Epidemiology and clinical features of community-onset bacteremia caused by extended-spectrum β-lactamase-producing Klebsiella pneumoniae. Microb Drug Resist. 2011;17:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Lob SH, Badal RE, Bouchillon SK, Hawser SP, Hackel MA, Hoban DJ. Epidemiology and susceptibility of Gram-negative appendicitis pathogens: SMART 2008-2010. Surg Infect (Larchmt). 2013;14:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Chan KW, Lee KH, Mou JW, Cheung ST, Sihoe JD, Tam YH. Evidence-based adjustment of antibiotic in pediatric complicated appendicitis in the era of antibiotic resistance. Pediatr Surg Int. 2010;26:157-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Jeon HG, Ju HU, Kim GY, Jeong J, Kim MH, Jun JB. Bacteriology and changes in antibiotic susceptibility in adults with community-acquired perforated appendicitis. PLoS One. 2014;9:e111144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Coccolini F, D’Amico G, Sartelli M, Catena F, Montori G, Ceresoli M, Manfredi R, Di Saverio S, Ansaloni L. Antibiotic resistance evaluation and clinical analysis of acute appendicitis; report of 1431 consecutive worldwide patients: A cohort study. Int J Surg. 2016;26:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Escolino M, Becmeur F, Saxena A, Till H, Holcomb GW 3rd, Esposito C. Endoloop versus endostapler: what is the best option for appendiceal stump closure in children with complicated appendicitis? Results of a multicentric international survey. Surg Endosc. 2018;32:3570-3575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Safavi A, Langer M, Skarsgard ED. Endoloop versus endostapler closure of the appendiceal stump in pediatric laparoscopic appendectomy. Can J Surg. 2012;55:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | van Rossem CC, van Geloven AA, Schreinemacher MH, Bemelman WA; snapshot appendicitis collaborative study group. Endoloops or endostapler use in laparoscopic appendectomy for acute uncomplicated and complicated appendicitis : No difference in infectious complications. Surg Endosc. 2017;31:178-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Naiditch J, Lautz T, Chin A, Browne M, Rowell E. Endoloop as the first line tool for appendiceal stump closure in children with appendicitis. Eur J Pediatr Surg. 2015;25:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Delgado-Valverde M, Sojo-Dorado J, Pascual A, Rodríguez-Baño J. Clinical management of infections caused by multidrug-resistant Enterobacteriaceae. Ther Adv Infect Dis. 2013;1:49-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Gupta N, Sohanlal T, Soman R, Shetty A, Rodrigues C. The relevance of ESBL producing isolates in patients surgically treated for acute appendicitis. J Assoc Physicians India. 2011;59:293-295. [PubMed] |
| 45. | Bhatia R, Narain JP. The growing challenge of antimicrobial resistance in the South-East Asia Region--are we losing the battle? Indian J Med Res. 2010;132:482-486. [PubMed] |