Published online Dec 26, 2018. doi: 10.12998/wjcc.v6.i16.1136
Peer-review started: September 10, 2018
First decision: October 11, 2018
Revised: October 17, 2018
Accepted: November 7, 2018
Article in press: November 7, 2018
Published online: December 26, 2018
Processing time: 106 Days and 11.9 Hours
To investigate the effect of clonidine on the cutaneous silent period (CSP) during spinal anesthesia.
A total of 67 adult patients were included in this randomized, prospective, single-center, double-blind trial. They did not have neurological disorders and were scheduled for inguinal hernia repair surgery. This trial was registered on ClinicalTrials.gov (NTC03121261). The patients were randomized into two groups with regards to the intrathecally administered solution: (1) 15 mg of 0.5% levobupivacaine with 50 µg of 0.015% clonidine, or (2) 15 mg of 0.5% levobupivacaine alone. There were 34 patients in the levobupivacaine-clonidine (LC) group and 33 patients in the levobupivacaine (L) group. CSP and its latency were measured four times: prior to the subarachnoid block (SAB), after motor block regression to the 0 level of the Bromage scale, with ongoing sensory blockade, and both 6 and 24 h after SAB.
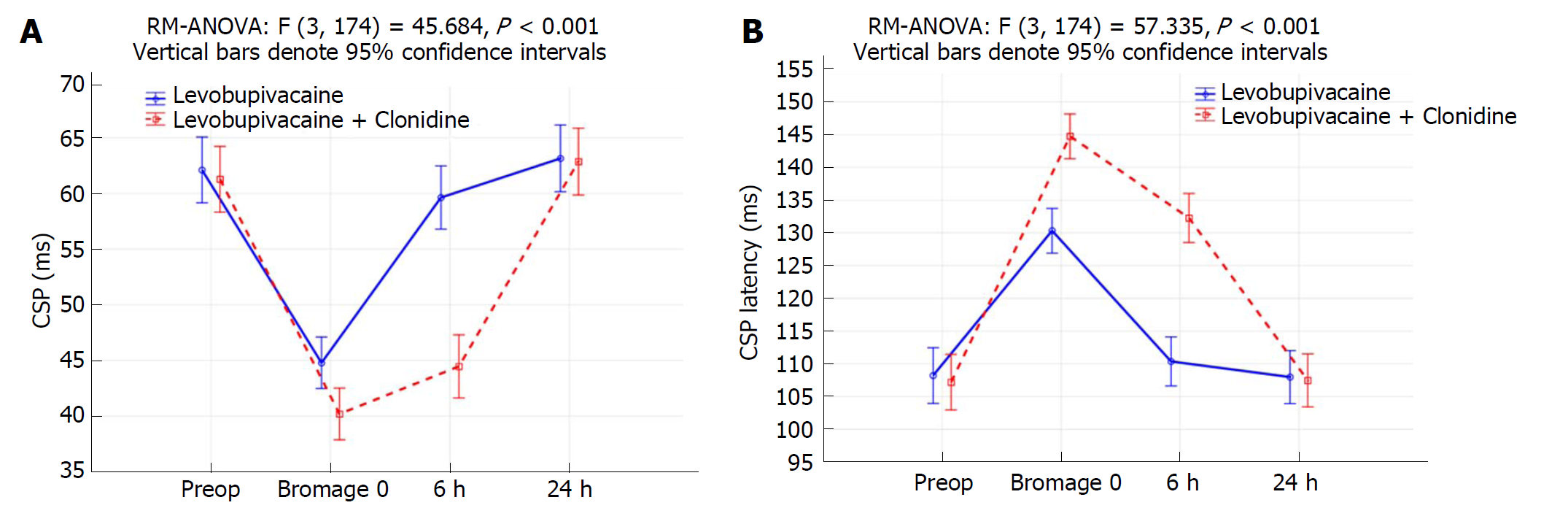
Only data from 30 patients in each group were analyzed. There were no significant differences between the groups investigated preoperatively and after 24 h. The CSP of the L group at the time point when the Bromage scale was 0 was 44.8 ± 8.1 ms, while in the LC group it measured 40.2 ± 3.8 ms (P = 0.007). The latency in the L group at the time point when the Bromage scale was 0 was 130.3 ± 10.2 ms, and in the LC group it was 144.7 ± 8.3 ms (P < 0.001). The CSP of the L group after 6 h was 59.6 ± 9.8 ms, while in the LC group it was 44.5 ± 5.0 ms (P < 0.001). The latency in the L group after 6 h was 110.4 ± 10.6 ms, while in LC group it was 132.3 ± 9.7 ms (P < 0.001).
Intrathecal addition of clonidine to levobupivacaine for SAB in comparison with levobupivacaine alone results in a diminished inhibitory tonus and shortened CSP.
Core tip: Cutaneous silent period (CSP) is an oligosynaptic spinal inhibitory reflex. The results of our study show that intrathecal administration of levobupivacaine with added clonidine, in comparison to levobupivacaine alone, yields a significantly shorter CSP and a significantly longer CSP latency during block regression following subarachnoid block (SAB) application. Accordingly, we can conclude that during SAB regression, a small dose of intrathecally administered clonidine ameliorates the inhibitory tonus and accelerates the conduction in the oligosynaptic spinal circuit.
- Citation: Zupcic SG, Zupcic M, Duzel V, Šimurina T, Milošević M, Basic S, Vuletic V, Kapural L. Effect of clonidine on the cutaneous silent period during spinal anesthesia. World J Clin Cases 2018; 6(16): 1136-1145
- URL: https://www.wjgnet.com/2307-8960/full/v6/i16/1136.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i16.1136
The cutaneous silent period (CSP) is an oligosynaptic spinal inhibitory reflex largely evoked through small-diameter Aδ-fibers[1,2]. The CSP begins after a noxious stimulus to a cutaneous sensory nerve during a voluntary muscle contraction and is evidenced by a transient lapse of electromyographic (EMG) activity[3]. According to some relevant studies, large-diameter afferent fibers with a low threshold action potential also contribute to EMG silencing through different mechanisms[4]. This is a non-invasive method that requires standard EMG equipment to investigate changes in the Aδ-fibers and further elucidate the understanding and organization of the spinal inhibitory circuit as an integral part of this reflex. Namely, the duration of the CSP and its latency are altered in polyneuropathy and various diseases of the central nervous system, which lead to damage of the corticospinal and spinothalamic pathways as well as extrapyramidal disorders, suggesting a possible supraspinal influence on the CSP[5-8].
Clonidine, a selective partial agonist of the alpha-2 receptors, when added to levobupivacaine and administered intrathecally, enhances the effect of the local anesthetic, prolongs the sensory and motor block during subarachnoid block (SAB) and also prolongs the duration of postoperative analgesia[9,10]. The analgesic effects of clonidine are achieved through the Aδ, C-fibers as well as the substantia gelatinosa of the spinal cord. However, considering that its analgesic effect is the strongest after intrathecal administration, it is deemed that the primary effective site of clonidine action is in the spinal cord[11,12]. Results from animal studies show the effect of clonidine on spinal reflexes[13-15]. Animal studies have shown that intrathecal administration of clonidine immediately resulted in a facilitation of the spinal reflexes in spinalized rats [mechanical or functional transection of the spinal cord (spinal block with procaine)][13]. Until now, the CSP has never been measured during neuraxial intrathecal blocking or after intrathecal administration of clonidine. We hypothesize that there could be a more pronounced and prolonged effect on the CSP after intrathecal administration of levobupivacaine and clonidine compared to levobupivacaine alone. The primary aim of this investigation was to demonstrate whether there is a difference in the duration and latency of CSP between a solution of levobupivacaine and clonidine together, in comparison with levobupivacaine alone, relative to the regression of the sensory and motor block and postoperative analgesia. A secondary aim was to determine whether there were differences in hemodynamic stability between the two studied groups, evidenced by changes in mean arterial pressure (MAP), heart rate (HR) and episodes of hypotension and bradycardia.
This was a randomized, prospective, single-center, double-blind trial conducted from May 2017 to October 2017 in Clinical Hospital Dubrava, Zagreb, Croatia. The trial was approved by the local ethics committee of Clinical Hospital Dubrava (registration code 2391-1/15) and was registered at the Clinical Trials registry (ClinicalTrials.gov; registration number NTC03121261). Informed consent was obtained from all individual participants included in the study.
The study included male and female patients scheduled for elective inguinal hernia repair surgery who were under spinal anesthesia. Inclusion criteria were: Signed informed consent, ages 18-60 years, body mass index 18.5-24.9 kg/m2, and American Society of Anesthesiologists physical status I or II (Table 1). Exclusion criteria were: allergy to medications used in the research, coagulation disorders, use of opioids, uncontrolled psychiatric disorders, use of psychotropic drugs, infections in areas of intended site of block application, central nervous system damage, diabetes, systemic inflammatory conditions, malignant disease, alcoholism and polyneuropathy.
| Patient data, duration of surgery | Group | |
| Levobupivacaine (n = 30) | Levobupivacaine + Clonidine (n = 30) | |
| Age (yr), mean ± SD | 43.5 ± 9.2 | 46.7 ± 10.3 |
| BMI (kg /m2), mean ± SD | 23.3 ± 0.9 | 23.5 ± 0.5 |
| Male gender | 26 (86.7) | 26 (86.7) |
| Female gender | 4 (13.3) | 4 (13.3) |
| ASA PS 2 | 10 (33.3) | 8 (26.7) |
| Hypertension | 3 (10.0) | 4 (13.3) |
| Hernia localization, left side | 17 (56.7) | 16 (53.3) |
| Duration of surgery (min), mean ± SD | 61.2 ± 12.9 | 62.8 ± 20.6 |
The patients were randomized through a free online randomization service [Urbaniak, G. C., and Plous, S. (2013). Research Randomizer (Version 4.0) Computer software] retrieved in May 2017 from http://www.randomizer.org. Eligible patients were randomly allocated to receive either a SAB with levobupivacaine and clonidine [levobupivacaine - clonidine group (LC group, n = 34)] or levobupivacaine [levobupivacaine group (L group, n = 33)]. The neurologists and anesthesiologists conducting the clinical part of the study were not aware of the randomization numbers of individual patients, which was known only to two anesthesia technicians who were not involved in either the selection or follow-up of patients. The control (L) group received 15 mg of 0.5% levobupivacaine (Chirocaine®, Abbott Laboratories, Dublin, Ireland) with 0.33 mL of 0.9% saline and 0.5 mL of 40% glucose, while the experimental (LC) group received 15 mg of 0.5% levobupivacaine with 50 µg of 0.015% clonidine (0.015% Catapressan; Boehringer Ingelheim KG, Germany) and 0.5 mL of 40% glucose. The CSP and its latency were measured 1 d before surgery in order to avoid an influence from benzodiazepine premedication. All patients received premedication of 5 mg midazolam (Dormicum®, Roche) intramuscularly. In the operating theatre, all patients were non-invasively monitored with noninvasive blood pressure cuffs (Dräger Medical GmbH, Lübeck, Germany), peripheral oxygen saturation (SpO2) and HR via a pulse oximetry finger probe (SpO2 Sensor, Adults, Reusable, Dräger Medical GmbH, Lübeck, Germany). The unilateral SAB in the theatre was performed by an anesthesiologist unaware of the assigned treatment, and the patient was also unaware of the group to which they were randomly assigned. The patients were positioned in the horizontal lateral position. The SAB was performed using a 27-gauge Whitacre spinal needle at the lumbar (L)3-L4 spinal level, after which the patients remained in the lateral position until full block onset. A successful block was confirmed by pinprick test for sensory block and Bromage scale for motor block on the operative side[16]. Once the block was confirmed, the patients were positioned in the supine position. In all patients, a standard inguinal hernia repair surgical technique was performed by the two same surgeons, each with more than ten years of experience. Perioperatively, the MAP and HR were measured every 5 min. If there was an episode of hypotension (blood pressure decreases of more than 30% of the basal values), the patients received a bolus of 250 mL of 0.9% saline intravenously (IV). In the case of persisting hypotension, the patients were given 5-10 mg of ephedrine hydrochloride (Ephedrine, Biotika, Prague, Czech Republic) IV, while bradycardia (HR < 50 beats/min) was treated with 0.5 mg of atropine IV. Postoperatively, the patients were admitted to the post-anesthesia care unit (PACU) for monitoring of vital functions and block regression. A blinded anesthesia technician recorded the times of block regression while testing the sacral (S) 1 dermatome bilaterally, using the pinprick test method every 10 min and motor block regression every 10 min with the Bromage scale[16]. The time of intrathecal administration of the solutions was considered the measurement starting time. Once the vital parameters fulfilled criteria for discharge from PACU, assessed by a blinded anesthesia technician, a blinded anesthesiologist estimated the motor block regression as Bromage 0 (ability to move the legs at the hip, knee and foot) while the sensory block was still present. At this time point, the second CSP measurement was performed in the EMG laboratory. After the CSP measurement, the patients were returned to the surgical ward where a ward technician recorded the pain intensity and return of sensation to the S 1 dermatome using a pinprick test. In accordance with previous studies, six hours after block application, analgesia was not present in the levobupivacaine group, while it was present in the levobupivacaine-clonidine group. The third measurement of CSP was conducted six hours after block application[9]. The fourth measurement of CSP was conducted 24 h after block application. The data were collected, coded and stored in a computer database. After statistical analysis, the blinding was broken.
Pain intensity was measured using the visual analogue scale (VAS, 0-10; VAS 0 = no pain; VAS 10 = maximal pain) prior to the surgical procedure, and every 3 h thereafter, during the 24-h postoperative period. The administration time of analgesics was also measured over the same period. In the case of moderate postoperative pain (> 3 VAS pain score < 6), the patients received 100 mg of ketoprofen (Ketonal, Sandoz) IV in 100 mL of 0.9% saline over 15 min. In the case of severe postoperative pain (VAS ≥ 6), the patients received 100 mg of tramadol hydrochloride (Tramal®, Herds) in 500 mL of 0.9 % saline over 30 min[17]. According to previous studies, tramadol significantly prolongs the duration of CSP, and these patients were excluded from the study[18].
All included patients were referred to the department of neurology, where a blinded neurologist conducted a measurement of CSP and its latency with an EMG device (Medelec Sinergy, United Kingdom). The ambient temperature was controlled and maintained at values of 25°C-26°C. The patients were lying horizontally and a stimulating ring electrode was placed on the hallux of the leg on the operated side, while a plain surface registering electrode was placed above the ipsilateral tendon of the extensor digitorum brevis muscle. Sweep duration was set at 500 ms sensitivity at 0.5 mV and filters were set at 50 Hz-5000 Hz. The patients were instructed to attempt a sustained isometric dorsi-flexion of the foot at about 50% of the maximum voluntary contraction (MVC) of the foot, considering that according to studies thus far, the mentioned strength of contraction does not affect the CSP[19]. MVC was determined by the manual muscle test, where 50% of MVC was determined by EMG activity, where MVC was shown over the entire screen and also followed by audiovisual feedback[20]. During a stable muscle contraction, a short pain stimulus of 250 V intensity and 0.3 ms duration was released and was shown to be the optimal duration for every individual stimulus at evoking a CSP[21]. In the case where EMG silence was not able to be evoked, the duration of an individual stimulus was gradually increased at a rate of 0.1 ms to 1 ms, with the aim of achieving a reproducible duration of CSP and its latency[21]. The absence of EMG activity was analyzed. The early latency duration was measured from the commencement of stimulation to the beginning of contraction suppression, and this was representative of CSP latency. Duration of late latency was measured from commencement of stimulation to the beginning of a new contraction. The difference between the two latencies is the duration of the CSP. The measurement was repeated up to 10 times in 30 s intervals, and the arithmetic mean of the three best measurements yielded a complete EMG silence, making it possible for the longest duration of CSP to be calculated.
Analysis of test power was made for variance analysis of repeated measurements according to the following parameters: two tested groups, four repeated measurements, significance α = 0.05, sample size consideration f = 0.25, and test power of 90%. This showed that 30 subjects per group were necessary to be included. Sample size consideration was made due to the presumption of a similar previously published study[9]. Power analysis was carried out with the software support of G*Power for Windows, version 3.1.2” (http://www.gpower.hhu.de/fileadmin/redaktion/Fakultaeten/Mathematisch-Naturwissenschaftliche_Fakultaet/Psychologie/AAP/gpower/GPowerManual.pdf).
Quantitative values are shown as the means, SD and 95% CI. Kolmogorov-Smirnov test was used to analyze the distribution of quantitative data, and we applied appropriate parametric tests according to the data obtained. The comparisons between quantitative values were made with independent and dependent t-tests. The differences in categorical values were analyzed with Fisher’s exact tests. A two-way repeated measured analysis of variance (RM-ANOVA) was conducted to evaluate the null hypothesis that there are no changes in CSP, latency of CSP, VAS score and secondary hemodynamic parameters (MAP, HR) before, during and after spinal anesthesia (time and treatment over time interaction). Overall and time-specific treatment differences were generated with adjustment for multiplicity - Bonferroni correction. All other P values less than 0.05 were considered significant. IBM SPSS Statistics, version 21.0 was used in the analysis (http://www.spss.com).
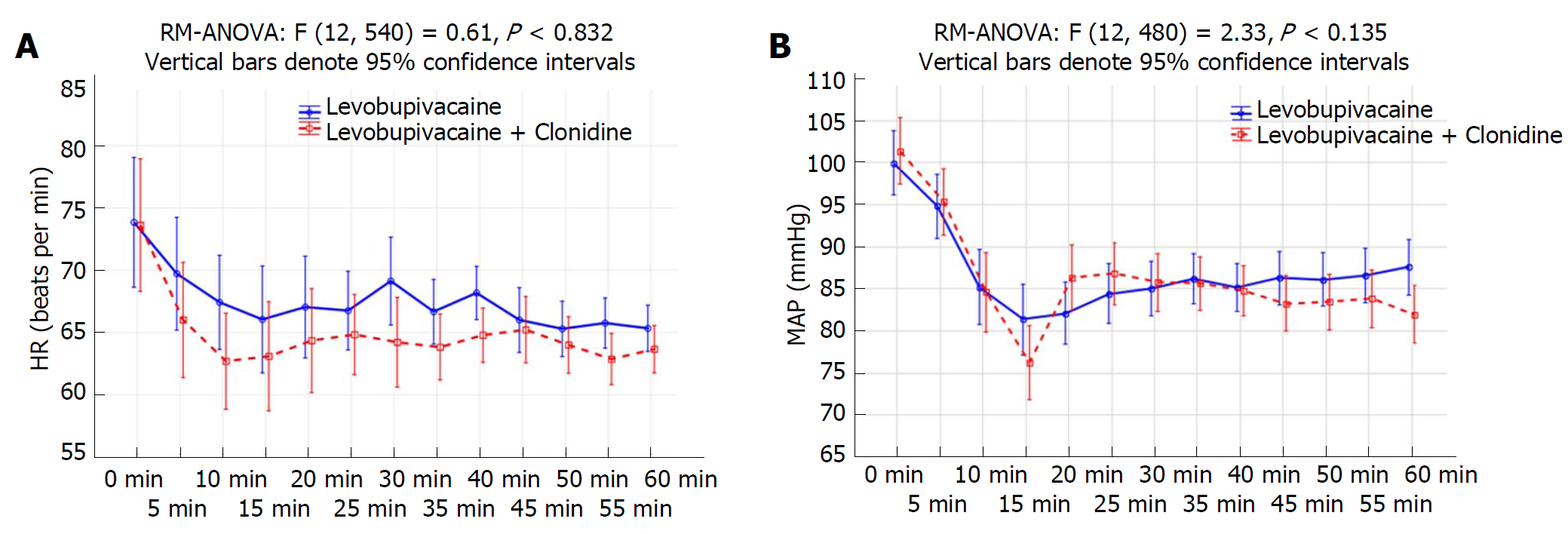
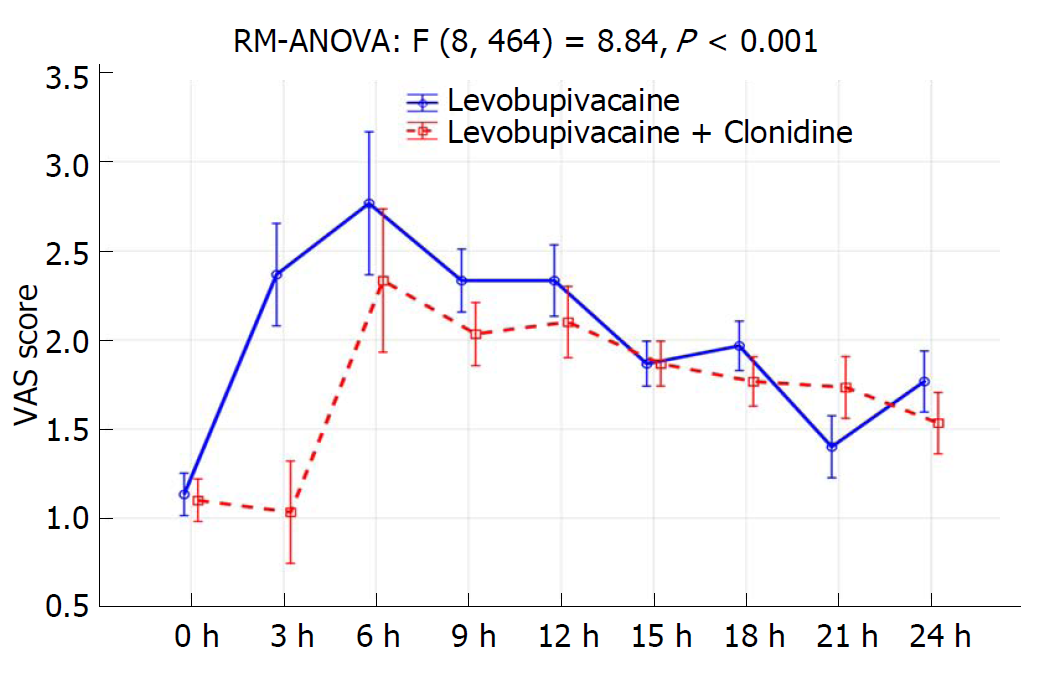
Four patients from the LC group and three from the L group were excluded from the trial due to discomfort during performance of the CSP test, and only 30 patients per group were included in the analysis. Data about patient characteristics and duration of surgery did not show statistically significant differences between groups (Table 1). There were no significant differences in CSP or its latency between investigated groups preoperatively and after 24 h (Table 2, Figure 1A and 1B). The CSP of the L group at the timepoint when the Bromage scale was 0 was 44.8 ± 8.1 ms, while in the LC group it measured 40.2 ± 3.8 ms (P = 0.007). The latency in the L group at the timepoint when the Bromage scale was 0 was 130.3 ± 10.2 ms, and in the LC group it was 144.7 ± 8.3 ms (P < 0.001). The CSP of the L group after 6 h was 59.6 ± 9.8 ms, while in the LC group it was 44.5 ± 5.0 ms (P < 0.001). The latency in the L group after 6 h was 110.4 ± 10.6 ms, while in LC group was 132.3 ± 9.7 ms (P < 0.001) (Table 2). When comparing preoperative and postoperative values of CSP duration, there was a statistically significant difference at timepoints of Bromage 0 in the L group (P < 0.001), the LC group (P = 0.007) and after 6 h in the LC group (P < 0.001) (Table 2 and Figure 1). When comparing the preoperative and postoperative values of CSP latency, there was a statistically significant difference at time points when the Bromage scale was 0 in both groups and after 6 h in the LC group (P < 0.001), while after 24 h there was no statistically significant difference in CSP duration or latency in either group (Table 2 and Figure 1). In addition, the LC group had a significantly longer regression time of the motor block (Bromage 0) on the operated side, a longer time of sensory regression in the S1 dermatome on the operated side (pinprick test), a longer duration of analgesia after SAB application, and longer times of first and second use of nonsteroidal anti-inflammatory drugs (NSAIDs) (P < 0.001; Table 3). Time over group interactions were highly significant in both CSP and its latency, indicating the significant difference in CSP and latency dynamics between the investigated groups (P < 0.001; Figure 1A and 1B). There were no significant changes in MAP and HR values (Figure 2B), while the VAS score over 24 h was significantly higher in the L group (P < 0.001; Figure 3). Between groups, there was no statistically significant difference in the incidence of hypotension of the L group [n (%)-5/30 (16.7%)] in comparison to the LC group [n (%)-10/30 (33.3%)], in bradycardia [(n (%)-2/30 (6.7%)] in both groups, nor in the administration of ephedrine in the L group [n (%)-2/30 (6.7%)] in comparison to the LC group [n (%)-4/30 (13.3%)] and atropine [n (%)-2/30 (6.7%)] in both groups, which were all subjected to Fisher’s exact tests.
| Levobupivacaine group(n = 30) | Levobupivacaine + Clonidine group(n = 30) | P1 | P2 | P3 | |
| CSP latency (ms) preoperative | 108.2 ± 11.6 | 107.2 ± 11.6 | 0.737 | ||
| CSP latency (ms) Bromage 0 | 130.3 ± 10.2 | 144.7 ± 8.3 | < 0.001a | < 0.001e | < 0.001e |
| CSP latency (ms) 6 h | 110.4 ± 10.6 | 132.3 ± 9.7 | < 0.001b | 0.459 | < 0.001f |
| CSP latency (ms) 24 h | 108.0 ± 10.6 | 107.5 ± 11.5 | 0.862 | 0.933 | 0.926 |
| CSP (ms) preoperative | 62.1 ± 9.5 | 61.3 ± 6.2 | 0.691 | ||
| CSP (ms) Bromage 0 | 44.8 ± 8.1 | 40.2 ± 3.8 | 0.007c | < 0.001g | 0.007g |
| CSP (ms) 6 h | 59.6 ± 9.8 | 44.5 ± 5.0 | < 0.001d | 0.325 | < 0.001h |
| CSP (ms) 24 h | 63.2 ± 9.9 | 62.9 ± 5.9 | 0.892 | 0.681 | 0.315 |
| Levobupivacaine group(n = 30) | Levobupivacaine + Clonidine group(n = 30) | P | |
| Regression time of the motor block (Bromage 0) - operated side | 162.3 ± 25.6 | 285.3 ± 55.2 | < 0.001 |
| The time of sense regression in S1 dermatome - operated side (pinprick test) | 195.0 ± 22.4 | 326.3 ± 49.4 | < 0.001 |
| Duration of analgesia after SAB application | 192.3 ± 20.3 | 364.3 ± 53.3 | < 0.001 |
| NSAID (iv.) 1st time (min) | 205.3 ± 22.2 | 377.0 ± 51.6 | < 0.001 |
| NSAID (iv.) 2nd time (min) | 388.3 ± 38.4 | 593.8 ± 54.9 | < 0.001 |
The results of our study show that intrathecal administration of levobupivacaine with added clonidine in comparison to levobupivacaine alone yields a significantly shorter CSP and longer CSP latency during block regression after SAB application (Table 2). It is known that intrathecal administration of small doses of clonidine (15-50 μg) as an adjuvant to local anesthetics in inguinal hernia repair surgery and hysterectomy can prolong the motor and sensory blockade as well as the duration of analgesia in comparison to local anesthetics alone, without significant differences in hemodynamic parameters (MAP and HR)[9,10,22,23]. In our study, we used a dose of 50 μg of clonidine in order to achieve a prolongation of the SAB, and showed the effects of intrathecal clonidine on the CSP without significant hemodynamic disturbances. In order to better portray the change in dynamics of CSP and latency, we conducted measurements at four different time points, as mentioned in the Methods section. The results of the first measurement of CSP and latency were similar between groups, which suggests adequate patient randomization (Table 2). In our study, we used a similar methodology as Svilpauskaite et al[21]. However, the results in our study showed a longer duration of CSP compared to the mentioned study, where the CSP duration was measured as 45.7 ± 11.8 ms for the left leg and 47.1 ± 10.3 ms for the right leg, which can be explained by differences in methodology. Namely, those authors used a maximal strength muscle contraction, while we used muscle contraction strength at 50% of the maximum, which could have influenced the duration of CSP in this study. According to the literature, CSP is shortest during a maximum muscle contraction when the CSP is most sensitive to changes and can disappear completely. According to published studies, muscle contractions at 10-50% MVC do not change the values of CSP duration[19]. The results in our study are similar to results obtained by Mota et al[24] (latency 112.8 ± 17.4 ms, CSP 69.5 ± 19.0 ms), which can be explained by the muscle contraction strength of 30% MVC, as was used in their investigation. In the second measurement, we confirmed a statistically significant shortening of CSP duration and significant prolongation of latency in each group compared to the first measurement. In the second measurement, the duration of CSP was significantly shorter in the LC group, and the latency significantly longer (Table 2). These results concur with the results of previous studies[24]. Namely, Mota et al[24] had established a shortening in the duration of CSP and a gradual prolongation of its latency with the administration of a local anesthetic in the popliteal fossa for peripheral nerve blockade. In the mentioned study, CSP could be evoked immediately prior to the onset of motor block, but it was of a shorter duration and prolonged latency. Contrary to the study by Mota et al[24], we measured the CSP duration and latency during SAB regression. Similar to their investigation, CSP was measured during the phase when the block was still maintained in terms of sensory blockade, but without a motor component. Thereby, the patients did not feel any pain in the S1 dermatome, but the EMG silence of shorter duration and prolonged latency could still be evoked. As explained by Mota et al[24], the EMG silence could be consequential to activation of large diameter fibers and is possibly an integral part of the cutaneomuscular reflex or a consequence of a small number of Aδ-fibers, whose function on nerve transmission is not hindered and is an integral part of the CSP[4]. The duration of the sensory block is determined by the return of sensation in the S1 dermatome (pinprick test). Since we stimulated the sensory nerves of the hallux (L4, L5, S1 roots) in order to evoke a CSP, it is plausible that the EMG silencing in this situation could be due to both the partial regression of blockade of the Aδ-fibers after intrathecal local anesthetic administration, as well as the activation of functionally recovered Aδ-fibers[22]. In the second measurement, there was a difference in the duration of the CSP and its latency between the L and LC groups. In the LC group, the latency was significantly prolonged and the duration of CSP was significantly shorter than in the L group. In the third measurement, there was a persistent prolongation of CSP latency with a shortened duration of CSP in the LC group, while in the L group, the duration and latency of the CSP did not differ significantly from the initial measurement prior to SAB. We consider that the observed changes in the duration and latency of the CSP in the LC group are a consequence of clonidine administration.
Clonidine is a mixed alpha-1 and alpha-2 adrenergic agonist with predominant alpha-2 activity, and has an antihypertensive, sedative and analgesic effect[11]. It exhibits a synergistic effect when combined with local anesthetics. Clonidine increases the influx of potassium ions in isolated neurons, resulting in an augmentation and prolongation of local anesthetic effects[25,26]. In vitro studies have shown that alpha-2 adrenergic agonists activate the postsynaptic alpha-2 adrenergic receptors and increase the influx of potassium ions, thereby causing a hyperpolarization of the neurons in the substantia gelatinosa. Meanwhile, the activation of presynaptic alpha-2 adrenergic receptors on the C and A-δ fibers inhibits the influx of calcium ions, resulting in a decreased release of neurotransmitters[27-29]. Noxious stimuli in rats cause a polysynaptic flexor reflex-hindlimb reflex, which is manifested by limb retraction and the tail-flick reflex. Animal studies have shown the effects of intrathecal administration of clonidine on the mentioned spinal reflexes, which depend on the functional integrity of the spinal cord and the administered dose of clonidine[13-15]. In an intact rat spine, the systemic administration of clonidine resulted in an attenuation of the flexor reflex, while in spinalized rats (mechanical or functional transection of the spinal column (spinal block with procaine)), the systemic or intrathecal administration of clonidine immediately resulted in a facilitation of the reflexor response[13,15,30,31]. Small doses of intrathecally-administered clonidine in spinalized mice resulted in facilitation of the mentioned reflex, while administration of larger doses resulted in an inhibition of the reflex[31]. Spinalization of mice resulted in predominant alpha-1 effects over alpha-2 effects of clonidine. In mice with intact spines, the preserved supraspinal pathways prevented the alpha-1 effects of clonidine on spinal reflex pathways, while a functional transection of the spinal cord caused a disinhibition of supraspinal effects, with predominant alpha-1 effects of clonidine, resulting in a facilitation of the flexor reflex[13]. In contrast to the flexor reflex, which is polysynaptic, the CSP is an oligosynaptic reflex and little is known about the spinal inhibitory circuit of the CSP. The results of this study are in accordance with those of animal studies where small doses of intrathecally-administered clonidine in spinalized animals resulted in a facilitated flexor reflex response[13,15,30,31]. In contrast to studies on spinalized animals, which measured the effect of intrathecally-administered clonidine on the polysynaptic spinal reflex, in this study we investigated the effect of intrathecally-administered clonidine with neuraxial intrathecal anesthesia on an oligosynaptic spinal reflex, the CSP. The results of this study show that a small dose of intrathecally-administered clonidine during neuraxial intrathecal block ameliorates the inhibitory tonus and accelerates conduction in the oligosynaptic spinal circuit. This is likely via predominant alpha-1 adrenergic effects. Since this is a constituent of the CSP, the end effect was a shortened CSP and a prolongation of its latency.
The results of our study confirm the results of a previous study by Singh et al[9], who claimed that the advantages of intrathecal administration of clonidine at a dose of 50 mg as an adjuvant to a local anesthetic include the prolongation of analgesia, motor blockade, and sensory blockade with minimal hemodynamic changes, as evidenced by minimal changes in MAP, HR and ephedrine administration. As in previous studies, the results of our study confirm that after intrathecal administration of clonidine, measured pain intensity VAS scores are lower during the 24 h postoperative period. Consequently, patients required less NSAID administration[9,22].
There are some limitations to our study. One limitation is that due to regression of the neuraxial block, there are a limited number of measurements of CSP duration and latency that can be conducted in order to avoid patient discomfort. This investigation did not measure the effect of intrathecally-administered clonidine on the CSP without local anesthetics, because in healthy subjects this would not be ethical and in surgical patients it would not provide sufficient anesthesia[22].
In conclusion, intrathecal administration of clonidine as an adjuvant to levobupivacaine for SAB, in comparison with levobupivacaine alone, results in a shorter duration of CSP and a prolongation of CSP latency. Accordingly, we can conclude that during SAB regression, a small dose of intrathecally-administered clonidine ameliorates the inhibitory tonus and accelerates the conduction in the oligosynaptic spinal circuit.
The silent cutaneous period (CSP) is an oligosynaptic spinal inhibitory reflex, largely mediated through small diameter Aδ-fibers. The CSP begins after a noxious stimulus of a cutaneous sensory nerve during a voluntary muscle contraction and is evidenced by a transient lapse of electromyographic (EMG) activity. This is a non-invasive method that requires standard EMG equipment to research changes in Aδ-fibers and can further elucidate the organization of the spinal inhibitory circuit as an integral part of this reflex.
The duration of CSP and its latency are altered in polyneuropathy and various diseases of the central nervous system, such as those that lead to damage of the corticospinal and spinothalamic pathways as well as extrapyramidal disorders. This suggests a possible supraspinal influence on the CSP. Clonidine, a selective partial agonist of alpha-2 receptors, when added to levobupivacaine and administered intrathecally, enhances the effect of the local anesthetic, prolongs the sensory and motor block during SAB, and prolongs the duration of postoperative analgesia. Until now, no other studies have measured the effect of intrathecally-administered clonidine on the CSP.
The research objective of this investigation was to examine the effect of clonidine on the CSP during SAB for inguinal herniorrhaphy.
A total of 67 adult patients were included in this randomized, prospective, single-center, double-blind trial. They had no neurological disorders and were scheduled for inguinal hernia repair surgery. The patients were randomized into two groups with regard to the intrathecally-administered solution: either levobupivacaine with clonidine or levobupivacaine alone (34 patients in the levobupivacaine-clonidine (LC) group and 33 patients in the levobupivacaine (L) group). CSP and its latency were measured four times: prior to the SAB, after motor block regression to Bromage scale level 0, with sensory blockade still present, and both 6 and 24 h after SAB.
The LC group had significantly lower CSP duration (P = 0.007) and higher CSP latency (P < 0.001) values at the timepoint when the Bromage scale was 0 and after 6 h (P < 0.001). Comparing preoperative and postoperative values of CSP duration, there was a statistically significant difference at time points Bromage 0 in the L group (P < 0.001), the LC group (P = 0.007) and after 6 h in the LC group (P < 0.001). When comparing the preoperative and postoperative values of CSP latency, there was a statistically significant difference at timepoints when the Bromage scale was 0 in both groups and after 6 h in the LC group (P < 0.001).
Intrathecal administration of clonidine as an adjuvant to levobupivacaine for SAB, in comparison with lavobupivacaine alone, results in a shorter duration of CSP and prolongation of CSP latency. Accordingly, we can conclude that during SAB regression, a small dose of intrathecally-administered clonidine ameliorates the inhibitory tonus and accelerates conduction in the oligosynaptic spinal circuit.
CSP is an oligosynaptic spinal inhibitory reflex. Little is known about the factors that influence the functions of its spinal inhibitory circuits, which should be the subject of further investigations.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Croatia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lizarraga I, Afzal M S- Editor: Dou Y L- Editor: Filipodia E- Editor: Wu YXJ
| 1. | Inghilleri M, Cruccu G, Argenta M, Polidori L, Manfredi M. Silent period in upper limb muscles after noxious cutaneous stimulation in man. Electroencephalogr Clin Neurophysiol. 1997;105:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Shefner JM, Logigian EL. Relationship between stimulus strength and the cutaneous silent period. Muscle Nerve. 1993;16:278-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Floeter MK. Cutaneous silent periods. Muscle Nerve. 2003;28:391-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Serrao M, Parisi L, Pierelli F, Rossi P. Cutaneous afferents mediating the cutaneous silent period in the upper limbs: evidences for a role of low-threshold sensory fibres. Clin Neurophysiol. 2001;112:2007-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Gilio F, Bettolo CM, Conte A, Iacovelli E, Frasca V, Serrao M, Giacomelli E, Gabriele M, Prencipe M, Inghilleri M. Influence of the corticospinal tract on the cutaneous silent period: a study in patients with pyramidal syndrome. Neurosci Lett. 2008;433:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Serrao M, Parisi L, Valente G, Martini A, Fattapposta F, Pierelli F, Rossi P. L-Dopa decreases cutaneous nociceptive inhibition of motor activity in Parkinson’s disease. Acta Neurol Scand. 2002;105:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Morkavuk G, Leventoglu A. Small fiber neuropathy associated with hyperlipidemia: utility of cutaneous silent periods and autonomic tests. ISRN Neurol. 2014;2014:579242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Kofler M, Kronenberg MF, Brenneis C, Felber A, Saltuari L. Cutaneous silent periods in intramedullary spinal cord lesions. J Neurol Sci. 2003;216:67-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Singh RB, Chopra N, Choubey S, Tripathi RK, Prabhakar , Mishra A. Role of Clonidine as adjuvant to intrathecal bupivacaine in patients undergoing lower abdominal surgery: A randomized control study. Anesth Essays Res. 2014;8:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, Bulbul M, Baraka AS. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 252] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 11. | Eisenach JC, De Kock M, Klimscha W. alpha(2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984-1995). Anesthesiology. 1996;85:655-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 513] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 12. | Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Han HJ, Beitz AJ, Lee JH. Intrathecal clonidine suppresses phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit in spinal dorsal horn neurons of rats with neuropathic pain. Anesth Analg. 2008;107:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Kehne JH, Gallager DW, Davis M. Spinalization unmasks clonidine’s alpha 1-adrenergic mediated excitation of the flexor reflex in rats. J Neurosci. 1985;5:1583-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Solomon RE, Brody MJ, Gebhart GF. Pharmacological characterization of alpha adrenoceptors involved in the antinociceptive and cardiovascular effects of intrathecally administered clonidine. J Pharmacol Exp Ther. 1989;251:27-38. [PubMed] |
| 15. | Luo L, Wiesenfeld-Hallin Z. Low-dose intrathecal clonidine releases tachykinins in rat spinal cord. Eur J Pharmacol. 1993;235:157-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Honca M, Dereli N, Kose EA, Honca T, Kutuk S, Unal SS, Horasanli E. [Low-dose levobupivacaine plus fentanyl combination for spinal anesthesia in anorectal surgery]. Rev Bras Anestesiol. 2015;65:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Župčić M, Graf Župčić S, Duzel V, Šimurina T, Šakić L, Fudurić J, Peršec J, Milošević M, Stanec Z, Korušić A. A combination of levobupivacaine and lidocaine for paravertebral block in breast cancer patients undergoing quadrantectomy causes greater hemodynamic oscillations than levobupivacaine alone. Croat Med J. 2017;58:270-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Pujia F, Coppola G, Anastasio MG, Brienza M, Vestrini E, Valente GO, Parisi L, Serrao M, Pierelli F. Cutaneous silent period in hand muscles is lengthened by tramadol: Evidence for monoaminergic modulation? Neurosci Lett. 2012;528:78-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Kofler M, Kumru H, Stetkarova I, Schindler C, Fuhr P. Muscle force up to 50% of maximum does not affect cutaneous silent periods in thenar muscles. Clin Neurophysiol. 2007;118:2025-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Fan E, Ciesla ND, Truong AD, Bhoopathi V, Zeger SL, Needham DM. Inter-rater reliability of manual muscle strength testing in ICU survivors and simulated patients. Intensive Care Med. 2010;36:1038-1043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Svilpauskaite J, Truffert A, Vaiciene N, Magistris MR. Electrophysiology of small peripheral nerve fibers in man. A study using the cutaneous silent period. Medicina (Kaunas). 2006;42:300-313. [PubMed] |
| 22. | Dobrydnjov I, Axelsson K, Thörn SE, Matthiesen P, Klockhoff H, Holmström B, Gupta A. Clonidine combined with small-dose bupivacaine during spinal anesthesia for inguinal herniorrhaphy: a randomized double-blinded study. Anesth Analg. 2003;96:1496-1503, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Rao KG, Shukla A, Misra S. Postoperative Analgesia After Panhysterectomy, Addition of Clonidine to Bupivacaine: Boon for the Patients. Anesth Essays Res. 2017;11:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Mota IA, Fernandes JB, Cardoso MN, Sala-Blanch X, Kofler M, Valls-Solé J. Temporal profile of the effects of regional anesthesia on the cutaneous reflexes of foot muscles. Exp Brain Res. 2015;233:2587-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Wolff M, Heugel P, Hempelmann G, Scholz A, Mühling J, Olschewski A. Clonidine reduces the excitability of spinal dorsal horn neurones. Br J Anaesth. 2007;98:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Nishiyama T, Hanaoka K. Intrathecal clonidine and bupivacaine have synergistic analgesia for acute thermally or inflammatory-induced pain in rats. Anesth Analg. 2004;98:1056-1061, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Kawasaki Y, Kumamoto E, Furue H, Yoshimura M. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology. 2003;98:682-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 139] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Sonohata M, Furue H, Katafuchi T, Yasaka T, Doi A, Kumamoto E, Yoshimura M. Actions of noradrenaline on substantia gelatinosa neurones in the rat spinal cord revealed by in vivo patch recording. J Physiol. 2004;555:515-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Funai Y, Pickering AE, Uta D, Nishikawa K, Mori T, Asada A, Imoto K, Furue H. Systemic dexmedetomidine augments inhibitory synaptic transmission in the superficial dorsal horn through activation of descending noradrenergic control: an in vivo patch-clamp analysis of analgesic mechanisms. Pain. 2014;155:617-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Advokat C. Spinal transection increases the potency of clonidine on the tail-flick and hindlimb flexion reflexes. Eur J Pharmacol. 2002;437:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Xu XJ, Puke MJ, Wiesenfeld-Hallin Z. The depressive effect of intrathecal clonidine on the spinal flexor reflex is enhanced after sciatic nerve section in rats. Pain. 1992;51:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |