Published online Dec 6, 2018. doi: 10.12998/wjcc.v6.i15.985
Peer-review started: September 12, 2018
First decision: October 15, 2018
Revised: October 24, 2018
Accepted: November 1, 2018
Article in press: November 1, 2018
Published online: December 6, 2018
Processing time: 85 Days and 21.2 Hours
To perform a meta-analysis to investigate the correlation between body mass index (BMI) and the short-term outcomes of laparoscopic gastrectomy (LG) for gastric cancer (GC) in Asian patients.
The PubMed, Cochrane, EMBASE, and Web of Science databases were searched for studies that focused on the impact of obesity on the short-term outcomes of LG for GC in Asian patients who were classified into a high BMI (BMI ≥ 25 kg/m2) or low BMI group (BMI < 25 kg/m2). The results are expressed using the pooled odds ratio (OR) for binary variables and standard mean difference (SMD) for continuous variables with 95% confidence interval (CI), and were calculated according to the fixed-effects model while heterogeneity was not apparent or a random-effects model while heterogeneity was apparent.
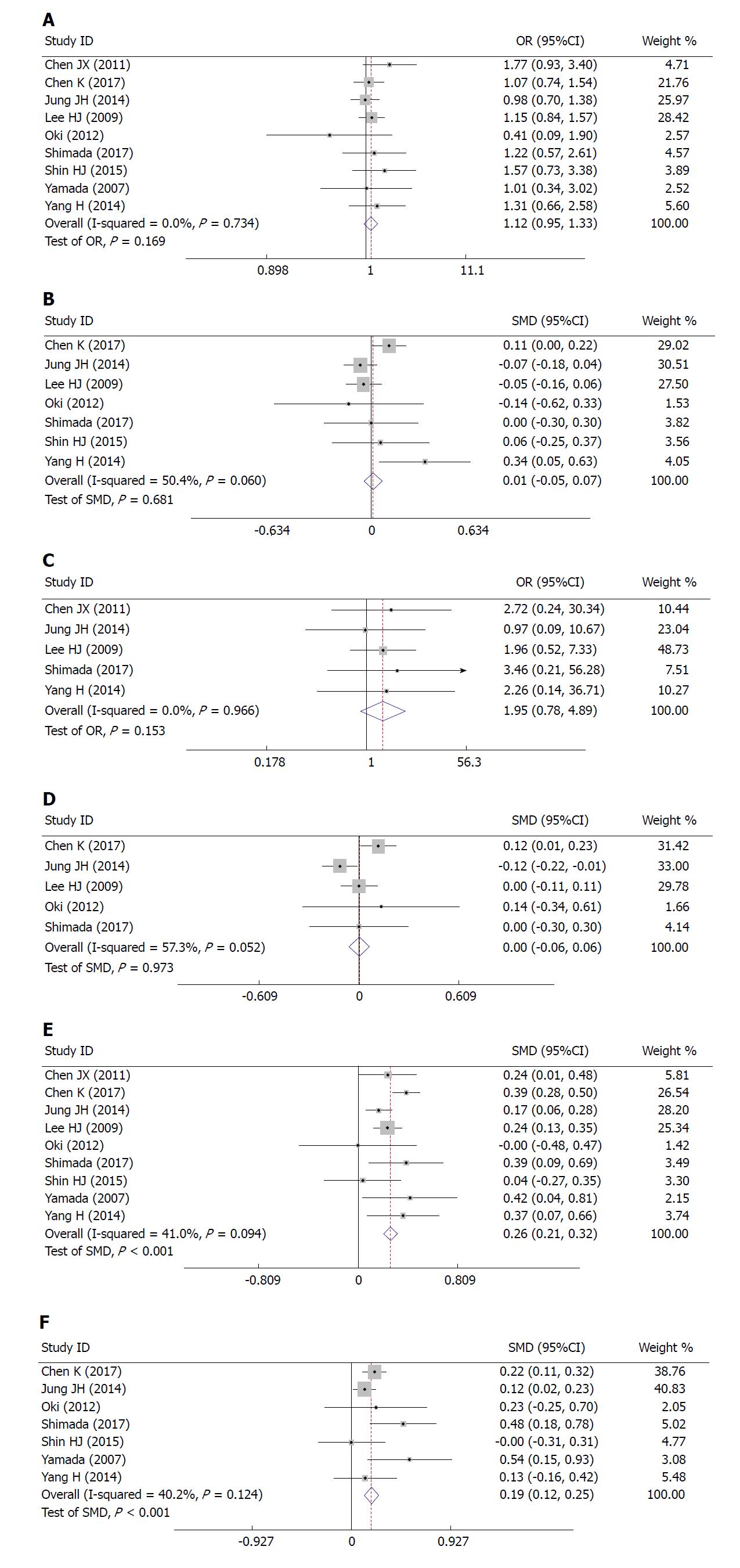
Nine studies, with a total sample size of 6077, were included in this meta-analysis. Compared with the low BMI group, the high BMI group had longer operative time (SMD = 0.26, 95%CI: 0.21 to 0.32, P < 0.001), greater blood loss (SMD = 0.19, 95%CI: 0.12 to 0.25, P < 0.001), and fewer retrieved lymph nodes (SMD = -0.13, 95%CI: 0.18 to 0.07, P < 0.001). There was no significant difference between the high and low BMI groups in postoperative complications (OR = 1.12, 95%CI: 0.95 to 1.33, P = 0.169), the duration of postoperative hospital stay (SMD = 0.681, 95%CI: -0.05 to 0.07, P = 0.681), postoperative mortality (OR = 1.95, 95%CI: 0.78 to 4.89, P = 0.153), or time to resuming food intake (SMD = 0.00, 95%CI: -0.06 to 0.06, P = 0.973).
Our meta-analysis provides strong evidence that despite being associated with longer operative time, greater blood loss, and fewer retrieved lymph nodes, BMI has no significant impact on the short-term outcomes of LG for GC in Asian patients, including postoperative complications, the duration of postoperative hospital stay, postoperative mortality, and time to resuming food intake. BMI may be a poor risk factor for short-term outcomes of LG. Other indices should be taken into account.
Core tip: The impact of body mass index (BMI) on the short-term outcomes of laparoscopic gastrectomy (LG) for gastric cancer in Asian patients have been controversial due to inconsistent results of previous studies. Our meta-analysis demonstrates that despite being associated with longer operative time, greater blood loss, and fewer retrieved lymph nodes, a high BMI could not be significantly associated with short-term outcomes of LG in Asian patients. BMI may be a poor risk factor for short-term outcomes of LG. Other indices should be taken into account.
- Citation: Chen HK, Zhu GW, Huang YJ, Zheng W, Yang SG, Ye JX. Impact of body mass index on short-term outcomes of laparoscopic gastrectomy in Asian patients: A meta-analysis. World J Clin Cases 2018; 6(15): 985-994
- URL: https://www.wjgnet.com/2307-8960/full/v6/i15/985.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i15.985
Gastric cancer (GC), the second most prevalent cause of cancer-related deaths worldwide, has been a source of increasing concern[1]. Since 1994, when laparoscopic technique was first used for GC[2-6], laparoscopic gastrectomy (LG) has become increasingly popular for treating early GC (EGC) due to decreased intraoperative blood loss, less pain, and shorter hospital duration[7-10]. The prevalence of obesity is increasing steadily in Asian countries. Obesity may increase the risk of health disorders, such as hypertension, cardiovascular disease, and type 2 diabetes mellitus[11,12], and is regarded as a risk factor for worse surgical outcomes of complicated surgical procedures[13]. Furthermore, patients with obesity have a higher risk of operative difficulties, as well as wound infection[14-16]. Recently, the impact of obesity on short-term outcomes of LG in patients has been controversial due to inconsistent results of several studies. Obesity leads to a longer duration of postoperative hospital stay and time to resuming food intake in studies performed by Chen et al[17] and Yang et al[18], while Jung et al[19] reported that obesity was not a risk factor for these impacts. The studies performed by Chen et al[20], Shimada et al[21], and Yamada et al[22] suggested that the association between obesity and LG was significant, while Shin et al[23] and Oki et al[24] reported the opposite conclusion.
To date, although several studies used the body mass index (BMI) to assess the impact of obesity on the short-term outcomes of LG, the results have been controversial and limited. Hence, we conducted this meta-analysis to summarize all of the available evidence.
The PubMed, Cochrane, EMBASE, and Web of Science databases were searched up to July 30, 2018 using the search terms (obesity) OR (metabolically benign) OR (obesity, morbid) OR (pediatric) OR (overweight) AND (body mass index) OR (index, body mass) OR (Quetelet index) OR (index, Quetelet) OR (Quetelet’s index) OR (Quetelets index) AND (stomach neoplasms) OR (neoplasm, stomach) OR (stomach neoplasm) OR (gastric neoplasms) OR (gastric neoplasm) OR (neoplasm, gastric) OR (neoplasms, gastric) OR (cancer of stomach) OR (stomach cancers) OR (gastric cancer) OR (cancer, gastric) OR (cancers, gastric) OR (gastric cancers) OR (stomach cancer) OR (cancer, stomach) OR (cancers, stomach) OR (cancer of the stomach) OR (gastric cancer, familial diffuse) AND (laparoscopy) OR (laparoscopies) OR (celioscopy) OR (celioscopies) OR (peritoneoscopy) OR (peritoneoscopies) OR (surgical procedures, laparoscopic) OR (laparoscopic surgical procedure) OR (procedure, laparoscopic surgical) OR (procedures, laparoscopic surgical) OR (surgery, laparoscopic) OR (laparoscopic surgical procedures) OR (laparoscopic surgery) OR (laparoscopic surgeries) OR (surgeries, laparoscopic) OR (surgical procedure, laparoscopic) AND within the “Title/Abstract” and “Asian” limits. No restrictions were applied for language, country, or publication date. Moreover, lists of all relevant review articles were manually screened to identify further studies.
Studies were included if they met the following predefined criteria: (1) all patients underwent LG; (2) all patients were diagnosed by esophagogastroduodenoscopy, postoperative pathological diagnosis, endoscopic ultrasound, or computed tomography (CT); (3) all studies were included without “age” or “pathological stage” limit; and (4) the risk estimates were adjusted for other confounding factors. Meeting abstracts, systematic reviews, case reports, studies without usable or extractable data, and those solely focusing on laparoscopic total gastrectomy (LTG) were all excluded. Publications with smaller data sets were excluded while the data were presented in more than one publication.
Operative time, blood loss, and the number of retrieved lymph nodes were defined as indices of difficulties in LG. Postoperative complications, the duration of postoperative hospital stay, postoperative mortality, and time to resuming food intake were estimated as indices to assess the impact of BMI on the short-term outcomes of LG. The cutoff points to divide patients into a high BMI and a normal BMI group were based on World Health Organization definitions (overweight, BMI ≥ 25 kg/m2; healthy-weight, BMI < 25 kg/m2)[25,26]. Postoperative complications were defined as those requiring surgical or conservative treatment according to the Clavein-Dindo classification system. All of the postoperative complications observed in the included studies conform to this definition.
Data were extracted from the included studies by two independent investigators, and disagreements were resolved through consensus or consultation with a third investigator. Study characteristics such as the authors’ names, year of publication, number of participants, operative time, blood loss, number of retrieved lymph nodes, postoperative complications, duration of postoperative hospital stay, postoperative mortality, and time to resuming food intake were recorded. The Newcastle-Ottawa Scale was used to assess the quality of the included studies.
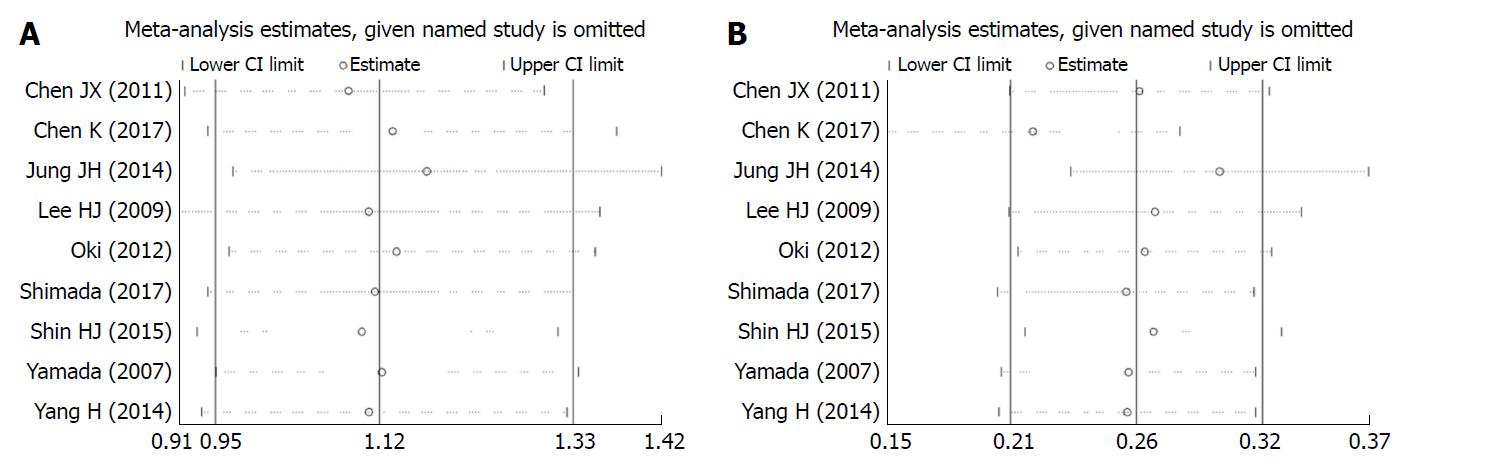
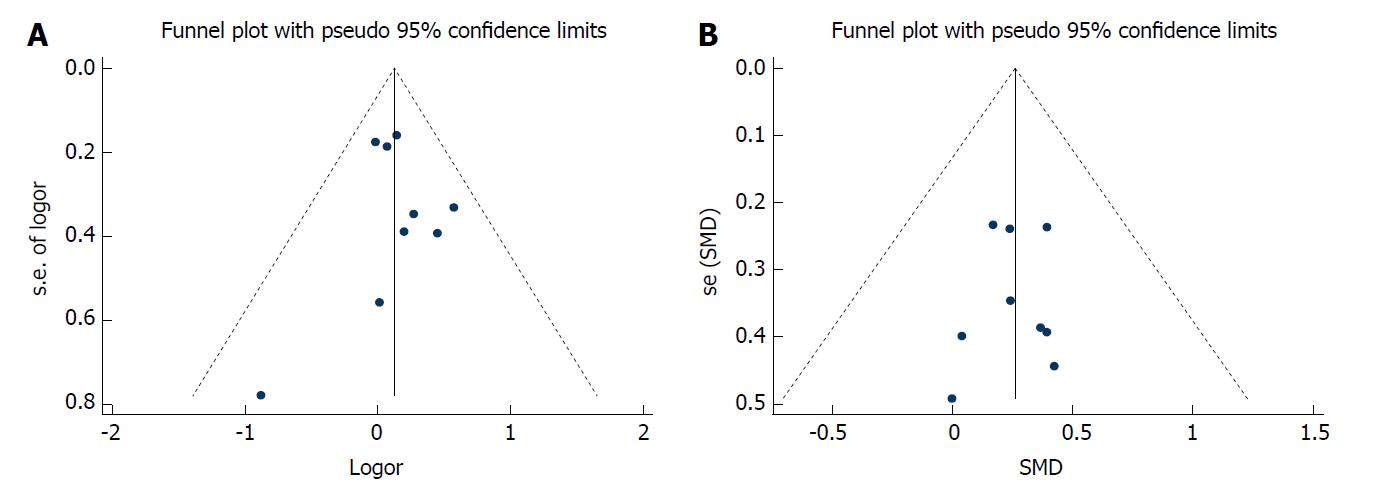
The pooled odds ratio (OR) for binary variables and standard mean difference (SMD) for continuous variables with 95% confidence interval (CI) were calculated using a fixed-effects model while heterogeneity was not apparent or a random-effects model while heterogeneity was apparent. P for I2 statistic was used to evaluate the heterogeneity in this meta-analysis, with P < 0.05 suggesting substantial heterogeneity among the included studies. The Galbraith plot test was performed to assess the potential source of heterogeneity. A sensitivity analysis was performed (when the number of included studies ≥ 9) to evaluate the stability of the results by excluding each study from the meta-analysis one by one. Publication bias was evaluated using funnel plots and the Egger’s test (when the number of included studies ≥ 9). Duval’s trim and fill method was used to solve publication bias. Statistical analyses were performed using STATA 12.1 (StataCorp, Texas, United States). A P-value < 0.05 indicated statistical significance.
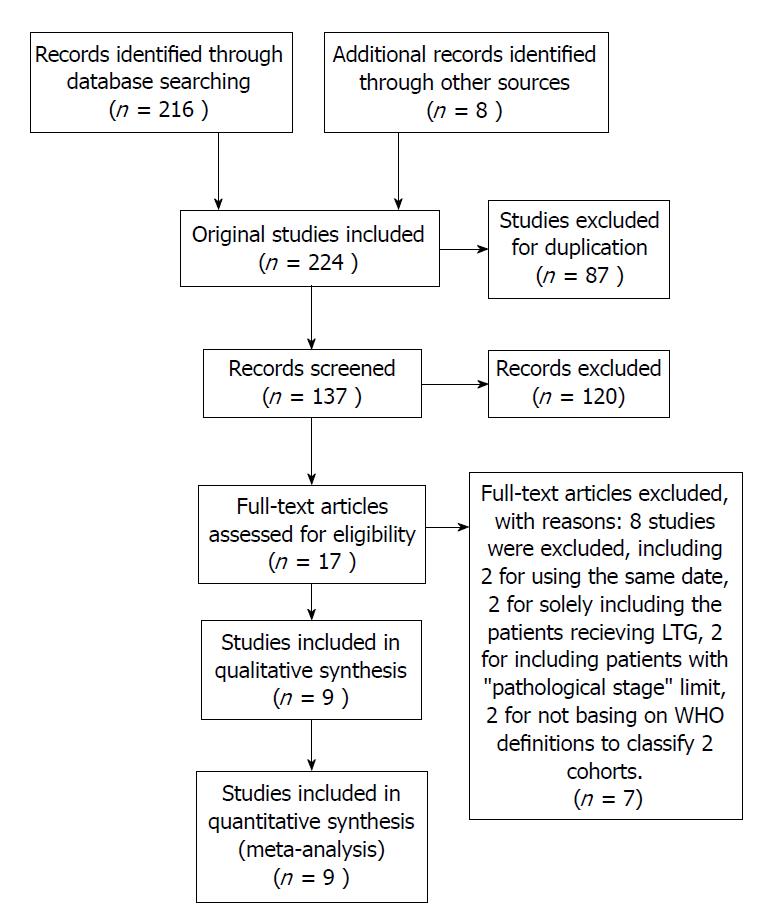
Of the 224 studies identified using the predefined search strategy, 207 were excluded after screening titles and abstracts because they did not meet the predefined criteria; they were duplicate; or their full-text could not be accessed and insufficient data to make calculations from the abstracts. After performing full-text evaluations, eight studies were excluded, including two for using the same date, two for solely including the patients receiving LTG, two for including patients with “pathological stage” limit, and two for not being based on WHO definitions to classify the two cohorts. Thus, nine studies[17-24,27] with a sample size of 6077 were included in the meta-analysis (Figure 1). The outcomes of the quality assessment are shown in Table 1. All of the included studies obtained at least eight points, meaning that they were defined as high-quality.
| Study | Country | Study type | Inclusion period | Sample size | Type of gastrectomy | BMI cutoff point | Adjustment | Quality |
| Chen et al[20] | China | RC | 2007-2010 | 531 | LG | 25 | 1, 3, 5 | 9 |
| Chen et al[17] | China | RC | 2004-2016 | 1691 | LAG TLG | 25 | 1, 2, 4, 5, 6, 7 | 8 |
| Jung et al[19] | South Korea | RC | 2006-2012 | 1512 | LDG | 25 | 1, 2, 3, 4, 6, 7 | 8 |
| Lee et al[27] | South Korea | RC | -2005 | 1485 | LAG | 25 | 1, 2, 3, 4, 5, 7 | 9 |
| Oki et al[24] | Japan | RC | 2005-2009 | 138 | TLDG | 25 | 1, 2, 4, 5, 6, 7 | 8 |
| Shimada et al[21] | Japan | RC | 2007-2014 | 173 | LADG | 25 | 1, 2, 3, 4, 5, 6, 7 | 8 |
| Shin et al[23] | South Korea | RC | 2003-2005 | 192 | LG | 25 | 1, 2, 5, 6, 7 | 8 |
| Yamada et al[22] | Japan | RC | 1999-2005 | 141 | LADG | 25 | 1, 5, 6, 7 | 9 |
| Yang et al[18] | China | RC | 2009-2012 | 214 | LAG | 25 | 1, 2, 3, 5, 6, 7 | 8 |
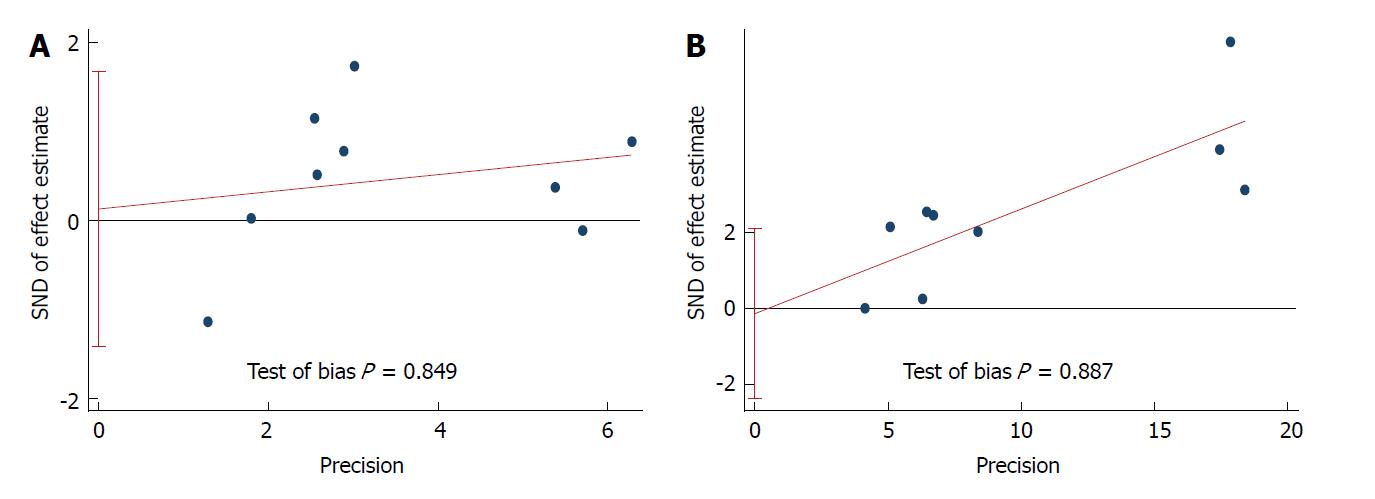
Correlation between BMI and short-term outcomes of LG: There was no significant difference between the two cohorts in overall postoperative complications (OR = 1.12, 95%CI: 0.95 to 1.33, P = 0.169; Figure 2A), various postoperative complications (Table 2), the duration of postoperative hospital stay (SMD = 0.681, 95%CI: -0.05 to 0.07, P = 0.681; Figure 2B), postoperative mortality (OR = 1.95, 95%CI: 0.78 to 4.89, P = 0.153; Figure 2C), or time to resuming food intake (SMD = 0.00, 95%CI: -0.06 to 0.06, P = 0.973; Figure 2D). Heterogeneity was not apparent in any of these outcome results according to the fixed-effects model. Sensitivity analysis demonstrated that no study could affect the pooled OR for postoperative complications (Figure 3A). Visual assessments of the funnel plots (Figure 4A) and the Egger’s test (Figure 5A) showed no evidence of publication bias for postoperative complications (P = 0.849).
| Complication variable | No. of studies | No. of pooled patients | Pooled OR | 95%CI | Test of heterogeneity | Test of overall effect |
| P value | P value | |||||
| Overall complications | 9 | 6077 | 1.12 | 0.95-1.33 | 0.734 | 0.169 |
| Anastomotic leakage | 6 | 3939 | 1.31 | 0.62-2.79 | 0.642 | 0.476 |
| Anastomotic stricture | 4 | 3587 | 0.85 | 0.27-2.66 | 0.489 | 0.775 |
| Anastomotic bleeding | 5 | 3798 | 0.63 | 0.27-1.45 | 0.942 | 0.277 |
| Abdominal abscess | 5 | 3801 | 1.56 | 0.91-2.67 | 0.615 | 0.103 |
| Pancreatic leakage | 6 | 3937 | 0.52 | 0.20-1.35 | 0.581 | 0.179 |
| Ileus | 4 | 3584 | 1.96 | 0.79-4.83 | 0.382 | 0.144 |
| Wound | 6 | 3939 | 1.77 | 0.92-3.42 | 0.289 | 0.087 |
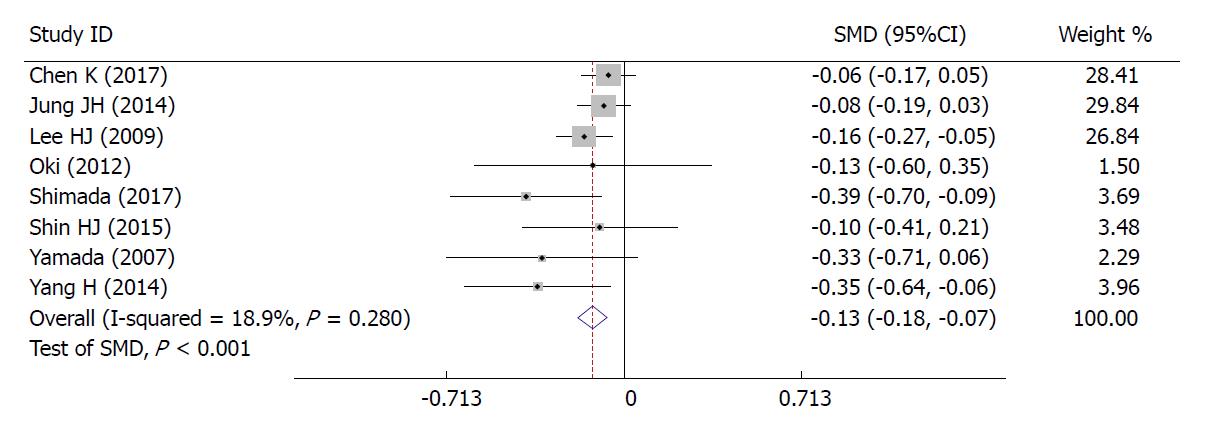
Correlation between BMI and difficulties in LG: The high BMI group had longer operative time (SMD = 0.26, 95%CI: 0.21 to 0.32, P < 0.001; Figure 2E), greater blood loss (SMD = 0.19, 95%CI: 0.12 to 0.25, P < 0.001; Figure 2F), and fewer retrieved lymph nodes (SMD = -0.13, 95%CI: 0.18 to 0.07, P < 0.001; Figure 6). Heterogeneity was not apparent in any of these outcome results according to the fixed-effects model. Sensitivity analysis demonstrated that no study could affect the pooled SMD for the operative time (Figure 3B). Visual assessment of the funnel plots (Figure 4B) and the Egger’s test (Figure 5B) showed no evidence of publication bias for the operative time (P = 0.887).
Obesity is traditionally considered a challenge for many surgeons who perform abdominal operations[28-30]. Until now, the impact of obesity on the short-term outcomes of LG for GC has been controversial[29,31]. This meta-analysis, including nine retrospective cohorts, aimed to investigate the correlation between obesity and the short-term outcomes of LG. Patients included were divided into a high BMI (≥ 25 kg/m2) and a normal BMI (< 25 kg/m2) group based on the World Health Organization definition of obesity. Splenic hilar lymph node dissection is necessary for LTG, but it is difficult to expose the deep location of the splenic hilum and complicated vessels. LTG has been regarded as a risk factor for short-term outcomes of LG for GC[17]; therefore, studies solely focusing on LTG were excluded. Additionally, owing to the insufficient representativeness of the sample in the study with “pathological stage” limit, we excluded two studies with this limit (one for solely including patients with early stage GC and the other for solely including patients with advanced GC).
Operative time, blood loss, and the number of retrieved lymph nodes were defined as indices of difficulties in LG. Our study has clearly demonstrated that the correlation between BMI and operative difficulties was significant. Patients undergoing LG with a high BMI have longer operative time (SMD = 0.26, 95%CI: 0.21 to 0.32, P < 0.001), greater blood loss (SMD = 0.19, 95%CI: 0.12 to 0.25, P < 0.001), and fewer retrieved lymph nodes (SMD = -0.13, 95%CI: 0.18 to 0.07, P < 0.001). Likely due to hindered exposure to the stomach and pancreas, LG performed in patients with obesity is more technically demanding. The thickened mesentery, omentum, and ligamentum are very common in obese patients under certain circumstances and may lead to difficulties in the operative procedure, especially in ligation, dissection, or anatomy of the vessels and lymph nodes. Fatty stomach and omentum can also result in severe challenges owing to the stomach pulled by the surgeon. Blood bleed during the operation, caused by excessive incrassate and fat mesenteries, is hard to stop in this narrow area surrounded by adipose tissue. It can be inferred that sensation recovery from anesthesia would be delayed in virtue of distribution of anesthetic agents affected by increased technical difficulties during exposure of an adequate operative field. Each of these potential factors contributes to longer surgical time and increased blood loss[19]. As to the lower number of retrieved lymph nodes, it may be conferred that dissection of lymph nodes for patients with obesity could be limited by excess adipose tissue and structures. Obtaining lymph nodes from a mass of fat tissue could be more difficult than normal tissue and may be regarded as the other contributing factor to fewer retrieved lymph nodes; however, in all studies, the number of retrieved lymph nodes in the obese group was greater than the standard (> 15) recommended by the National Comprehensive Cancer Network (NCCN) guidelines, which suggests that LG can also ensure the curative effect in obese patients with GC.
Postoperative complications, the duration of postoperative hospital stay, postoperative mortality, and time to resuming food intake were estimated as the impact of BMI on the short-term outcomes of LG. Our meta-analysis provided strong evidence that the correlation between BMI and postoperative short-term outcomes was not significant. In addition, anastomotic leakage, anastomotic stricture, anastomotic bleeding, abdominal abscess, pancreatic leakage, ileus, and wound infection would also be included in subgroup analysis. The results showed that BMI is not significantly associated with either overall postoperative complications or a particular one. The most critical factor contributing to this condition would be anastomosis. The excessive torsion of remnant stomach and duodenum could be avoided during the procedure of anastomosis performed by an experienced surgeon, especially in totally laparoscopic gastrectomy (TLG) which is becoming prevalent in Asian areas[25]. In addition, the lower number of retrieved lymph nodes resulting from increased surgical difficulties also indicated that surgeons would be more careful and cautious during the operative procedure, and the injury of tissues and structures would be avoided to some extent. High-quality pre- and postoperative management could also help surgeons improve the patients’ overall conditions and identify minor problems through observed indices on time, such as the amount of intro-abdominal bile drainage and the wound healing condition. Milder procedures and smaller wounds as well as careful pre- and postoperative management result in lower rates of postoperative complications[21]. Furthermore, to an extent, the results suggest that the assessment of obesity based solely on BMI may be insufficient. The distribution and amount of abdominal fat in an individual patient may not be accurately reflected by BMI, since it is a simple calculation based on weight and height[23]. For instance, patients with a large amount of subcutaneous fat may have normal amounts of visceral and intra-abdominal fat that would not alter the difficulties and short-term outcomes of LG. Recent studies confer that visceral fat area is a more accurate predictor of intro- and postoperative outcomes than high BMI in obese patients due to its feasibility to evaluate the distribution of intra-abdominal fat[32-34].
Although we have searched recent publications using a rigorous search strategy, there are still some limitations to this meta-analysis. First, although all of the included studies obtained at least eight points, almost all of them were retrospective cohort studies. Although the randomized clinical trials (RCTs) are the gold standard for study design, it is hardly feasible to allocate patients with different BMIs randomly. Second, nine studies were included with a total sample size of 6077, which is relatively small. A larger sample size is needed to support the evidence. Third, as patients in Western countries have a higher BMI compared to Asian patients, our results should be considered carefully when being applied to other races, and more relevant studies should be performed worldwide.
In conclusion, our meta-analysis clearly supports that although high BMI in Asian patients with GC could increase the difficulties in LG with regard to operative time, blood loss, and the number of retrieved lymph nodes, there was no significant association between BMI and postoperative short-term outcomes, including postoperative complications, the duration of the postoperative hospital stay, postoperative mortality, and time to resuming food intake in Asian patients. It strongly demonstrates that a high BMI may not be a risk factor for short-term outcomes of patients undergoing LG if performed by an experienced surgeon with careful pre- and post-operative management, in contrast with the perspectives reported by previous studies. It may not be enough to estimate difficulties and postoperative outcomes in patients with GC undergoing LG using BMI as the only index to assess obesity. Other indices, for instance, VSA, should be taken into account.
Gastric cancer (GC) is the second most prevalent cause of cancer-related deaths worldwide. Since 1994, laparoscopic gastrectomy (LG) has become increasingly popular for treating early GC in patients. The prevalence of obesity is increasing steadily in Asian countries. Obesity is regarded as a risk factor for worse surgical outcomes of complicated surgical procedures. Furthermore, patients with obesity have a higher risk of operative difficulties, as well as wound infection.
Recently, the impact of obesity on the short-term LG in patients has been controversial due to several studies. For instance, some studies reported that the association between obesity and LG was significant, while others reported the opposite conclusion.
To date, although several studies evaluating the body mass index (BMI) as an index to assess obesity and short-term outcomes of LG, the results have been controversial and limited. Hence, we conducted this meta-analysis to summarize all of the available evidence.
The PubMed, Cochrane, EMBASE, and Web of Science databases were searched for studies that focused on the impact of obesity on the short-term outcomes of LG for GC in Asian patients who were classified into a high BMI (BMI ≥ 25 kg/m2) or low BMI group (BMI < 25 kg/m2). The results are expressed using the pooled odds ratio (OR) for binary variables and standard mean difference (SMD) for continuous variables with 95% confidence interval (CI), and were calculated according to the fixed-effects model while heterogeneity was not apparent or a random-effects model while heterogeneity was apparent.
Nine studies, with a total sample size of 6077, were included in this meta-analysis. Compared with the low BMI group, the high BMI group had longer operative time (SMD = 0.26, 95%CI: 0.21 to 0.32, P < 0.001), greater blood loss (SMD = 0.19, 95%CI: 0.12 to 0.25, P < 0.001), and fewer retrieved lymph nodes (SMD = -0.13, 95%CI: 0.18 to 0.07, P < 0.001). There was no significant difference between the high and low BMI groups in postoperative complications (OR = 1.12, 95%CI: 0.95 to 1.33, P = 0.169), the duration of postoperative hospital stay (SMD = 0.681, 95%CI: -0.05 to 0.07, P = 0.681), postoperative mortality (OR = 1.95, 95%CI: 0.78 to 4.89, P = 0.153), or time to resuming food intake (SMD = 0.00, 95%CI: -0.06 to 0.06, P = 0.973).
Our meta-analysis provides strong evidence that despite the longer operative time, greater blood loss, and fewer retrieved lymph nodes, the association between BMI and the short-term outcomes of laparoscopic gastrectomy for GC, including postoperative complications, the duration of postoperative hospital stay, postoperative mortality, and time to resuming food intake was not significant. BMI could be a poor risk factor for short-term outcomes of LG. Other indices should be taken into account.
We are grateful to Professor Xu, Jin-Fu Zhuang, Jing-Zhou Wang, Yi-Ling Lin, Chun-Lin lin, Hai-Tao Yang, Tian Zou, and Ying Wang for their useful advice, guidance, and encouragement. In addition, Heng-Kai Chen especially wishes to thank his families and Dan Lin, his wife, for giving him complete spiritual support over the past years.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kocazeybek B, Musella M S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Bian YN
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25540] [Article Influence: 1824.3] [Reference Citation Analysis (7)] |
| 2. | Adachi Y, Shiraishi N, Shiromizu A, Bandoh T, Aramaki M, Kitano S. Laparoscopy-assisted Billroth I gastrectomy compared with conventional open gastrectomy. Arch Surg. 2000;135:806-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 288] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 3. | Lin JX, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Jun L, Chen QY, Lin M, Tu R. Evaluation of laparoscopic total gastrectomy for advanced gastric cancer: results of a comparison with laparoscopic distal gastrectomy. Surg Endosc. 2016;30:1988-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Lee HJ, Yang HK, Ahn YO. Gastric cancer in Korea. Gastric Cancer. 2002;5:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Kim YW, Bae JM, Lee JH, Ryu KW, Choi IJ, Kim CG, Lee JS, Rho JY. The role of hand-assisted laparoscopic distal gastrectomy for distal gastric cancer. Surg Endosc. 2005;19:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Lee JH, Ryu KW, Doh YW, Bae JS, Kim YW, Bae JM. Liver lift: A simple suture technique for liver retraction during laparoscopic gastric surgery. J Surg Oncol. 2007;95:83-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Hayashi H, Ochiai T, Shimada H, Gunji Y. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc. 2005;19:1172-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 259] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 8. | Kim MC, Kim KH, Kim HH, Jung GJ. Comparison of laparoscopy-assisted by conventional open distal gastrectomy and extraperigastric lymph node dissection in early gastric cancer. J Surg Oncol. 2005;91:90-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Mochiki E, Nakabayashi T, Kamimura H, Haga N, Asao T, Kuwano H. Gastrointestinal recovery and outcome after laparoscopy-assisted versus conventional open distal gastrectomy for early gastric cancer. World J Surg. 2002;26:1145-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Noshiro H, Nagai E, Shimizu S, Uchiyama A, Tanaka M. Laparoscopically assisted distal gastrectomy with standard radical lymph node dissection for gastric cancer. Surg Endosc. 2005;19:1592-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Pereira MA. Diet beverages and the risk of obesity, diabetes, and cardiovascular disease: a review of the evidence. Nutr Rev. 2013;71:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | National Task Force on the Prevention and Treatment of Obesity. Overweight, obesity, and health risk. Arch Intern Med. 2000;160:898-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 718] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 13. | Lee JH, Paik YH, Lee JS, Ryu KW, Kim CG, Park SR, Kim YW, Kook MC, Nam BH, Bae JM. Abdominal shape of gastric cancer patients influences short-term surgical outcomes. Ann Surg Oncol. 2007;14:1288-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Aronne LJ, Brown WV, Isoldi KK. Cardiovascular disease in obesity: A review of related risk factors and risk-reduction strategies. J Clin Lipidol. 2007;1:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Mozaffarian D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation. 2016;133:187-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1337] [Cited by in RCA: 1472] [Article Influence: 163.6] [Reference Citation Analysis (0)] |
| 16. | Nepogodiev D, Chapman SJ, Glasbey J, Kelly M, Khatri C, Drake TM, Kong CY, Mitchell H, Harrison EM, Fitzgerald JE. Determining Surgical Complications in the Overweight (DISCOVER): a multicentre observational cohort study to evaluate the role of obesity as a risk factor for postoperative complications in general surgery. BMJ Open. 2015;5:e008811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Chen K, Pan Y, Zhai ST, Cai JQ, Chen QL, Chen DW, Zhu YP, Zhang Y, Zhang YP, Maher H. Laparoscopic gastrectomy in obese gastric cancer patients: a comparative study with non-obese patients and evaluation of difference in laparoscopic methods. BMC Gastroenterol. 2017;17:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Yang H, Xing J, Cui M, Zhang C, Yao Z, Zhang N, Su X. [Efficacy evaluation of laparoscopy-assisted radical gastrectomy in obese patients with gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2014;17:776-780. [PubMed] |
| 19. | Jung JH, Ryu SY, Jung MR, Park YK, Jeong O. Laparoscopic distal gastrectomy for gastric cancer in morbidly obese patients in South Korea. J Gastric Cancer. 2014;14:187-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Chen JX, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Lin JX. [Impact of obesity on laparoscopic-assisted radical gastrectomy for gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2011;14:781-784. [PubMed] |
| 21. | Shimada S, Sawada N, Ishiyama Y, Nakahara K, Maeda C, Mukai S, Hidaka E, Ishida F, Kudo SE. Impact of obesity on short- and long-term outcomes of laparoscopy assisted distal gastrectomy for gastric cancer. Surg Endosc. 2018;32:358-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Yamada H, Kojima K, Inokuchi M, Kawano T, Sugihara K. Effect of obesity on technical feasibility and postoperative outcomes of laparoscopy-assisted distal gastrectomy--comparison with open distal gastrectomy. J Gastrointest Surg. 2008;12:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Shin HJ, Son SY, Cui LH, Byun C, Hur H, Lee JH, Kim YC, Han SU, Cho YK. Is There any Role of Visceral Fat Area for Predicting Difficulty of Laparoscopic Gastrectomy for Gastric Cancer? J Gastric Cancer. 2015;15:151-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Oki E, Sakaguchi Y, Ohgaki K, Saeki H, Chinen Y, Minami K, Sakamoto Y, Toh Y, Kusumoto T, Okamura T. The impact of obesity on the use of a totally laparoscopic distal gastrectomy in patients with gastric cancer. J Gastric Cancer. 2012;12:108-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1-452. [PubMed] |
| 26. | Seidell JC, Flegal KM. Assessing obesity: classification and epidemiology. Br Med Bull. 1997;53:238-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 273] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Lee HJ, Kim HH, Kim MC, Ryu SY, Kim W, Song KY, Cho GS, Han SU, Hyung WJ, Ryu SW; Korean Laparoscopic Gastrointestinal Surgery Study Group. The impact of a high body mass index on laparoscopy assisted gastrectomy for gastric cancer. Surg Endosc. 2009;23:2473-2479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Dhar DK, Kubota H, Tachibana M, Kotoh T, Tabara H, Masunaga R, Kohno H, Nagasue N. Body mass index determines the success of lymph node dissection and predicts the outcome of gastric carcinoma patients. Oncology. 2000;59:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 123] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Inagawa S, Adachi S, Oda T, Kawamoto T, Koike N, Fukao K. Effect of fat volume on postoperative complications and survival rate after D2 dissection for gastric cancer. Gastric Cancer. 2000;3:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Tsujinaka T, Sasako M, Yamamoto S, Sano T, Kurokawa Y, Nashimoto A, Kurita A, Katai H, Shimizu T, Furukawa H. Influence of overweight on surgical complications for gastric cancer: results from a randomized control trial comparing D2 and extended para-aortic D3 lymphadenectomy (JCOG9501). Ann Surg Oncol. 2007;14:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Bickenbach KA, Denton B, Gonen M, Brennan MF, Coit DG, Strong VE. Impact of obesity on perioperative complications and long-term survival of patients with gastric cancer. Ann Surg Oncol. 2013;20:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Kunisaki C, Makino H, Takagawa R, Sato K, Kawamata M, Kanazawa A, Yamamoto N, Nagano Y, Fujii S, Ono HA. Predictive factors for surgical complications of laparoscopy-assisted distal gastrectomy for gastric cancer. Surg Endosc. 2009;23:2085-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Hiki N, Fukunaga T, Yamaguchi T, Ogura T, Miyata S, Tokunaga M, Ohyama S, Sano T. Increased fat content and body shape have little effect on the accuracy of lymph node retrieval and blood loss in laparoscopic distal gastrectomy for gastric cancer. J Gastrointest Surg. 2009;13:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Ueda J, Ichimiya H, Okido M, Kato M. The impact of visceral fat accumulation on laparoscopy-assisted distal gastrectomy for early gastric cancer. J Laparoendosc Adv Surg Tech A. 2009;19:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |