Published online Dec 6, 2018. doi: 10.12998/wjcc.v6.i15.1059
Peer-review started: July 12, 2018
First decision: October 11, 2018
Revised: November 21, 2018
Accepted: November 23, 2018
Article in press: November 24, 2018
Published online: December 6, 2018
Processing time: 148 Days and 8 Hours
Suprachoroidal hemorrhage (SCH) is a rare but potentially catastrophic ocular event. Surgery for SCH is often challenging because of the difficulty in resolving the retinal and choroidal detachment. Here, we describe a novel surgical technique in which urokinase is administered by sub-Tenon’s injection to target an organized clot in SCH prior to drainage.
A consecutive case series of four eyes with serous and hemorrhagic choroidal detachments secondary to cataract surgery or trauma was documented to evaluate the feasibility of using a sub-Tenon’s urokinase injection-assisted 23-gauge and 20-gauge incision to drain choroidal detachments. Urokinase (2000 IU) was given by sub-Tenon’s injection one day before surgery for clot liquefaction. A 23-gauge infusion line was placed in the anterior chamber. A 20-gauge incision was created in the suprachoroidal space 3.5 mm from the limbus. After drainage, pars plana vitrectomy was performed because of concomitant pathology that demanded this additional procedure. Visual acuity, ocular findings, the timing of surgical interventions, surgical procedures, and outcomes were retrospectively reviewed in four patients. Postoperative follow-up of the patients ranged from 6 to 24 mo (mean, 13 mo). After the treatment, all patients achieved excellent anatomical recovery.
Sub-Tenon’s urokinase injection-assisted vitrectomy makes clot liquefaction happen in the early treatment stage, resulting in marked stability during the procedure.
Core tip: We report a consecutive case series of four eyes with serous and hemorrhagic choroidal detachments secondary to cataract surgery or trauma to evaluate the feasibility of using a sub-Tenon’s urokinase injection-assisted 23-gauge and 20-gauge incision to drain choroidal detachments. The primary advantage of this technique is that it makes clot liquefaction happen in the early treatment stage and allows a slower and semiautomated controlled mechanism to be achieved, resulting in marked stability during the procedure.
- Citation: Chai F, Ai H, Deng J, Zhao XQ. Sub-Tenon’s urokinase injection-assisted vitrectomy in early treatment of suprachoroidal hemorrhage: Four cases report. World J Clin Cases 2018; 6(15): 1059-1066
- URL: https://www.wjgnet.com/2307-8960/full/v6/i15/1059.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i15.1059
Suprachoroidal hemorrhage (SCH) is a vision-threatening complication associated with ocular trauma or certain surgical procedures, such as cataract extraction, glaucoma filtering surgery, penetrating keratoplasty, and vitreoretinal surgery[1-5]. It has also been reported to occur spontaneously[6-8]. Sudden reductions in intraoperative and postoperative intraocular pressure (IOP) and/or sustained low IOP due to various causes are important causes of fulminant SCH. Risk factors such as advanced age, hypertension, arteriosclerosis, diabetes and hemorrhagic disorders, glaucoma, and high myopia are all predisposing factors for SCH[9-13]. Although Verhoeff reported treatment by scleral incision in 1915, the effect was poor, and eventually many patients needed eye-excavation. The advance of vitreous surgery in recent years, especially the application of heavy water and silicone oil, has made the treatment of SCH possible. Through eyeball reconstruction, it not only effectively reduces the chance of eyeball atrophy, but even retains certain visual functions. However, what treatment is most appropriate for choroidal detachment and SCH remains largely controversial[14-16].
To improve therapeutic effects, we developed a novel method of sub-Tenon’s urokinase injection-assisted vitrectomy drainage for serous and hemorrhagic choroidal detachments. We sought to prospectively evaluate this method for safety and efficacy in patients treated for massive SCH and choroidal detachment. In this study, we describe a novel surgical technique in which urokinase is administered sub-Tenon’s to target an organized clot in SCH prior to drainage. After drainage, pars plana vitrectomy (PPV) was performed because concomitant pathology demanded this additional procedure.
All cases who underwent sub-Tenon’s urokinase injection-assisted vitrectomy in our institution between April 2016 and December 2017 were collected. The surgical treatments were performed by the same surgeon (Dr. Xi-Quan Zhao) to minimize bias due to different procedures and levels of experience. Informed consent was obtained from all individual participants included in the study. All patients are female, and the other details of the patient characteristics are given in Table 1.
| Patient No. | Age (yr) | Chief complaints | History of present illness | History of past illness | Physical examination | Laboratory testing |
| 1 | 73 | Vision loss for 10 d (L) | Phaco 10 d before | Glucoma for more than 30 yr | VA: LP | (-) |
| IOP: 6.7 mmHg | ||||||
| Aphakia | ||||||
| Retinal detachment | ||||||
| 2 | 56 | Vision loss for 5 d (L) | ECCE 5 d before | Hypertension | VA: HM | (-) |
| IOP: 9.6 mmHg | ||||||
| Aphakia | ||||||
| Iridocoloboma | ||||||
| Aphakia | ||||||
| Vitreous hemorrhage | ||||||
| 3 | 61 | Vision loss for 7 d (R) | Phaco 8 d before | High myopia | VA: HM | (-) |
| IOP: 9.2 mmHg | ||||||
| Vitreous incarceration | ||||||
| Aphakia | ||||||
| Choroidal detachment | ||||||
| 4 | 52 | Vision loss for 12 d (R) | Trauma 12 d before, and the wound was sutured | Trauma | VA: LP | (-) |
| IOP: 6.7 mmHg | ||||||
| Hyphema | ||||||
| Vitreous incarceration | ||||||
| Aphakia | ||||||
| Vitreous hemorrhage |
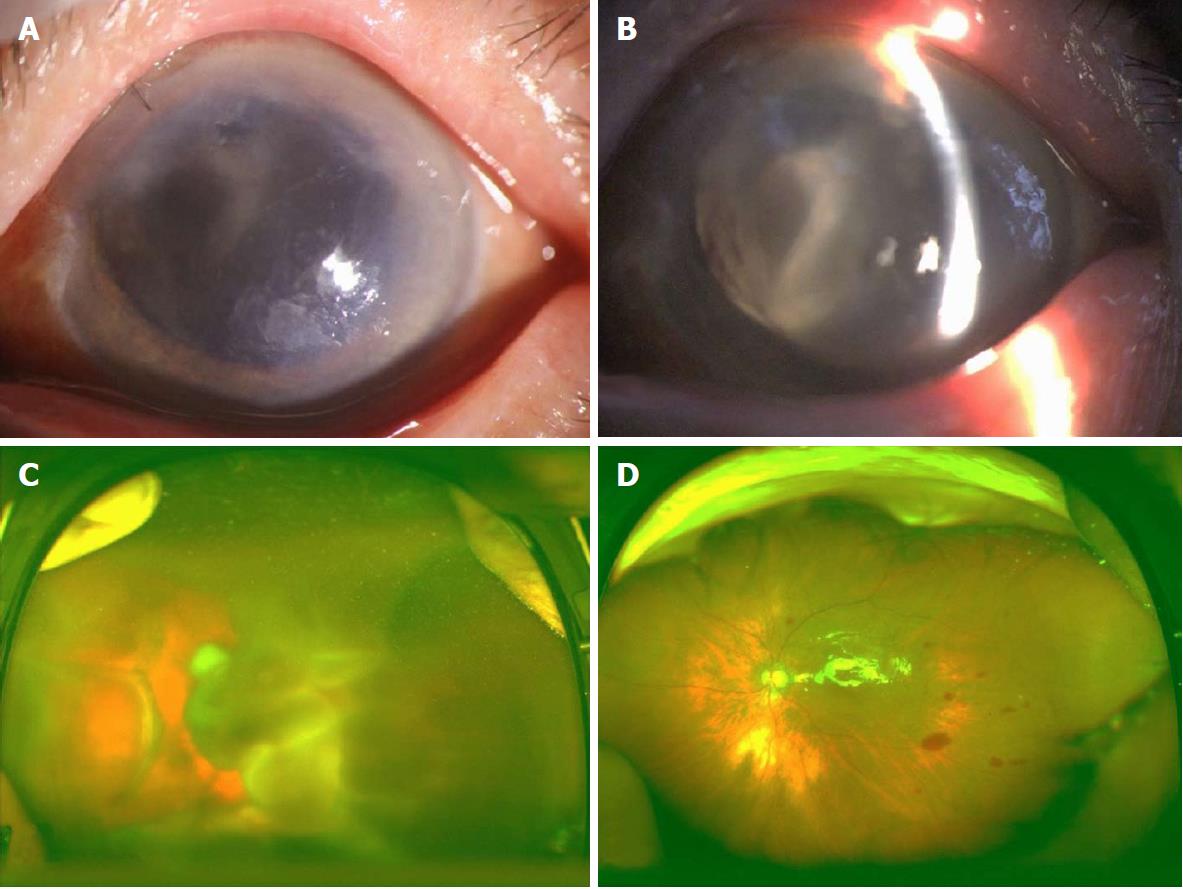
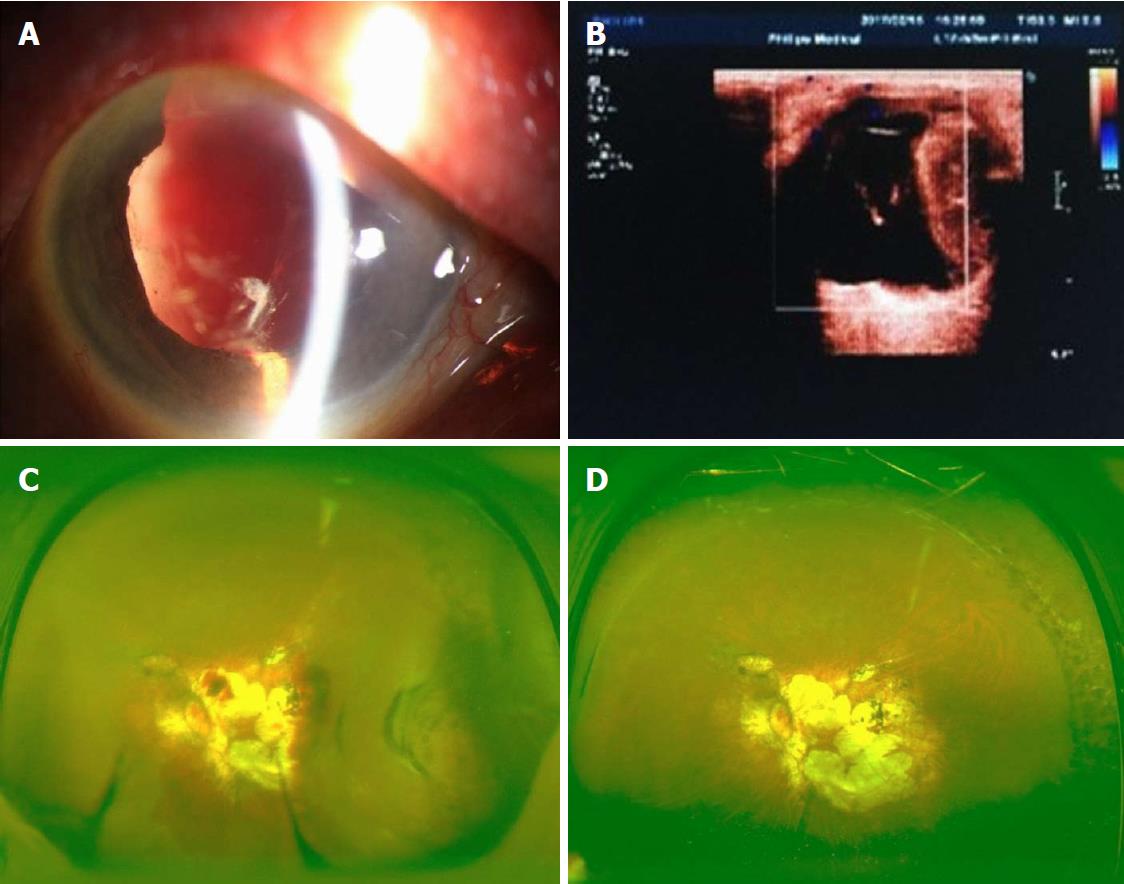
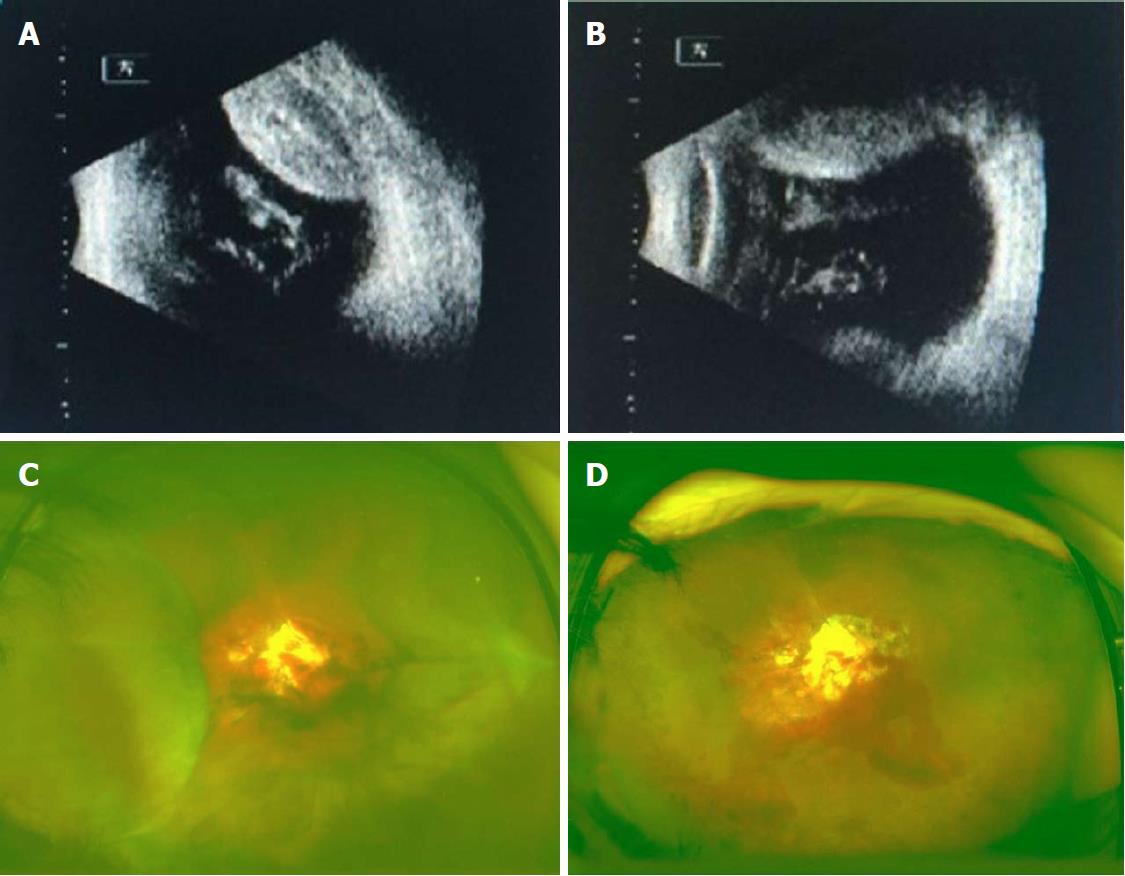
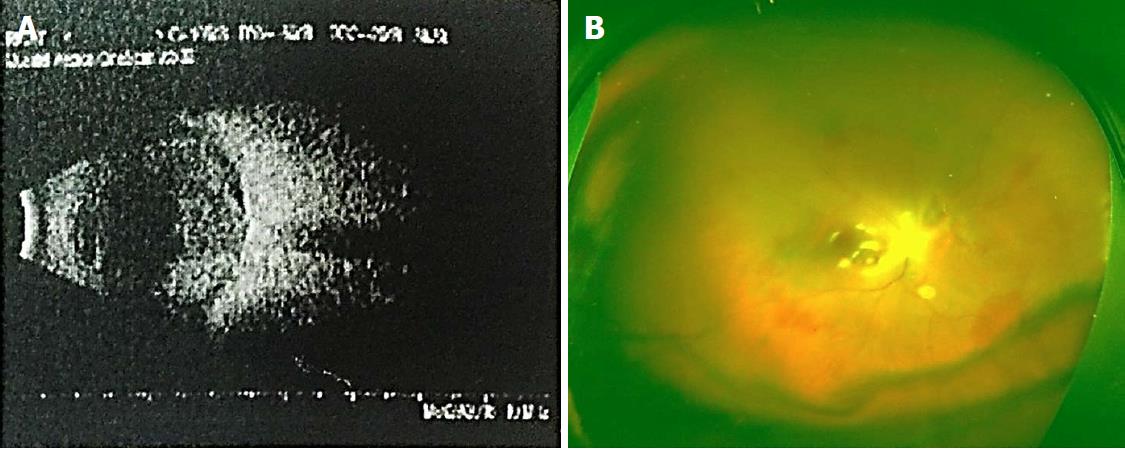
Details of the physical examination and imaging examination are given in Figures 1-4.
With the clear history, eye examination, and ultrasound findings, all the four patients in this group were diagnosed with SCH.
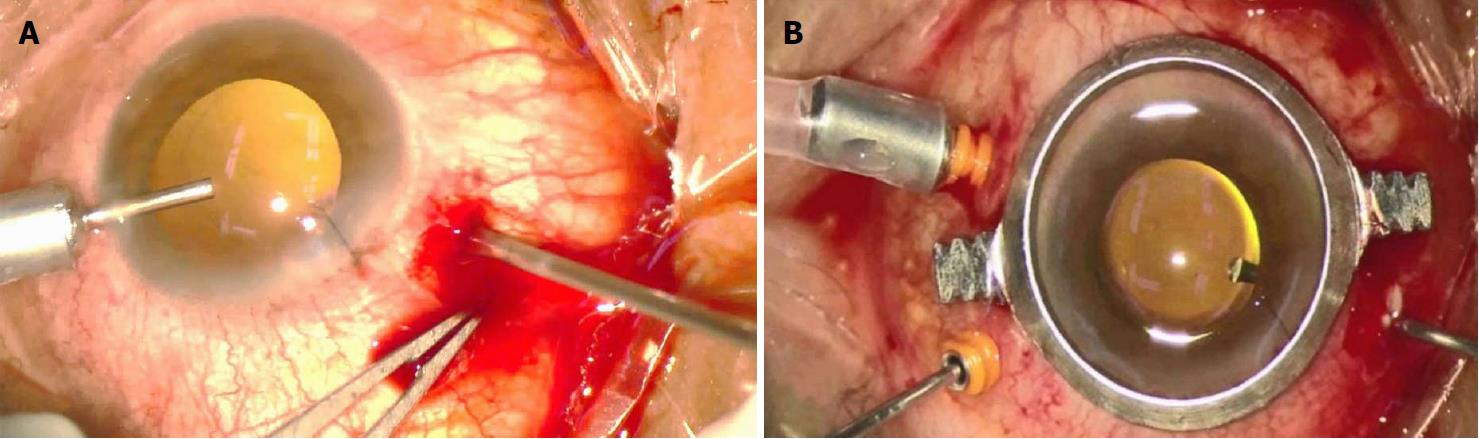
The urokinase was supplied fresh in ampoules as a lyophilized powder. A 10000-unit urokinase solution was made by mixing the powder with 1 mL of sterile saline. A sub-Tenon’s injection was performed for clot liquefaction with 0.2 mL of urokinase solution and 0.05 mL of 2% lidocaine. The next day, PPV was performed using 20G and 23G vitrectomy cannulas that were placed 3.5 mm from the limbus (Figure 5A). The 20G cannula was left open, and the infusion line was placed in the anterior chamber through a clear corneal paracentesis with a bottle height of 40 mmHg. As soon as the infusion line was opened, a copious, thick flux of blood flowed out of the 20G cannula. As the blood flow continued, the choroidal detachment visibly regressed. Vitrectomy, fibrovascular membrane peeling, and liquid gas exchange combined with silicone oil tamponade were performed later. During infusion and vitrectomy, the sclerotomies remained functional and permitted continuous blood flow out of the suprachoroidal space (Figure 5B). The drainage during surgery went smoothly and resulted in excellent final anatomical results. We performed PPV and tamponade with silicone oil instillation in three cases and no tamponade in one case (Table 2).
| Patient No. | 1 | 2 | 3 | 4 |
| Lens status at the time of intervention | Aphakia | Aphakia | Aphakia | Aphakia |
| Preoperative findings | Retinal detachment | Vitreous hemorrhage | Vitreous incarceration, subretinal hemorrhage | Hyphema, vitreous hemorrhage |
| Drainage during surgery | Good drainage of blood | Partial drainage of blood | Good drainage of blood | Good drainage of blood |
| Instillation of PFCL | No | No | No | No |
| Tamponade | Silicone oil | Silicone oil | None | Silicone oil |
| Anatomic success | Retinal and choroidal reattachment | Choroidal reattachment | Choroidal reattachment | Choroidal reattachment |
Two cases of SCH were completely discharged during operation, and two cases of hemorrhage were absorbed in 7-12 mo after operation. The patients were followed for 6-24 mo, and excellent final anatomical results were achieved in all four cases. Preoperative visual acuity (VA) was light perception in two eyes and hand motion in two eyes. At final presentation, VA improved in two cases and remained the same in case 4, whereas in case 1, light perception was lost (Table 3). In this group, two patients had low IOP (average IOP, 5.9 mmHg), and the IOP was normal after 3 mo of follow-up. The silicone oil tamponade was removed in case 1 and case 3 at 2 mo postoperatively, at which time the IOP was increased while the retina was in place. Proliferative vitreoretinopathy occurred 3 mo postoperatively in case 4, and vitrectomy combined with intraoperative injection of silicone oil was performed. The retina was attached and no complications occurred.
| Patient No. | Prior to SCD | 1 mo follow-up | Last follow-up (mo) |
| 1 | LP | NLP | NLP (7) |
| 2 | HM | CF | 20/1000 (14) |
| 3 | HM | CF | 20/1000 (6) |
| 4 | LP | LP | LP (24) |
Although the incidence of SCH is very low, sudden bleeding can force the eye content to escape from the open wound, and the blood can seep into the subretinal, vitreous, and anterior chambers. In the later stage, intraocular blood mechanization causes retinal and ciliary body detachment, which can cause complete loss of vision and even atrophy of the eyeball[1-5]. In our study, the patients had one to three systemic or ocular risk factors for developing SCH, including hypertension, glaucoma, and aphakia, which are consistent with other studies[10-13,17]. For such patients, the systemic condition should be actively improved before surgery, such as controlling blood pressure and blood sugar, improving cardiopulmonary function, stopping oral anticoagulant drugs, etc. It may be safer to perform surgery after the general condition is improved.
In the event of SCH during surgery, the incision should be quickly closed to control IOP. Local or systemic application of corticosteroids to reduce intraocular inflammation, and the use of carbonic anhydrase inhibitors, sedatives, etc., can be applied according to systemic conditions. Surgical treatment should be taken in the cases with a large amount of bleeding in the suprachoroidal space, especially the generation of kiss signs, difficult to control high IOP, persistent pain, and patients with other vitreoretinal complications (such as a large amount of vitreous hemorrhage, retinal detachment, or retinal incarceration)[18,19].
The timing of surgery is important when performing a sclerectomy. Some authors believe that the time of liquefaction is 7-14 d after bleeding. If the operation time is too early, the blood is not fully liquefied, and drainage is difficult. If the delay is too long, the blood clot will cause retinal proliferation, and the success rate of surgery is low. Therefore, if the blood clot can liquefy earlier, the success rate of operation and surgical effect may be better. Some authors have also used suprachoroidal cavity injection of tissue plasminogen activator 4-5 d after SCH to liquefy the clot[20-22]. Suprachoroidal cavity injection is an intraocular operation which can be performed in some complications, such as retinal detachment, vitreoretinal traction, and vitreous hemorrhage. However, sub-Tennon’s injection is much more easy and safe to perform. Urokinase catalyzes the cleavage of plasminogen to plasmin and may degrade fibrin clots by thrombolysis, producing rapid and positive results. It has a short half-life of approximately 16 min, improves vascular adenosine diphosphate (ADP) activity, inhibits ADP-induced platelet aggregation, and prevents thrombosis. Urokinase has been reported in the successful treatment of various vitreoretinal diseases, including traumatic hyphema[23], vitreous hemorrhage[17], branch retinal artery occlusion[24], and central retinal artery occlusion[25]. At the same time, urokinase is a common clinical drug, and the price is appropriate, which is more suitable for clinical treatment in developing countries.
We took advantage of 20G and 23G vitrectomy cannulas to ensure sclerotomies of known and reliable diameter and consistent patency throughout all surgical maneuvers. The cannulas also allow the very quick, safe, and easy closure of the sclerotomy when needed, and this method has also been described in other studies that used 23G or 25G cannulas[15,16]. The advantage of vitrectomy for SCH is that its closed surgical system maintains a stable IOP. Surgery can remove the incarcerated anterior vitreous body and relieve the pulling action, thereby reducing the vitreous hemorrhage. When the vitreous body is removed, the perfusate is injected into the eyeball, which forces the SCH to be further discharged through the scleral incision. We performed PPV and tamponade with silicone oil instillation in three cases and no tamponade in one case. A silicone oil tamponade has been shown provide advantages over a balanced salt solution or gas filling because it protects against choroidal re-bleeding and prevents the development of chronic hypotony[3,26]. While none of our patients developed postoperative hypotony, other studies have reported a frequency for hypotony ranging from 24% to 71%[1,13,27]. The reduction in aqueous humor production followed by low IOP may be the cause of rebleeding. The injection of silicone oil prevents this from happening and avoids reoperation. Silicone oil can be removed if the patient’s vision is well recovered or silicone oil-related complications occur.
There are important points during surgery that require our attention. The first is the location of drainage. The traditional suprachoroidal effusion is usually performed by 5-11 mm scleral puncture after the limbus[9,28], and in this study, patients were cut from the flat part of the ciliary body. This method can avoid unnecessary surgical incision and reduce tissue damage. The second is intraocular perfusion. Neither is it IOP during drainage nor intraocular perfusion during vitrectomy, the most important thing is to ensure that the perfusion needle is located in the vitreous cavity. Otherwise it will only increase the retinal and choroidal detachment, leading to surgery failure. Third, there are many reports of heavy water, intraocular laser and silicone oil filling. It is necessary to flexibly apply various filling materials and techniques during surgery to restore the retina as much as possible and close the retinal tear. Finally, retinal proliferative lesions will occur in some patients after surgery. Close follow-up is needed to select the appropriate surgical timing for re-operation to achieve therapeutic goals. In our study, the mean time from the occurrence of SCH to surgical intervention was 8.5 d (range, 5-12 d). In other studies, the mean time interval was 11 d with a similar range (6-20 d)[3,9]. Generally, a longer duration of appositional SCH has been shown to result in poorer visual outcomes[24].
The recovery of vision is mainly related to the amount of bleeding and the range and height of bleeding choroidal detachment. And also, the prognosis is related to whether the treatment after the bleeding is correct. Rapid closure of the incision during surgery, selection of appropriate surgical timing, and reasonable surgical procedures are useful to preserve the optimal vision. A face-up posture, the disappearance of the anterior chamber, increasing IOP and, finally, optic nerve atrophy were the underlying causes of the poor visual outcome achieved in this patient. While the VA of case 4 remained light perception with a large central chorioretinal scar, cases 2 and 3 had better results, including a final VA of 20/1000. Patient 1 was the only case of intraoperative SCH included in our study, and an immediate tamponade was performed by quickly suturing all surgical incisions. Despite the poor visual outcome (no light perception) achieved in case 1, the anatomical outcome at the final presentation (7 mo postoperatively) was good and showed a reattached choroidea and retina. Excellent final anatomical results were also achieved in cases 1 and 3, and good choroidal reattachment following absorption of the hemorrhage 1 year postoperatively was observed in case 2. Anatomical recovery reduces the eyeball from being phthisical or eviscerated/enucleated, which were a considerable proportion of events in the literature reported[29].
The primary advantage of this technique is that it makes clot liquefaction happen in the early treatment stage and allows a slower and semiautomated controlled mechanism to be achieved, resulting in marked stability during the procedure. At the same time, this study is a pilot study that was undertaken to examine the use of sub-Tenon’s urokinase injection-assisted vitrectomy in the timely treatment of massive SCH complicating cataract surgery or trauma, and the absence of a control group is a limitation of this study. In the future, more cases will be included, according to the preliminary data obtained in the present study.
It is important to ensure that the perfusion needle is located in the vitreous cavity. Otherwise, it will only aggravate the retinal and choroidal detachment, leading to surgery failure. It is necessary to flexibly apply various filling materials and techniques during surgery to restore the retina as much as possible and close the retinal tear. Close follow-up is needed to select the appropriate surgical timing for re-operation to achieve therapeutic goals.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Vaudo G, Sergi C, Rong G S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Wu YXJ
| 1. | Ling R, Cole M, James C, Kamalarajah S, Foot B, Shaw S. Suprachoroidal haemorrhage complicating cataract surgery in the UK: epidemiology, clinical features, management, and outcomes. Br J Ophthalmol. 2004;88:478-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Eriksson A, Koranyi G, Seregard S, Philipson B. Risk of acute suprachoroidal hemorrhage with phacoemulsification. J Cataract Refract Surg. 1998;24:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Meier P, Wiedemann P. Massive suprachoroidal hemorrhage: secondary treatment and outcome. Graefes Arch Clin Exp Ophthalmol. 2000;238:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Chandra A, Xing W, Kadhim MR, Williamson TH. Suprachoroidal hemorrhage in pars plana vitrectomy: risk factors and outcomes over 10 years. Ophthalmology. 2014;121:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Purcell JJ Jr, Krachmer JH, Doughman DJ, Bourne WM. Expulsive hemorrhage in penetrating keratoplasty. Ophthalmology. 1982;89:41-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Cheung AY, David JA, Ober MD. Spontaneous bilateral hemorrhagic choroidal detachments associated with malignant hypertension. Retin Cases Brief Rep. 2017;11:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Gressel MG, Parrish RK 2nd, Heuer DK. Delayed nonexpulsive suprachoroidal hemorrhage. Arch Ophthalmol. 1984;102:1757-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 105] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Mafee MF, Peyman GA. Choroidal detachment and ocular hypotony: CT evaluation. Radiology. 1984;153:697-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Lavinsky F, Moisseiev J, Levkovitch-Verbin H. The surgical management of massive intraoperative and postoperative suprachoroidal hemorrhage: anatomic and functional outcomes. Arq Bras Oftalmol. 2013;76:212-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Obuchowska I, Mariak Z. Risk factors of massive suprachoroidal hemorrhage during extracapsular cataract extraction surgery. Eur J Ophthalmol. 2005;15:712-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Speaker MG, Guerriero PN, Met JA, Coad CT, Berger A, Marmor M. A case-control study of risk factors for intraoperative suprachoroidal expulsive hemorrhage. Ophthalmology. 1991;98:202-209; discussion 210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 132] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Piper JG, Han DP, Abrams GW, Mieler WF. Perioperative choroidal hemorrhage at pars plana vitrectomy. A case-control study. Ophthalmology. 1993;100:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Lakhanpal V, Schocket SS, Elman MJ, Nirankari VS. A new modified vitreoretinal surgical approach in the management of massive suprachoroidal hemorrhage. Ophthalmology. 1989;96:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Laube T, Brockmann C, Bornfeld N. Massive suprachoroidal hemorrhage: Surgical management and outcome. GMS Ophthalmol Cases. 2015;5:Doc10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 15. | Rizzo S, Tartaro R, Faraldi F, Franco F, Finocchio L, Barca F, Caporossi T. Two-Stage Surgery to Manage Massive Suprachoroidal Hemorrhage. Retina. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Rossi T, Boccassini B, Iossa M, Lesnoni G, Tamburrelli C. Choroidal hemorrhage drainage through 23-gauge vitrectomy cannulas. Retina. 2010;30:174-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Peyman GA, Huamonte FU, Goldberg MF, Koziol J. Urokinase in the management of vitreous haemorrhage. Br J Ophthalmol. 1978;62:70-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 18. | Hsiao SF, Shih MH, Huang FC. Spontaneous suprachoroidal hemorrhage: Case report and review of the literature. Taiwan J Ophthalmol. 2016;6:36-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Goff MJ, Jumper JM, Yang SS, Fu AD, Johnson RN, McDonald HR, Ai E. Intravitreal triamcinolone acetonide treatment of macular edema associated with central retinal vein occlusion. Retina. 2006;26:896-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Fei P, Jin HY, Zhang Q, Li X, Zhao PQ. Tissue plasminogen activator-assisted vitrectomy in the early treatment of acute massive suprachoroidal hemorrhage complicating cataract surgery. Int J Ophthalmol. 2018;11:170-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Matsumoto K, Matsumoto CS, Shinoda K, Watanabe E, Mizota A. Tissue plasminogen activator-assisted vitrectomy for ruptured eye with suprachoroidal hemorrhage. Case Rep Ophthalmol. 2012;3:258-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Novelli FJD, Preti RC, Monteiro MLR, Nóbrega MJ, Takahashi WY. A New Method of Subretinal Injection of Tissue Plasminogen Activator and Air in Patients With Submacular Hemorrhage. Retina. 2017;37:1607-1611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Rakusin W. Urokinase in the management of traumatic hyphaema. Br J Ophthalmol. 1971;55:826-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Chai F, Du S, Zhao X, Wang R. Reperfusion of occluded branch retinal arteries by transluminal Nd:YAG laser embolysis combined with intravenous thrombolysis of urokinase. Biosci Rep. 2018;38:pii: BSR20170930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Wang R, Qian L, Wang Y, Zheng Y, Du S, Lei T, Lv P, Long T, Wang W. Evaluation of Ophthalmic Artery Branch Retrograde Intervention in the Treatment of Central Retinal Artery Occlusion (CRAO). Med Sci Monit. 2017;23:114-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Abrams GW, Azen SP, McCuen BW 2nd, Flynn HW Jr, Lai MY, Ryan SJ. Vitrectomy with silicone oil or long-acting gas in eyes with severe proliferative vitreoretinopathy: results of additional and long-term follow-up. Silicone Study report 11. Arch Ophthalmol. 1997;115:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 148] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Scott IU, Flynn HW Jr, Schiffman J, Smiddy WE, Murray TG, Ehlies F. Visual acuity outcomes among patients with appositional suprachoroidal hemorrhage. Ophthalmology. 1997;104:2039-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Chen SN, Ho CL, Ho JD, Guo YH, Chen TL, Chen PF. Acute angle-closure glaucoma resulting from spontaneous hemorrhagic retinal detachment in age-related macular degeneration: case reports and literature review. Jpn J Ophthalmol. 2001;45:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Kuhn F, Morris R, Mester V. Choroidal detachment and expulsive choroidal hemorrhage. Ophthalmol Clin North Am. 2001;14:639-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |