Published online Dec 6, 2018. doi: 10.12998/wjcc.v6.i15.1047
Peer-review started: August 6, 2018
First decision: October 5, 2018
Revised: November 8, 2018
Accepted: November 14, 2018
Article in press: November 15, 2018
Published online: December 6, 2018
Processing time: 125 Days and 8.2 Hours
This report describes a 52-year-old male patient with blunt abdominal traumatic rupture of the spleen due to injuries sustained in an automobile accident. Following splenectomy, the patient developed a gastric fistula. He underwent a long period of conservative treatment, including antibiotics and total parenteral nutrition, which was ineffective. The fistula could not be closed and titanium clip closure using a gastroscopy was then performed in order to close the fistula. After endoscopic therapy and clipping surgery, the patient’s general condition improved significantly, and he had no post-procedural abdominal complications. On post-clipping day 6, the gastric fistula was completely closed as shown by X-ray examination of the upper digestive tract. The patient was discharged from hospital and no complications were observed during the six-month follow-up period. Our report suggests that titanium clip closure using endoscopy may be the choice of treatment in patients with a gastric fistula.
Core tip: Gastric fistula after splenectomy is an uncommon complication, and is difficult to treat and cure. The current management of gastric fistula mainly includes conservative treatment and surgery. There are only a few reports concerning gastric fistula treatment using endoscopy. This is the first report of successful treatment of a gastric fistula after splenectomy using titanium endoscopic clipping.
- Citation: Yu J, Zhou CJ, Wang P, Wei SJ, He JS, Tang J. Endoscopic titanium clip closure of gastric fistula after splenectomy: A case report. World J Clin Cases 2018; 6(15): 1047-1052
- URL: https://www.wjgnet.com/2307-8960/full/v6/i15/1047.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i15.1047
Splenectomy is an important life-saving treatment in patients with massive hemorrhage due to splenic trauma[1]. However, post-splenectomy patients with gastric or pancreatic fistula and/or incomplete intestinal obstruction and other complications are very rare and these complications are difficult to cure[2,3]. At present, post-splenectomy patients with gastric fistula are usually treated with conservative or surgical treatment, whereas there are very few reports on endoscopic titanium clipping for the treatment of gastric fistula after splenectomy. To the best of our knowledge, this is the first report on successful titanium endoscopic clipping of a gastric fistula after splenectomy.
A 52-year-old male patient was admitted to our hospital in May 2017 due to post-splenectomy complications for 21 d. He reported abdominal pain and high fever lasting 19 d at a local hospital. Before admission to our hospital, he had undergone splenectomy and a peritoneal drainage tube was placed in the splenic recess due to traumatic rupture of the spleen with massive hemorrhage after an automobile accident. On post-operative days 1 and 2, the patient was almost normal, and the drainage tube outflow was a pale red liquid (approximately 300 mL/d). However, on post-operative day 3, the patient complained of moderate fever and obvious pain in the left upper quadrant of the abdomen. When the patient ate, he would feel severe abdominal pain and the food drained out of the drainage tube, so he had not been able to eat after splenectomy. In addition, the peritoneal drainage volume had increased to approximately 700 mL/d and the liquid had become purulent. Following conservative treatment at the local hospital for nearly 3 wk, the patient’s condition showed no obvious improvement, and he was transferred to our hospital.
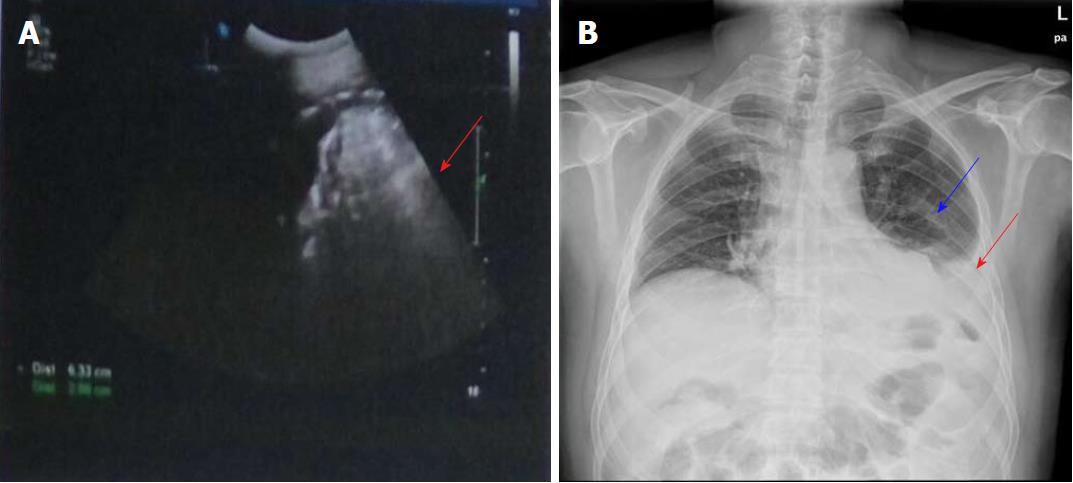
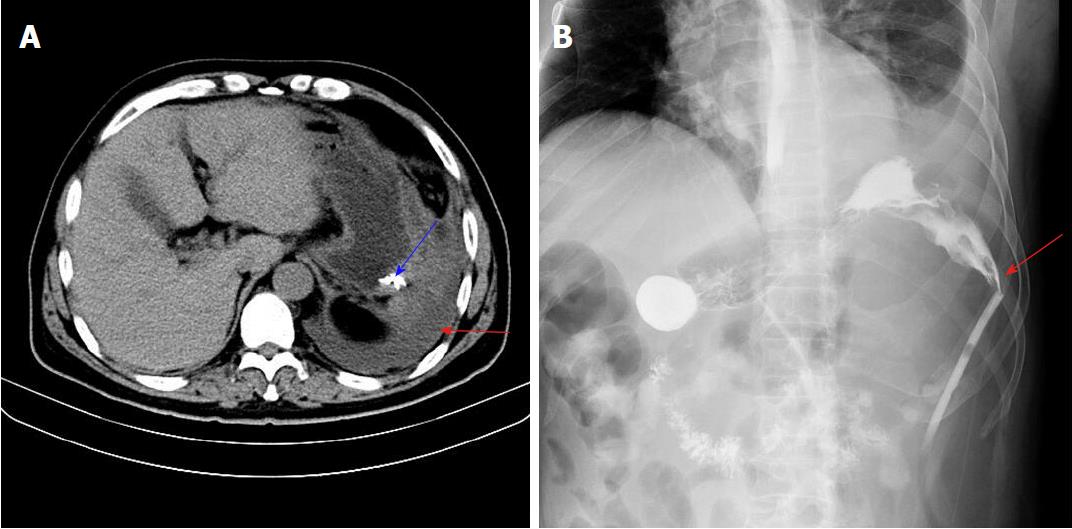
Physical examination revealed obvious tenderness and moderate rebound pain over the left upper quadrant of the abdomen, with a body temperature of 38.8 °C, pulse frequency of 112 bpm, and blood pressure of 154/96 mmHg. Laboratory examination showed that the white blood cell count was 29500/mm3 (normal range 4000-10000/mm3), percentage of neutrophils was 86.90% (normal range 50%-70%), percentage of lymphocytes was 5.20% (normal range 20%-40%), and high sensitivity C-reactive protein level was 57.90 mg/L (normal range 0.068-8.20 mg/L). These results showed that the patient had a severe infection. An abdominal B-ultrasonography examination revealed a limited effusion (about 200 mL) in the splenic fossa area (Figure 1A). Chest X-ray examination showed a moderate amount of effusion in the left chest and intra-abdominal intestinal pneumatosis (Figure 1B). Furthermore, an upper abdominal computed tomography (CT) scan revealed that there was left abdominal wall swelling. A drainage tube and fluid were present in the left side of the abdomen, in addition to partial abdominal mesenteric edema and bowel wall thickening (Figure 2A).
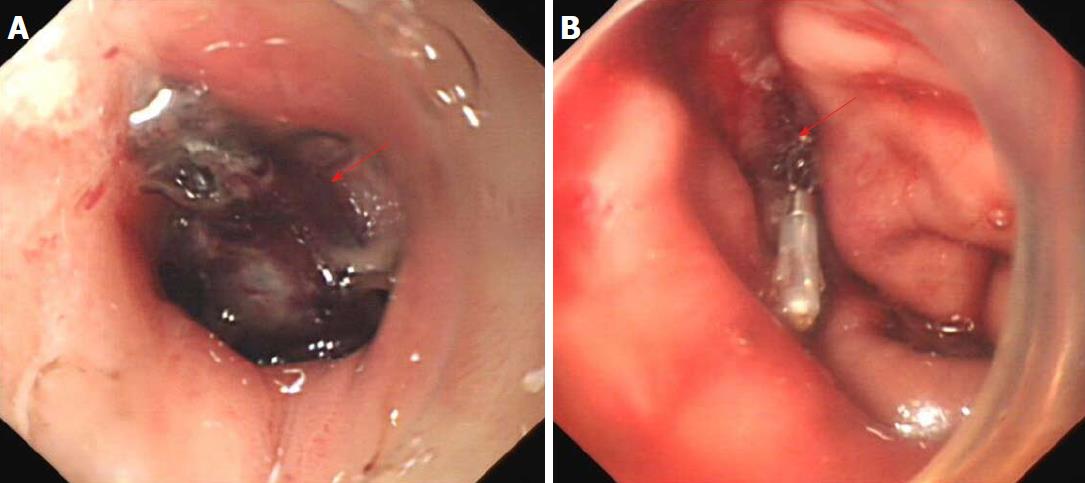
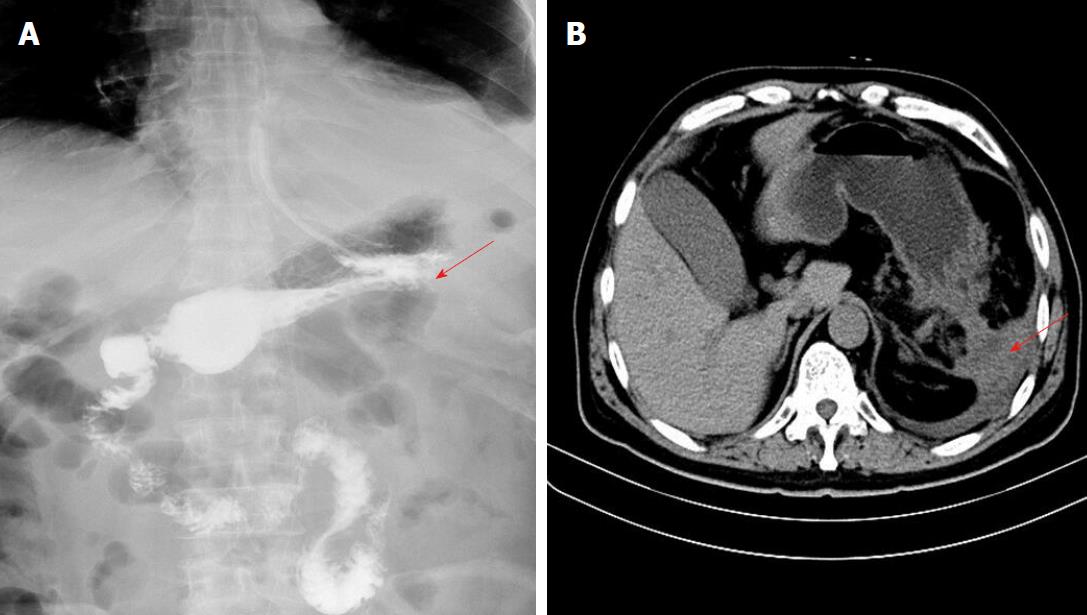
An upper digestive tract ioversol angiography showed that contrast agent had overflowed from the greater curvature of the gastric body into the abdominal cavity, and had then entered the drainage tube (Figure 2B). Therefore, we considered that the patient had a gastric fistula and an abdominal infection. After more than a week of anti-infective treatment and nutritional support, the fistula did not heal, which was confirmed by oral methylene blue examination. Thus, we attempted to carry out endoscopic titanium clip closure of the gastric corpus fistula for approximately 30 min (Figure 3A and 3B). On post-clipping day 2, the patient’s condition started to improve, his abdominal pain was significantly relieved, his body temperature returned to normal, and the drainage tube outflow volume (approximately 100 mL/d) was significantly reduced compared with previous days. On post-clipping day 6, an upper digestive tract ioversol angiography showed no contrast agent overflow into the abdominal cavity, suggesting that the gastric fistula had been closed (Figure 4A). On post-clipping day 7, the patient started a liquid diet, and no abdominal pain, fever, or other symptoms were observed. On post-clipping day 13, the drainage tube was removed as no liquid was observed in the tube, and CT examination showed almost no remaining liquid in the abdominal cavity (Figure 4B). The patient was then discharged, and no complications were detected during the six-month follow-up period (data not shown).
It is well known that splenectomy is necessary in patients with massive hemorrhage following rupture of the spleen[1]. However, gastric or pancreatic fistula, pseudohyperkalemia, and other complications in post-splenectomy patients are difficult to treat[3-5]. Gastric fistula after splenectomy is often due to the condition and surgical factors caused by unfavorable anatomy. As the operative field during emergency splenectomy is usually filled with blood, it is easy to damage the splenic pedicle and short gastric vein in the gastric muscle layer. Poor visibility during surgery can result in gastric tissue being crushed by forceps, causing tissue ischemia and necrosis, and a post-operative gastric fistula can occur. Furthermore, poor post-operative gastric tube drainage and eating too early can aggravate the injured area, leading to the formation of a gastric fistula[6,7]. It has been reported that gastric contents can leak and cause local secondary infections, fever, left upper abdominal pain, and other symptoms; however, the diffusion of gastric contents into the abdominal cavity can result in peritonitis and aggravation of the infection[8]. Inappropriate or untimely treatment of the infection can lead to septic shock and multiple organ failure resulting in patient death[9].
Therefore, the choice of treatment in this situation is critical for patients with a gastric fistula. It has been documented that the current treatment of gastric fistula is as follows: (1) Non-surgical treatment: Full drainage is the key to the treatment of gastric fistula. Following splenectomy, the drainage tube is generally placed in the splenic fossa in patients with post-operative bleeding and gastric fistula, which can also guarantee drainage of stomach contents and prevent diffusion of gastric contents in the abdominal cavity[10,11]. Gastrointestinal decompression is also an important treatment for gastric fistula, and can greatly reduce the volume of gastric leakage[12]. It is known that supportive treatment can promote fistula healing, and supportive treatment should include fresh blood, plasma, and albumin. Good nutrition and vitamins are also necessary in these patients[13,14]; (2) Surgical repair should be performed if the gastric fistula is large, or a long period of conservative therapy was ineffective. However, this type of surgery is usually difficult due to severe adhesions in the abdominal cavity secondary to serious infection, and is also prone to causing new accessory injury of organs[15]; and (3) An over-the-scope clip has recently been used for endoscopic closure of perforations, leaks, fistulas, and endoscopic hemostasis[16].
In the present study, the patient experienced abdominal pain and high fever for approximately 3 wk during conservative treatment before admission to our hospital. Following physical and laboratory examinations, and taking into account the patient’s mild and limited peritonitis signs, he received anti-infective treatment and nutritional support for 1 wk. However, the fistula did not heal, and the patient was unable to eat. Moreover, the patient may have had serious adhesions in the abdominal cavity, which was indicated by an abdominal enhanced CT scan (data not shown), and surgery would have been very difficult. Therefore, we attempted to perform endoscopy-assisted titanium clip closure of the fistula, which was successful. The patient recovered well after this treatment, and an X-ray examination of the upper digestive tract showed that the gastric fistula had completely closed.
Abdominal enhanced CT and both upper gastrointestinal radiography and endoscopy are important diagnostic methods for gastric fistula, and are of great significance in the assessment of fistula severity and treatment choice[17,18]. In this report, a patient with abdominal pain and high fever after splenectomy was admitted to our hospital, and ultrasound examination did not reveal the cause of peritoneal effusion. Further examinations, including abdominal CT, and gastrointestinal radiography and endoscopy were performed, and a definite diagnosis of gastric fistula was established. Gastroscopy showed that the fistula was of medium size (about 2.0 cm x 2.5 cm in diameter) and there was no obvious edema and necrosis in the surrounding tissues of the fistula; therefore, for the first time, we used titanium clips to treat this patient.
In review of the literature, there are very few reports on endoscopic closure of fistula. Uesato et al[19] reported endoscopic occlusion using an endobronchial Watanabe spigot was performed to close a long-term esophago-bronchiole fistula after esophagectomy. Tsai et al[20] showed that gastrogastric fistula could be closed by using endoscopic Apollo Overstitch system. Our present report indicates that the key to successful endoscopic titanium clipping of the fistula is that the tissue around the fistula must be healthy enough to be held in place by the metal clip. If the tissue around the fistula is fragile or necrotic, the metal clip will not be able to achieve alignment of the tissue. Therefore, endoscopic closure can be performed for the long-term fistula.
In summary, our report indicates that if signs of peritonitis are mild, and a long period of conservative treatment for gastric fistula has been ineffective, endoscopic titanium clip closure may be a good treatment choice. This technique has the advantages of reduced trauma, shorter operative time, and fewer complications, and was effective in the treatment of gastric fistula.
A 52-year-old male patient with blunt abdominal traumatic rupture of the spleen developed a gastric fistula after splenectomy. Following conservative treatment in a local hospital for almost 3 wk that was ineffective, he was transferred to our hospital.
The patient was diagnosed with a gastric fistula and abdominal infection.
Pancreatic fistula should be excluded.
Laboratory examination showed that the white blood cell count, percentage of neutrophils, and high sensitivity C-reactive protein level were significantly increased.
A fistula of the greater curvature of the gastric body accompanied by abdominal infection was confirmed by upper digestive tract ioversol angiography and dynamic abdominal computed tomography scanning.
Anti-infective treatment and nutritional support was ineffective for the fistula of the patient. Therefore, endoscopic titanium clip closure was performed and the gastric fistula was successfully closed.
As conservative treatment may be ineffective for medium-sized gastric fistulas after splenectomy, endoscopic titanium clipping is a good and safe treatment choice, which avoids the risk of re-operation.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chen JQ, Unek T S- Editor: Wang JL L- Editor: Filipodia E- Editor: Tan WW
| 1. | Johnsen NV, Betzold RD, Guillamondegui OD, Dennis BM, Stassen NA, Bhullar I, Ibrahim JA. Surgical Management of Solid Organ Injuries. Surg Clin North Am. 2017;97:1077-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Yamamoto N, Oshima T, Sato T, Makino H, Nagano Y, Fujii S, Rino Y, Imada T, Kunisaki C. Upper abdominal body shape is the risk factor for postoperative pancreatic fistula after splenectomy for advanced gastric cancer: a retrospective study. World J Surg Oncol. 2008;6:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Qu Y, Ren S, Li C, Qian S, Liu P. Management of postoperative complications following splenectomy. Int Surg. 2013;98:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Tsutsumi N, Tomikawa M, Akahoshi T, Kawanaka H, Ota M, Sakaguchi Y, Kusumoto T, Ikejiri K, Hashizume M, Maehara Y. Pancreatic fistula after laparoscopic splenectomy in patients with hypersplenism due to liver cirrhosis: effect of fibrin glue and polyglycolic acid felt on prophylaxis of postoperative complications. Am J Surg. 2016;212:882-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Wilson R, Skelly RT. Pseudohyperkalaemia: a rare complication of splenectomy. Ann R Coll Surg Engl. 2017;99:e52-e53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Zheng X, Yao Y, Liu Q. Gastric perforation after laparoscopic splenectomy and esophagogastric devascularization for portal hypertension: report of a case. Surg Laparosc Endosc Percutan Tech. 2011;21:e209-e212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Martinez CA, Waisberg J, Palma RT, Bromberg SH, Castro MA, Santos PA. Gastric necrosis and perforation as a complication of splenectomy. Case report and related references. Arq Gastroenterol. 2000;37:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Wuthisuthimethawee P, Sangkhathat S, Ruegklinag C, Patrapinyokul S, Laoprasopwathana K. Gastropleural fistula following a splenectomy for splenic abscess: a case report. J Med Assoc Thai. 2008;91:1291-1295. [PubMed] |
| 9. | Rausa E, Bonavina L, Asti E, Gaeta M, Ricci C. Rate of Death and Complications in Laparoscopic and Open Roux-en-Y Gastric Bypass. A Meta-analysis and Meta-regression Analysis on 69,494 Patients. Obes Surg. 2016;26:1956-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Farach SM, Danielson PD, McClenathan DT, Wilsey MJ, Chandler NM. Endoscopic closure of persistent gastrocutaneous fistula in children. Pediatr Surg Int. 2015;31:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Soufron J. Leak or Fistula After Sleeve Gastrectomy: Treatment with Pigtail Drain by the Rendezvous Technique. Obes Surg. 2015;25:1979-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Abou Rached A, Basile M, El Masri H. Gastric leaks post sleeve gastrectomy: review of its prevention and management. World J Gastroenterol. 2014;20:13904-13910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 141] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 13. | Vasant DH, Lal S, Blackett BD, Paine PA. Closure of a large high-output gastrocutaneous fistula with combined postpyloric feeding and aggressive medical therapy. BMJ Case Rep. 2012;2012:bcr2012007267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Chauhan A, Perry I, Veitch A, Li P, Rattehalli D, Brookes MJ. Gastropericardial fistula: a potential role for conservative treatment. Eur J Gastroenterol Hepatol. 2012;24:1341-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Blot C, Mouly C, Rebibo L, Dhahri A, Régimbeau JM. Conservative surgical management of persistent leak after sleeve gastrectomy by Roux-en-Y gastro-jejunostomy to the fistulous orifice. J Visc Surg. 2015;152:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Goenka MK, Rai VK, Goenka U, Tiwary IK. Endoscopic Management of Gastrointestinal Leaks and Bleeding with the Over-the-Scope Clip: A Prospective Study. Clin Endosc. 2017;50:58-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Sandrasegaran K, Rajesh A, Lall C, Gomez GA, Lappas JC, Maglinte DD. Gastrointestinal complications of bariatric Roux-en-Y gastric bypass surgery. Eur Radiol. 2005;15:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Graif A, Conde K, DeMauro CA. Imaging of a gastrobronchial fistula after gastric bypass surgery and the contrast dilemma. Del Med J. 2015;87:113-116. [PubMed] |
| 19. | Uesato M, Kono T, Akutsu Y, Murakami K, Kagaya A, Muto Y, Nakano A, Aikawa M, Tamachi T, Amagai H. Endoscopic occlusion with silicone spigots for the closure of refractory esophago-bronchiole fistula after esophagectomy. World J Gastroenterol. 2017;23:5253-5256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Tsai C, Kessler U, Steffen R, Merki H, Zehetner J. Endoscopic Closure of Gastro-gastric Fistula After Gastric Bypass: a Technically Feasible Procedure but Associated with Low Success Rate. Obes Surg. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |