Published online Dec 6, 2018. doi: 10.12998/wjcc.v6.i15.1024
Peer-review started: August 17, 2018
First decision: October 8, 2018
Revised: October 31, 2018
Accepted: November 7, 2018
Article in press: November 7, 2018
Published online: December 6, 2018
Processing time: 113 Days and 1.5 Hours
Primary immune thrombocytopenia (ITP) is a rare autoimmune disease associated with a high bleeding risk. For those patients with gastric cancer, surgical treatment may be the only option for therapy. Here, we present the first case of gastric cancer with severe and medically refractory ITP treated by radical resection of the gastric cancer and splenectomy.
A 54-year-old female patient was admitted to our surgical department with a 2 mo history of decreased appetite, nausea, vomiting, and weight loss, which progressed to difficulty in feeding 3 d prior to her visit. According to her medical history, she was diagnosed with refractory ITP [platelets (PLT), 3000-8000/μL] 10 years ago. After admission, the patient underwent a splenectomy and a distal subtotal gastrectomy (D2 radical resection) with Roux-en-Y reconstruction simultaneously. She had an uneventful postoperative course with a slight increase in her PLT count. This case is unique in terms of the patient’s complication of severe and medically refractory ITP.
Simultaneous splenectomy, preoperative PLT transfusion, and early enteral nutrition were important treatment methods for helping this patient recover.
Core tip: Immune thrombocytopenia (ITP) is a rare autoimmune disease with a reduced platelet count. Severe and medically refractory thrombocytopenia is an absolute contraindication to chemotherapy or radiotherapy. For those patients with a malignant tumor, surgical treatment may be the only option despite a high risk of bleeding. This case might contribute to improving our understanding of the behavior and perioperative management of severe and medically refractory ITP patients with gastric cancer.
- Citation: Zhao ZW, Kang WM, Ma ZQ, Ye X, Yu JC. Gastric cancer with severe immune thrombocytopenia: A case report. World J Clin Cases 2018; 6(15): 1024-1028
- URL: https://www.wjgnet.com/2307-8960/full/v6/i15/1024.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i15.1024
Primary immune thrombocytopenia (ITP), also known as idiopathic thrombocytopenia purpura, is an autoimmune disease associated with a reduced platelet (PLT) count without any obvious initiating and/or underlying cause. Severe and medically refractory thrombocytopenia is an absolute contraindication to chemotherapy or radiotherapy. For those patients with gastric cancer, surgical treatment may be the only option for therapy despite a high risk of bleeding. This uncommon condition can be worrisome for surgeons. Here, aiming to lay a foundation for future clinical work, we report the first case of gastric cancer complicated by severe and medically refractory ITP treated by subtotal gastrectomy and splenectomy and a review of the related literature.
A 54-year-old woman was admitted to our hospital with a 2-mo history of decreased appetite, nausea, vomiting, and weight loss, which progressed to difficulty in feeding 3 d prior to her visit. A rapid urease test and serum antibody testing demonstrated that she was negative for Helicobacter pylori (H. pylori). An upper gastrointestinal endoscopy showed irregular erosion on the pylorus with pyloric stenosis. Multiple mucosal biopsies were obtained, and a histological analysis revealed gastric adenocarcinoma. Serum levels of tumor markers were significantly elevated (CA19-9 273.1 U/mL and CA242 > 150.0 U/mL). Abdominal contrast-enhanced computed tomography revealed a thickening wall of the gastric antrum with significantly enhanced, multiple small lymph nodes around the stomach but no obvious retroperitoneal lymph nodes, and a normal spleen. She worked in a plastics factory and was exposed to chemicals and radioactive materials for several years. She was diagnosed with ITP (PLT 3000-8000/μL) 10 years ago. Prednisolone therapy (80 mg/d for 2 wk) was started; and her PLT increased to 50000-60000/μL. However, PLT decreased to 3000-7000/μL immediately after reduction of corticosteroids. Then, dexamethasone therapy (40 mg/d for 4 d) was started. PLT increased to 170000/μL temporarily but decreased to 3000-7000/μL 3 d after therapy. Other medications including immunoglobulins (10 g/d for 4 d), androgen derivatives (danazol 400 mg/d for 3 mo), cyclosporine A (200 mg/d for 2 wk), and thrombopoietin (50 μg/d for 7 d) were administered respectively. However, she no longer responded to any of these medical therapies. Other previous medical history included congenital ventricular septal defect and subclinical hypothyroidism. Her family history was unremarkable. On admission, her physical examination and blood biochemistry laboratory results were within normal limits. Hematological tests revealed a decreased PLT count of 5000/μL and a normal hemoglobin (HGB) level of 111 g/L. Subsequently, she presented with melena and bloody drainage which was observed in the nasogastric tube; the HGB and PLT decreased to 85 g/L and 1000/μL, respectively. Her general condition was examined before surgery. Because of decreased appetite and difficulty in feeding, her weight dropped to 66 kg from her normal weight of 80 kg (body mass index dropped from 30.1 kg/m2 to 24.8 kg/m2). Although both cardiac function and pulmonary function were at normal levels, her high nutritional risk may affect the postoperative recovery.
The patient underwent a splenectomy and a distal subtotal gastrectomy (D2 radical resection) with a Roux-en-Y reconstruction simultaneously. A needle catheter jejunostomy was performed to ensure postoperative enteral nutrition (EN). In total, four pheresis units (one pheresis unit contains approximately 2.5-4.0 × 1011 PLTs, equal to 10-12 whole blood donor units) of PLT were transfused. The patient’s PLT counts fluctuated between 30000-60000/μL during the surgery and PLT were administered accordingly (one pheresis unit was administered 1 h before induction of anesthesia; two pheresis units administered during surgery; and one pheresis unit 2 h after surgery).
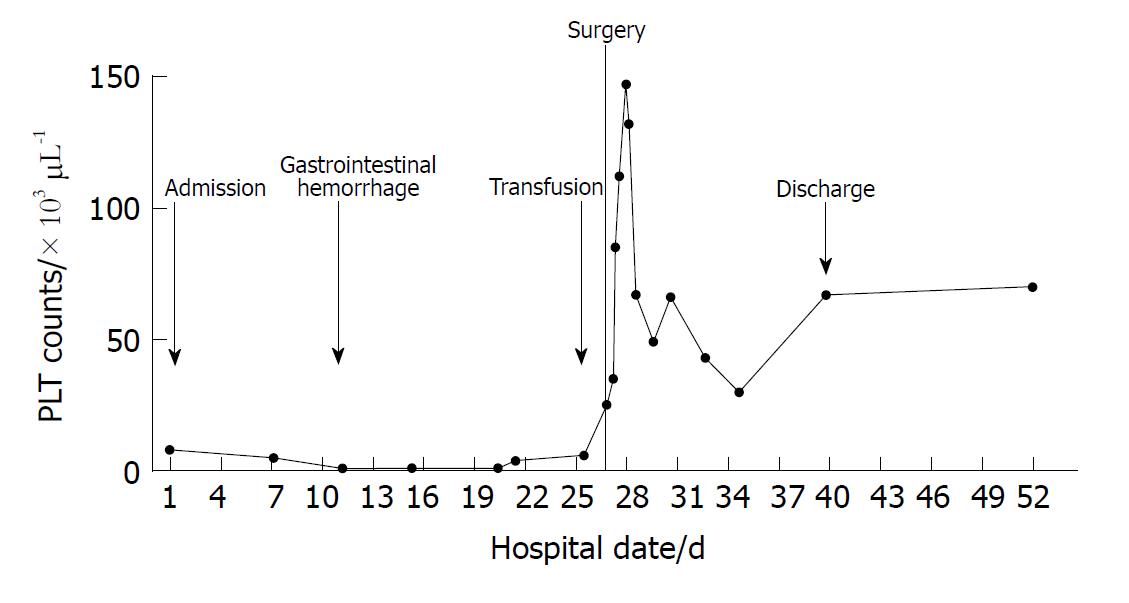
The patient resumed EN (oligopeptide, low-fat, isocaloric, non-residue diet; Peptisorb, Nutricia, Schiphol, The Netherlands) on the morning of the 2nd postoperative day. She had an uneventful postoperative course with a slight increase in the PLT count (Figure 1) and was discharged on the 15th postoperative day. The histopathological examination after the subtotal gastrectomy revealed a poorly differentiated gastric adenocarcinoma which had reached the serosal layer. The cancer had also metastasized to 3/30 lymph nodes. The pathological stage was pT4aN2M0, IIIA. There was no evidence of recurrence, and she showed a consistent and stable increase in the PLT count around 20000/μL for about 2 years after the operation.
ITP is an immune-mediated disease associated with a reduced PLT count lower than 100000/μL[1]. The mechanism of ITP includes that PLT membrane proteins become antigenic and stimulate the immune system resulting in thrombocytopenia due to immune-mediated PLT destruction and/or suppression of PLT production[2]. Some studies defined very severe thrombocytopenia as PLT count < 10000/μL[3]; however, some researchers suggest that the severity of the disease is based clinically on bleeding scores rather than a PLT count[4,5]. In this case, the patient presented with gastrointestinal hemorrhage accompanied with acute anemia. According to Khellaf’s bleeding score[4], this patient had a high bleeding score (> 8), which indicated a severe ITP with a high risk of life-threatening hemorrhage. Patients are considered to have medically refractory ITP when they require treatment following failure to respond to medical treatment. Response was defined as PLT count > 30000/μL and at least a 2-fold increase in the baseline count and absence of bleeding[1]. In this case, the patient was considered nonresponse to medication.
To the best of our knowledge, only five cases of ITP patients suffering with gastric malignant tumors have been reported in English in MEDLINE (Table 1)[6-10]. Patients in these cases suffered mild ITP with a PLT level larger than 20000/μL. The commonest pathology is gastric cancer and gastric mucosa associated lymphoid tissue (MALT) lymphoma. H. pylori may play a key role in the pathogenesis of both gastric malignant tumors and ITP. Noda et al[7] reported a case of regression of ITP after resection of gastric MALT lymphoma and eradicating treatment of H. pylori. In terms of treatment, endoscopic resection could be performed only when the tumor is restricted to the mucosa during eradication therapy. Subtotal/total gastrectomy combined with splenectomy is the most appropriate treatment when tumors invade the submucosa. And this is the first case of gastric cancer with severe and medically refractory ITP. The extremely low PLT level led to a high risk of bleeding, especially during the perioperative period.
| Ref. | PLT level (/μL) | Gastric tumor | H. pylori | Treatment for gastric tumor | Prognosis of gastric tumor |
| Bachmeyer et al[6], 2000 | 40000 | Gastric MALT lymphoma | Positive | Chemotherapy | - |
| Noda et al[7], 2004 | 27000 | Gastric MALT lymphoma | Positive | Endoscopic mucosal resection | Without recurrence in 2 yr |
| Wakata et al[8], 2006 | 73000-10800 | Gastric cancer | - | Subtotal gastrectomy and splenectomy | Without recurrence in 2 yr |
| Villias et al[9], 2008 | 76000 | Gastric cancer and GIST | - | Subtotal gastrectomy and splenectomy | - |
| Hamabe et al[10], 2011 | 52000 | Gastric cancer and gastric MALT lymphoma | Positive | Total gastrectomy and splenectomy | Without recurrence in 2 yr |
Corticosteroids with or without intravenous immunoglobulin (IVIg) are the standard first-line treatment for ITP patients with a PLT count < 30000/μL[11]. For patients who have failed corticosteroid therapy, a splenectomy is recommended as a second-line treatment. According to recent research, a short-term response to a splenectomy was achieved in approximately 87% of ITP patients[12-14]. Whereas in patients with a PLT count on admission of < 40000/μL, only 40% may achieve a long-term stable response[14]. In our patient, the thrombocytopenia continued with PLT counts fluctuating between 3000-8000/μL and she showed a poor response to preoperative medical boosting. Considering its satisfactory and high response rates in short-term postoperative time, it is reasonable for patients to undergo a splenectomy in terms of low complication rates and low bleeding risk.
According to some studies, PLT transfusions were recommended only in a few life-threatening cases that require a rapid rise in PLT count to achieve hemostasis, such as intracranial hemorrhage or major surgery[1,15]. Despite the short-term efficacy, PLT transfusion every 30 min to 8 h and a PLT transfusion in conjunction with IVIg or steroids have been effective for increasing PLT levels in emergency situations[16-18]. Traditionally, for safety, most guidelines recommend a PLT count of at least 30000-50000/μL for prophylaxis during surgery[19,20]. Recently, some researchers reported that a perioperative PLT transfusion might be unnecessary for a laparoscopic splenectomy in ITP patients[21,22]. However, there is still a lack of evidence to guide preoperative PLT transfusions used as prophylaxis for surgery, especially for those with a high bleeding risk. In this case, a PLT transfusion resulted in a rapid rise of PLT count ranging from 30000 to 60000/μL during the operation and ensured a successful surgery.
Postoperative patients with advanced gastric cancer generally suffer from various complications, such as infection and malnutrition[23]. Early EN is important to implement as a way to accelerate rehabilitation of intestinal function and immune response in patients undergoing a gastrectomy, especially in patients with severe complications[24]. Needle catheter jejunostomy was reported to be safe and progressive EN support could be implemented successfully[25,26]. In this patient, the step-by-step EN feeding program was initiated on the 2nd postoperative day using the needle catheter jejunostomy.
In conclusion, we report a case of advanced gastric cancer complicated with severe and medically refractory ITP that was successfully cured by radical resection of the gastric cancer. Simultaneous splenectomy, preoperative PLT transfusion, and early EN were important assistances to the treatment of this patient.
The patient suffered from decreased appetite, nausea, vomiting, and weight loss for 2 mo with a past medical history of thrombocytopenia, which progressed to difficulty in feeding and gastrointestinal hemorrhage.
The patient was diagnosed with gastric cancer accompanied with severe and medically refractory immune thrombocytopenia.
Mucosal biopsy from endoscopy was useful for differential diagnosis and histological analysis revealed a gastric adenocarcinoma.
Laboratory findings revealed elevated tumor markers (CA19-9 and CA242), low platelet count, and decreased hemoglobin.
Abdominal contrast-enhanced computed tomography revealed a thickening wall of the gastric antrum with significantly enhanced, multiple small lymph nodes around the stomach but no obvious retroperitoneal lymph nodes and a normal spleen.
The histopathological examination after the subtotal gastrectomy revealed a poorly differentiated gastric adenocarcinoma which had reached the serosal layer and the cancer had also metastasized to 3/30 lymph nodes.
The patient underwent a splenectomy and a distal subtotal gastrectomy (D2 radical resection) with a Roux-en-Y reconstruction simultaneously.
Five cases of immune thrombocytopenia (ITP) patients suffering with gastric malignant tumors have been reported in English in MEDLINE. Patients in these cases suffered mild ITP with a platelet (PLT) level larger than 25000/μL. The commonest pathologies are gastric cancer and gastric mucosa associated lymphoid tissue (MALT) lymphoma. Helicobacter pylori (H. pylori) may play a key role in the pathogenesis of both gastric malignant tumors and ITP. Noda et al[7] reported a case of regression of ITP after resection of gastric MALT lymphoma and eradicating treatment of H. pylori. In terms of treatment, endoscopic resection could be performed only when the tumor is restricted to the mucosa during eradication therapy. Subtotal/total gastrectomy combined with splenectomy is the most appropriate treatment when tumors invade the submucosa.
For patients with cancer and medical refractory ITP, surgical treatment may be the only option for therapy despite a high risk of bleeding. Simultaneous splenectomy, preoperative PLT transfusion, and early enteral nutrition are important treatment methods for postoperative recovery.
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Morini S, Zhu X, Zhu YL S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Bian YN
| 1. | Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong BH, Cooper N. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386-2393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1569] [Cited by in RCA: 1847] [Article Influence: 108.6] [Reference Citation Analysis (0)] |
| 2. | Shan NN, Dong LL, Zhang XM, Liu X, Li Y. Targeting autophagy as a potential therapeutic approach for immune thrombocytopenia therapy. Crit Rev Oncol Hematol. 2016;100:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Gupta S, Kalayarasan R, Chandrasekar S, Gnanasekaran S, Pottakkat B. Laparoscopic Splenectomy for Immune Thrombocytopenic Purpura (ITP) Patients with Very Severe Thrombocytopenia. Indian J Hematol Blood Transfus. 2018;34:535-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Khellaf M, Michel M, Schaeffer A, Bierling P, Godeau B. Assessment of a therapeutic strategy for adults with severe autoimmune thrombocytopenic purpura based on a bleeding score rather than platelet count. Haematologica. 2005;90:829-832. [PubMed] |
| 5. | Page LK, Psaila B, Provan D, Michael Hamilton J, Jenkins JM, Elish AS, Lesser ML, Bussel JB. The immune thrombocytopenic purpura (ITP) bleeding score: assessment of bleeding in patients with ITP. Br J Haematol. 2007;138:245-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Bachmeyer C, Audouin J, Bouillot JL, Coutarel P, Mougeot-Martin M, Delmer A. Immune thrombocytopenic purpura as the presenting feature of gastric MALT lymphoma. Am J Gastroenterol. 2000;95:1599-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Noda M, Mori N, Nomura K, Kojima K, Mitsufuji S, Yamane I, Misawa S, Okanoue T. Regression of idiopathic thrombocytopenic purpura after endoscopic mucosal resection of gastric mucosa associated lymphoid tissue lymphoma. Gut. 2004;53:1698-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Wakata N, Kiyozuka T, Konno S, Nakazora H, Nomoto N, Sugimoto H, Nemoto H. Autoimmune thrombocytopenic purpura, autoimmune hemolytic anemia and gastric cancer appeared in a patient with myasthenia gravis. Intern Med. 2006;45:479-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Villias C, Gourgiotis S, Veloudis G, Sampaziotis D, Moreas H. Synchronous early gastric cancer and gastrointestinal stromal tumor in the stomach of a patient with idiopathic thrombocytopenic purpura. J Dig Dis. 2008;9:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Hamabe A, Omori T, Oyama T, Akamatsu H, Yoshidome K, Tori M, Ueshima S, Tsujimoto M, Nishida T. A case of Helicobacter pylori infection complicated with gastric cancer, gastric mucosa-associated lymphoid tissue lymphoma, and idiopathic thrombocytopenic purpura successfully treated with laparoscopy-assisted total gastrectomy and splenectomy. Asian J Endosc Surg. 2011;4:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Provan D, Newland AC. Current Management of Primary Immune Thrombocytopenia. Adv Ther. 2015;32:875-887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 12. | Montalvo J, Velazquez D, Pantoja JP, Sierra M, López-Karpovitch X, Herrera MF. Laparoscopic splenectomy for primary immune thrombocytopenia: clinical outcome and prognostic factors. J Laparoendosc Adv Surg Tech A. 2014;24:466-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Qu Y, Xu J, Jiao C, Cheng Z, Ren S. Long-term outcomes of laparoscopic splenectomy versus open splenectomy for idiopathic thrombocytopenic purpura. Int Surg. 2014;99:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Rijcken E, Mees ST, Bisping G, Krueger K, Bruewer M, Senninger N, Mennigen R. Laparoscopic splenectomy for medically refractory immune thrombocytopenia (ITP): a retrospective cohort study on longtime response predicting factors based on consensus criteria. Int J Surg. 2014;12:1428-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA; American Society of Hematology. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190-4207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 1298] [Article Influence: 92.7] [Reference Citation Analysis (0)] |
| 16. | Bavunoğlu I, Eşkazan AE, Ar MC, Cengiz M, Yavuzer S, Salihoğlu A, Öngören Ş, Tunçkale A, Soysal T. Treatment of patients with immune thrombocytopenia admitted to the emergency room. Int J Hematol. 2016;104:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Salama A, Kiesewetter H, Kalus U, Movassaghi K, Meyer O. Massive platelet transfusion is a rapidly effective emergency treatment in patients with refractory autoimmune thrombocytopenia. Thromb Haemost. 2008;100:762-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Spahr JE, Rodgers GM. Treatment of immune-mediated thrombocytopenia purpura with concurrent intravenous immunoglobulin and platelet transfusion: a retrospective review of 40 patients. Am J Hematol. 2008;83:122-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the use of platelet transfusions. Br J Haematol. 2003;122:10-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 385] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 20. | Habermalz B, Sauerland S, Decker G, Delaitre B, Gigot JF, Leandros E, Lechner K, Rhodes M, Silecchia G, Szold A. Laparoscopic splenectomy: the clinical practice guidelines of the European Association for Endoscopic Surgery (EAES). Surg Endosc. 2008;22:821-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 21. | Chen X, Peng B, Cai Y, Zhou J, Wang Y, Wu Z, Chen S. Laparoscopic splenectomy for patients with immune thrombocytopenia and very low platelet count: is platelet transfusion necessary? J Surg Res. 2011;170:e225-e232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Cai Y, Liu X, Peng B. Should we routinely transfuse platelet for immune thrombocytopenia patients with platelet count less than 10 × 109/L who underwent laparoscopic splenectomy? World J Surg. 2014;38:2267-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Takeuchi D, Koide N, Suzuki A, Ishizone S, Shimizu F, Tsuchiya T, Kumeda S, Miyagawa S. Postoperative complications in elderly patients with gastric cancer. J Surg Res. 2015;198:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Li B, Liu HY, Guo SH, Sun P, Gong FM, Jia BQ. Impact of early postoperative enteral nutrition on clinical outcomes in patients with gastric cancer. Genet Mol Res. 2015;14:7136-7141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Young MT, Troung H, Gebhart A, Shih A, Nguyen NT. Outcomes of laparoscopic feeding jejunostomy tube placement in 299 patients. Surg Endosc. 2016;30:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Senkal M, Koch J, Hummel T, Zumtobel V. Laparoscopic needle catheter jejunostomy: modification of the technique and outcome results. Surg Endosc. 2004;18:307-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |