Published online Dec 6, 2018. doi: 10.12998/wjcc.v6.i15.1018
Peer-review started: September 18, 2018
First decision: October 8, 2018
Revised: November 1, 2018
Accepted: November 7, 2018
Article in press: November 7, 2018
Published online: December 6, 2018
Processing time: 83 Days and 9.3 Hours
The optimal therapeutic strategy in treating thyroid metastasis from renal cell carcinoma (RCC) has not been clearly established. Here we describe a case of didactic surgical experience of the disease which caused massive intraoperative bleeding.
A 59-year-old male patient presented with a thyroid left lobe soft mass detected by chest computed tomography scans prior to the surgical treatment of RCC of the left kidney. The thyroid mass was initially considered to be benign, then he underwent left radical nephrectomy. One year after the nephrectomy, stereotactic radiosurgery was performed for brain metastasis. During follow-up, the thyroid nodule gradually grew, and the patient manifested swallowing discomfort. Under a clinical diagnosis of thyroid follicular neoplasm, left hemithyroidectomy was performed. Although hemithyroidectomy is usually a safe and straightforward procedure, massive bleeding from markedly developed tumor vessels made the operation very difficult. The thyroid tumor was finally diagnosed as metastasis from clear cell RCC.
For proper timing of the surgery, a clinician should take into consideration the possibility of thyroid metastasis of RCC when a thyroid lesion is found in patients with RCC or in patients with a previous history of RCC. We recommend that thyroid metastasis of RCC should be resected as early as possible even if a patient has other metastatic sites.
Core tip: A didactic surgical experience of thyroid metastasis from renal cell carcinoma (RCC) which caused massive intraoperative bleeding is presented. Based on this experience, we recommend that thyroid metastasis of RCC should be resected as early as possible even if a patient has other metastatic sites, unless the patient has appropriate reasons to avoid surgery.
- Citation: Yamauchi M, Kai K, Shibamiya N, Shimazu R, Monji M, Suzuki K, Kakinoki H, Tobu S, Kuratomi Y. Didactic surgical experience of thyroid metastasis from renal cell carcinoma: A case report. World J Clin Cases 2018; 6(15): 1018-1023
- URL: https://www.wjgnet.com/2307-8960/full/v6/i15/1018.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i15.1018
Thyroid metastasis is a clinically rare entity, accounting for only 1.4% to 3.0% of all thyroid malignancy[1]. The kidneys (renal cell carcinoma, RCC) are the most common primary site (33%) followed by the lungs (16%), breast (16%), esophagus (9%), and uterus (7%)[2]. Although there are several case reports and review articles about thyroid metastasis from RCC[3,4] these have mainly focused on the diagnostic challenges, and thus an optimal therapeutic strategy has not been clearly established. Here we present a case of thyroid metastatic tumor from RCC that was accompanied by massive intraoperative bleeding. Based on this experience, we recommend that thyroid metastasis of RCC be resected as early as possible.
Hematuria.
A 59-year-old Japanese man visited a nearby hospital for the examination of hematuria. Ultrasonographic (US) examination revealed a mass lesion at the left kidney and he was referred to our hospital for further examination and surgical treatment.
Unremarkable.
A solid and painless 3 cm × 2 cm mass was palpable on the left thyroid lobe without lymphadenopathy.
The patient showed no alterations in thyroid function tests and other serum laboratory tests.
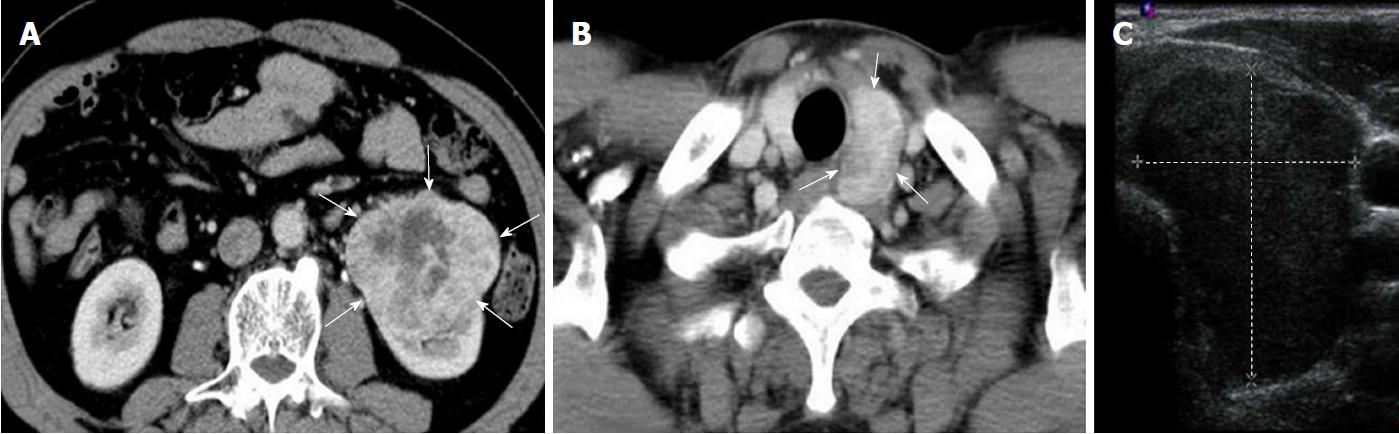
The preoperative computed tomography (CT) scans revealed an exophytic mass lesion measuring 8.1 cm × 6.2 cm at the lower pole of the left kidney (Figure 1A) and a mass lesion with heterogeneous contrast-enhancement measuring 4.1 cm × 2.4 cm at the left lobe of the thyroid (Figure 1B). Radiologically, the renal mass lesion was considered to be RCC (cT3N0M0, Stage III). The findings of US for the thyroid mass were consistent with a follicular lesion at that time (Figure 1C).
The patient underwent left radical nephrectomy.
The postoperative clinical course was uneventful. The pathological diagnosis of the renal nodule was clear cell RCC of Fuhrman grade 2. The tumor invaded into the perirenal and renal sinus fat tissue (pT3a). All surgical margins were free from tumor invasion.
One year after the surgery, the patient became aware of memory disturbance. Head CT scans revealed a brain mass lesion. From the findings of head magnetic resonance imaging (MRI), this mass lesion was considered a metastasis of RCC. The patient was treated with stereotactic radiosurgery for brain metastasis and a complete response was realized. During the treatment for brain metastasis, the thyroid mass was gradually enlarged in plain CT scans and the patient manifested swallowing discomfort.
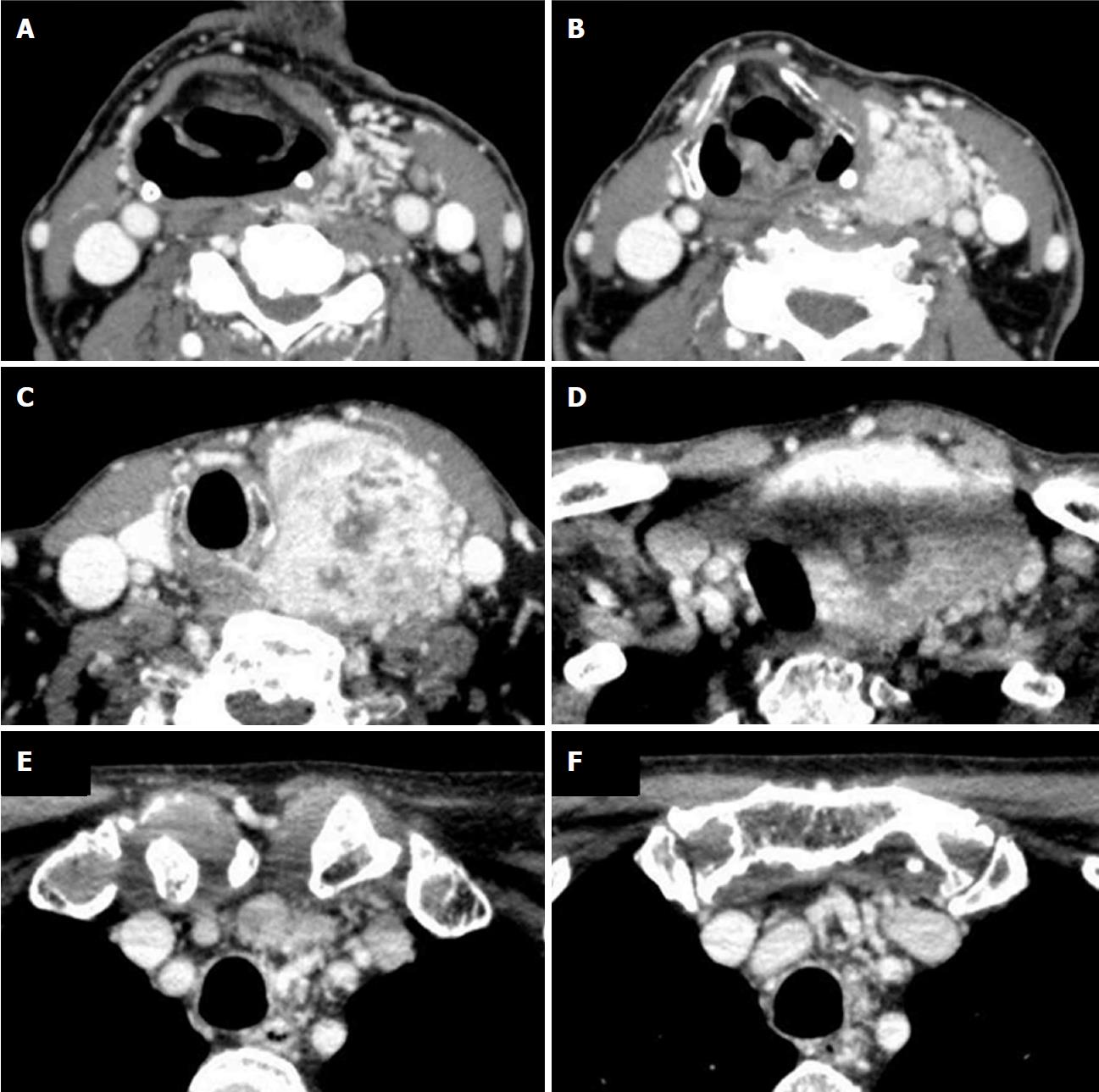
The patient was examined with contrast-enhanced CT scan. The CT scan images four years after initial surgery showed a mass lesion measuring 6.6 cm × 5.8 cm × 9.2 cm in the left lobe of the thyroid gland with heterogeneous strong contrast enhancement and enriched vasculature around the left thyroid lobe (Figure 2). These findings were not apparent in the previous contrast-enhanced CT scans that were carried out for preoperative examination of RCC. It was difficult to diagnose whether the thyroid mass lesion was benign or malignant from the radiological findings.
Although fine-needle aspiration cytology was performed, the materials were insufficient and only a small amount of blood cells were found in the cytological specimens. The patient underwent left hemithyroidectomy under a clinical diagnosis of thyroid follicular neoplasm. Intraoperatively, the thyroid gland was extremely highly hemorrhagic, although the tumor was not exposed to the exterior of the thyroid gland. The left lobe was fixed on the deep cervical fascia by a tumor vessel derived from the inferior thyroid artery and measuring approximately 5 mm in diameter. The perioperative bleeding was almost 3000 mL and the operative time exceeded 7 h. The patient manifested hoarseness due to left recurrent nerve paralysis after the surgery.
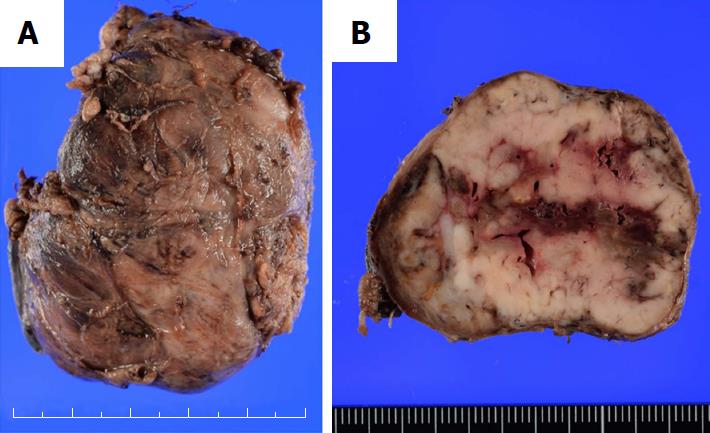
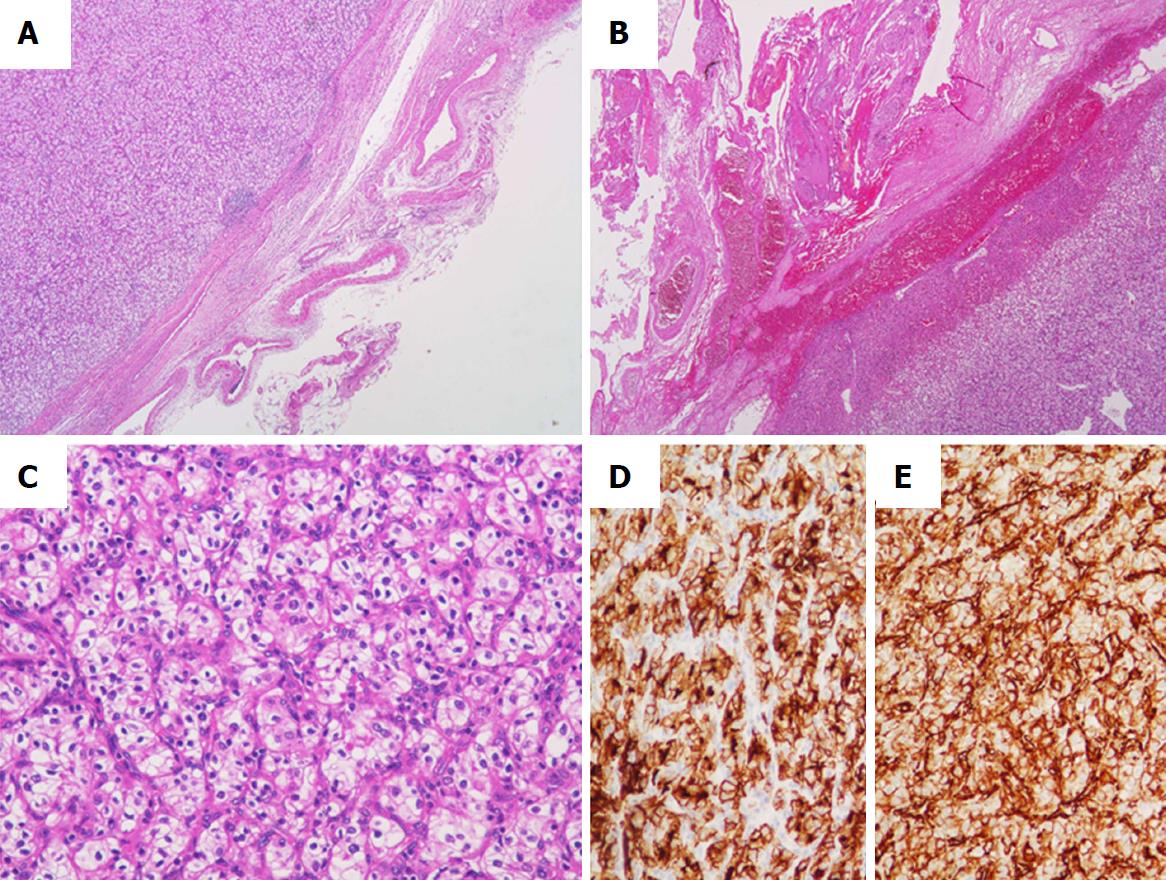
In pathological examination of the resected specimens, the left lobe of the thyroid was markedly enlarged, measuring 6.5 cm × 5.0 cm (Figure 3A). The cut surface of the resected specimen showed a whitish and partially hemorrhagic solid tumor (Figure 3B). Histologically, many markedly developed blood vessels were found at the surface of the resected thyroid (Figure 4A). Some of these abnormal vessels showed signs of bleeding (Figure 4B). The tumor was composed of atypical cells with clear or eosinophilic cytoplasm, suggesting metastasis of the clear cell RCC (Figure 4C). In immunohistochemical analysis, the tumor cells were diffusely positive for CD10 and vimentin (Figure 4D and E). From these findings, a pathological diagnosis of thyroid metastasis from clear cell RCC was finally made.
One month after the hemithyroidectomy, CT scans revealed a small nodule in the left lung which had increased in size compared to the previous examination. The nodule was clinically diagnosed as lung metastasis from RCC, and targeted molecular therapy (TMT) with sunitinib was initiated. After 5 mo of the sunitinib therapy, the lung nodule had regressed in the CT scan examination. The patient is currently free of disease at 28 mo after the surgery for thyroid metastasis.
RCC is a common malignancy that comprises 3% of adult cancers. It has been reported that nearly 20% to 30% of RCC patients have a metastatic lesion at the time of initial diagnosis and 20% to 30% of patients undergoing nephrectomy for localized RCC develop metastatic disease[5]. Common metastatic sites of RCC are the lungs (45.2%), bone (29.5%), lymph nodes (21.8%), liver (20.3%), adrenal gland (8.9%) and brain (8.1%)[6]. Metastasis of RCC to the thyroid gland is quite rare. The mean time from diagnosis of primary tumor to metastasis to thyroid is considerably long, ranging from 106 to 113 mo[2,7].
Although thyroid metastasis from RCC has some characteristic US findings, such as oval-shaped hypoechoic solid nodules with well-defined smooth margins, no calcifications, prominent chaotic intra-tumoral vascularity and tumor thrombus, these findings are not specific to this disease[8,9]. Thus, it is necessary to perform fine-needle aspiration cytology (FNAC) and to obtain information on the previous history of RCC. When a previous history of RCC is recognized, cytological findings and immunocytochemistry on FNAC-obtained material would be helpful for preoperative diagnosis[10]. However, it is reported that an RCC metastasis was correctly suspected in only 21 of 37 cases (57%) by preoperative FNAC[11]. Thus, a clinician should keep in mind the possibility of metastatic disease to the thyroid gland even when FNAC is negative or inconclusive.
The prognosis for patients with metastatic RCC is generally poor, with a 2-year survival of 10% to 20%[12,13]. Gravis et al[5] reported that the presence of at least one glandular metastatic site (pancreas, breast, parotid, thyroid, or contralateral adrenal gland) in the development of metastatic RCC has been associated with a significantly longer overall survival among patients with metastatic RCC, and thus patients with metastatic RCC with glandular metastases should receive more aggressive treatment with a potential for long-term survival.
Although recent advances of TMTs have improved the progression-free survival of RCC[14], cytoreductive surgery still plays an important role in the management of patients with advanced disease[15]. Cytoreductive nephrectomy (CN) refers to radical nephrectomy as a treatment option in metastatic RCC prior to immunotherapy (IT) or TMT. Evidence of the efficacy of CN has been provided by two large randomized controlled trials, which showed a survival benefit and delayed time-to-progression in patients who underwent CN followed by IT compared to patients with IT alone[16]. Not only the surgical resection of the primary tumor, but that of the metastatic foci prior to the IT or TMT is associated with prolongation of survival when technically feasible[17]. Therefore, metastatic RCC of the thyroid should be resected unless the patients have disseminated metastases or cannot tolerate surgery under general anesthesia.
Thyroid surgery is usually safe, with almost 0% mortality and a low complication rate[18]. Although total thyroidectomy involves a potential risk of serious complications, such as cervical hematoma followed by airway compromise requiring urgent surgical treatment, bilateral recurrent laryngeal nerve injury, and hypoparathyroidism, hemithyroidectomy can be performed safely for most patients. In the present case, massive bleeding from the tumor due to markedly developed tumor vessels made the operation very difficult, even though hemithyroidectomy is usually a safe and straightforward procedure. Based on our experience, we consider that resection of thyroid metastasis from RCC should be performed as early as possible. If the metastatic tumor involves adjacent cervical structures (e.g., internal jugular vein invasion, recurrent laryngeal nerve invasion and involvement of cervical lymph nodes) which is a strong adverse prognostic factor, extensive surgery should be embedded in a systemic treatment concept[11,19].
The reasons for the delay of the surgery in the present case were as follows: first, we prioritized the therapy for brain metastasis over the long-term prognosis of the patient; second, we could not properly diagnose the thyroid mass lesion before operation. As a result, the tumor grew and developed a strong feeding vasculature.
In conclusion, we have presented our didactic surgical experience of a case of thyroid metastasis from RCC which caused massive intraoperative bleeding. Based on our experience, we recommend that resection be performed early whenever possible, even if a patient with thyroid metastasis is asymptomatic and has other metastatic sites at the time of diagnosis, unless the patient has appropriate reasons to avoid surgery. Finally, in order to determine the appropriate timing for surgery, a clinician should take into consideration the possibility of thyroid metastasis when a thyroid mass lesion is found in patients with RCC or in patients having a previous history of RCC.
We experienced a case of thyroid metastasis from RCC which caused massive intraoperative bleeding: (1) The possibility of thyroid metastasis should be taken into consideration when a thyroid mass lesion is found in patients with a history of RCC; and (2) The resection of thyroid metastasis of RCC should be performed as early as possible, even if the patient is asymptomatic and has other metastatic sites, unless there are appropriate reasons to avoid surgery.
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cheungpasitporn W, Ekpenyong CEE S- Editor: Ji FF L- Editor: A E- Editor: Bian YN
| 1. | Chung AY, Tran TB, Brumund KT, Weisman RA, Bouvet M. Metastases to the thyroid: a review of the literature from the last decade. Thyroid. 2012;22:258-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 2. | Nakhjavani MK, Gharib H, Goellner JR, van Heerden JA. Metastasis to the thyroid gland. A report of 43 cases. Cancer. 1997;79:574-578. [PubMed] |
| 3. | Jackson G, Fino N, Bitting RL. Clinical Characteristics of Patients With Renal Cell Carcinoma and Metastasis to the Thyroid Gland. Clin Med Insights Oncol. 2017;11:1179554917743981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | De Stefano R, Carluccio R, Zanni E, Marchiori D, Cicchetti G, Bertaccini A, Sensi L, Pedrini L, Martorana G, Marlia E. Management of thyroid nodules as secondary involvement of renal cell carcinoma: case report and literature review. Anticancer Res. 2009;29:473-476. [PubMed] |
| 5. | Gravis G, Chanez B, Derosa L, Beuselinck B, Barthelemy P, Laguerre B, Brachet PE, Joly F, Escudier B, Harrison DJ. Effect of glandular metastases on overall survival of patients with metastatic clear cell renal cell carcinoma in the antiangiogenic therapy era. Urol Oncol. 2016;34:167.e17-167.e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh QD, Briganti A, Tian Z, Schmitges J, Graefen M, Perrotte P. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 481] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 7. | Hegerova L, Griebeler ML, Reynolds JP, Henry MR, Gharib H. Metastasis to the thyroid gland: report of a large series from the Mayo Clinic. Am J Clin Oncol. 2015;38:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Song OK, Koo JS, Kwak JY, Moon HJ, Yoon JH, Kim EK. Metastatic renal cell carcinoma in the thyroid gland: ultrasonographic features and the diagnostic role of core needle biopsy. Ultrasonography. 2017;36:252-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Kobayashi K, Hirokawa M, Yabuta T, Fukushima M, Masuoka H, Higashiyama T, Kihara M, Ito Y, Miya A, Amino N. Metastatic carcinoma to the thyroid gland from renal cell carcinoma: role of ultrasonography in preoperative diagnosis. Thyroid Res. 2015;8:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Rizzo M, Rossi RT, Bonaffini O, Scisca C, Sindoni A, Altavilla G, Benvenga S. Thyroid metastasis of clear cell renal carcinoma: Report of a case. Diagn Cytopathol. 2009;37:759-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Iesalnieks I, Machens A, Bures C, Krenz D, Winter H, Vorländer C, Bareck E, Alesina PF, Musholt T, Steinmüller T. Local recurrence in the neck and survival after thyroidectomy for metastatic renal cell carcinoma. Ann Surg Oncol. 2015;22:1798-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Vogl UM, Zehetgruber H, Dominkus M, Hejna M, Zielinski CC, Haitel A, Schmidinger M. Prognostic factors in metastatic renal cell carcinoma: metastasectomy as independent prognostic variable. Br J Cancer. 2006;95:691-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Motzer RJ, Bacik J, Schwartz LH, Reuter V, Russo P, Marion S, Mazumdar M. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 601] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 14. | Bedke J, Gauler T, Grünwald V, Hegele A, Herrmann E, Hinz S, Janssen J, Schmitz S, Schostak M, Tesch H. Systemic therapy in metastatic renal cell carcinoma. World J Urol. 2017;35:179-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 15. | Thomas AZ, Adibi M, Borregales LD, Karam JA, Wood CG. Cytoreductive surgery in the era of targeted molecular therapy. Transl Androl Urol. 2015;4:301-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Abel EJ, Wood CG. Cytoreductive nephrectomy for metastatic RCC in the era of targeted therapy. Nat Rev Urol. 2009;6:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Dabestani S, Marconi L, Hofmann F, Stewart F, Lam TB, Canfield SE, Staehler M, Powles T, Ljungberg B, Bex A. Local treatments for metastases of renal cell carcinoma: a systematic review. Lancet Oncol. 2014;15:e549-e561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 247] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 18. | Fortuny JV, Guigard S, Karenovics W, Triponez F. Surgery of the thyroid: recent developments and perspective. Swiss Med Wkly. 2015;145:w14144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Machens A, Dralle H. Outcome after thyroid surgery for metastasis from renal cell cancer. Surgery. 2010;147:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |