Published online Dec 6, 2018. doi: 10.12998/wjcc.v6.i15.1007
Peer-review started: September 19, 2018
First decision: October 4, 2018
Revised: November 8, 2018
Accepted: November 14, 2018
Article in press: November 15, 2018
Published online: December 6, 2018
Processing time: 78 Days and 20.9 Hours
Gangrenous cholecystitis (GC) is a severe and potentially deadly complication of acute cholecystitis. We present a 83-year-old gentleman with a past medical history of type 2 diabetes mellitus with significant associated neuropathy, presenting to a community hospital in a major metropolitan area with 10 days nausea and vomiting and a benign abdominal exam. While the patient was admitted for hyperglycemia, he was subsequently found to have severe GC requiring urgent surgical intervention.
Core tip: We present an 83-year-old gentleman with type 2 diabetes mellitus and associated neuropathy who was found to have severe gangrenous cholecystitis (GC) requiring urgent surgical intervention but without any of the cardinal symptoms of GC.
- Citation: Mehrzad M, Jehle CC, Roussel LO, Mehrzad R. Gangrenous cholecystitis: A silent but potential fatal disease in patients with diabetic neuropathy. A case report. World J Clin Cases 2018; 6(15): 1007-1011
- URL: https://www.wjgnet.com/2307-8960/full/v6/i15/1007.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i15.1007
Diabetes mellitus (DM) is a chronic disease caused by deficiency in production of insulin by the pancreas, or by of the insulin produced. This subsequently leads to an increased concentrations of glucose in the blood[1]. The prevalence of DM has markedly increased over the past few decades with nearly 300 million people worldwide with the disease today[2]. DM is itself a specific group of metabolic disorders that share common phenotype of hyperglycemia. The two broad categories, or types, of DM are classified by the underlying pathogenic process leading to elevated blood sugar levels. Type 1 DM exhibits almost complete insulin deficiency, while type 2 DM is an intricate constellation of pathology including insulin resistance, increased glucose production and impaired insulin secretion[3]. Important complications from DM include cardiovascular disease, stroke, diabetes retinopathy, renal failure and neuropathy[4].
Gangrenous cholecystitis (GC) is a severe and potentially deadly progression of acute cholecystitis that occurs in up to 30% of cases[5]. It is the end result of persistent and severe inflammation, where there is such significant distension of the gallbladder that the wall becomes ischemic[6]. Risk factors include male gender, age > 45 years, history of diabetes and heart disease[7]. Although clinical signs of peritonitis are sometimes absent, these patients typically present with at least one or more of the following symptoms: right upper quadrant abdominal pain, loss of appetite, jaundice, and/or fever[8]. These patients almost universally undergo emergent cholecystectomy to avoid fatal complications[6].
Diabetic neuropathy is among the most common complications of DM, and is clinically important because it can diminish symptoms of life-threatening such as myocardial ischemia[9]. We present an 83-year-old gentleman who was admitted for hyperglycemia but was later found to have a severe GC without an exam findings concerning for peritonitis. To our knowledge, this is the fourth case ever reported of GC in a patient with DM presenting with non-specific abdominal symptoms and reassuring physical exam[10-12].
An 83-year-old male with a 20 year history of type 2 DM, with advanced diabetic neuropathy, hypertension and hyperlipidemia presented to his primary care physician’s office with a chief complaint of nausea, vomiting, and diarrhea for 10 d. Point of care glucose monitor showed the patient’s blood sugar to be > 600 mg/dL, and the patient was transferred to the emergency department (ED) of the associated community hospital.
The patient attributed the nausea and vomiting to a beef sandwich he ate four days preceding the symptoms. He had not been able tolerate food and reported very limited fluid intake. Additionally, the patient noted three loose brown bowel movements daily without gross blood or melena which resolved three days prior to admission. The patient denied abdominal pain, hematemesis, chest pain, shortness of breath or any other associated symptoms. A complete review of systems was otherwise negative.
The patient had a strong family history of both type 1 and 2 DM including his mother, two brothers, and other distant relatives. The patient’s social history was significant for occasional alcohol use of 2-3 beers per month. The patient denied tobacco and illicit drug use. The patient’s home medications were Pravastatin 20 mg PO daily, Glyburide 1.25 mg PO daily, Lisinopril 10 mg PO daily, and Omeprazole 20 mg PO daily.
In the ED his vital signs were a temperature of 98.2 F, heart rate of 89/min, respiratory rate of 18/min, blood pressure 147/79 mmHg, and O2 Saturation by pulse oximetry 93% on room air. Laboratory results were significant for a white blood count (WBC) of 16.5 k/uL, Sodium 132 mmol/L, Chloride 93 mmol/L, Glucose 598 mg/dL, AST 49 U/L, Alkaline Phosphatase 196 U/L, Albumin 3.1 g/dL, Albumin/Globulin Ratio 0.6 L. Other labs were within normal limits, and urinalysis was negative for ketones.
Physical examination was documented as normal. The patient was alert and oriented to person, time and place. Respiratory exam was clear to auscultation bilaterally. Cardiovascular exam revealed regular rate and rhythm, with no murmurs, rubs or gallop. Abdomen was soft, non-tender, non-distended, with normal bowel sounds, and no organomegaly. Neurological exam showed intact cranial nerves 2-12, reflexes were 2+ and symmetric at the biceps, triceps, knees, and ankles. Plantar responses for flexor, light touch, pinprick, position sense, and vibration sense was impaired in fingers and toes, Rapid alternating movements and fine finger movements are intact. There was no dysmetria on finger-to-nose and heel-knee-shin. There were no abnormal or extraneous movements. Romberg was absent. The skin was warm, dry, with no identified rashes. Imaging in the ED was limited to a plain film of the chest which demonstrated atelectasis vs scarring in the lateral left base, without infiltrates.
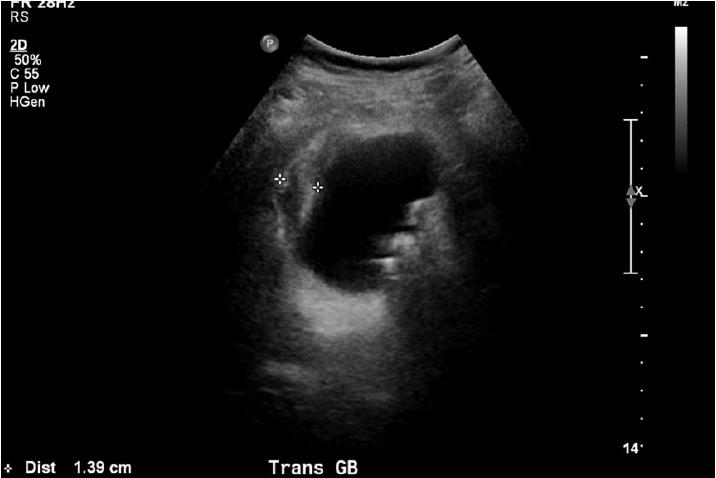
The patient was admitted to the general medicine ward for treatment of hyperglycemia. He was initially given 0.2 units/kg of Lantus once, and then put on insulin sliding scale with blood glucose measurements every 6 h. A second abdominal examination was performed several hours after admission because of an elevated alkaline phosphatase, without any concerning findings. Additionally, an abdominal ultrasound was performed which exhibited hepatomegaly with the liver measuring to 18 cm in length. While no hepatic biliary tract obstruction was noted on ultrasound, his gallbladder was found to have a thickened wall, with pericholecystic fluid and multiple hyper-echoic calculi (Figure 1). These findings were strongly concerning for acute cholecystitis.
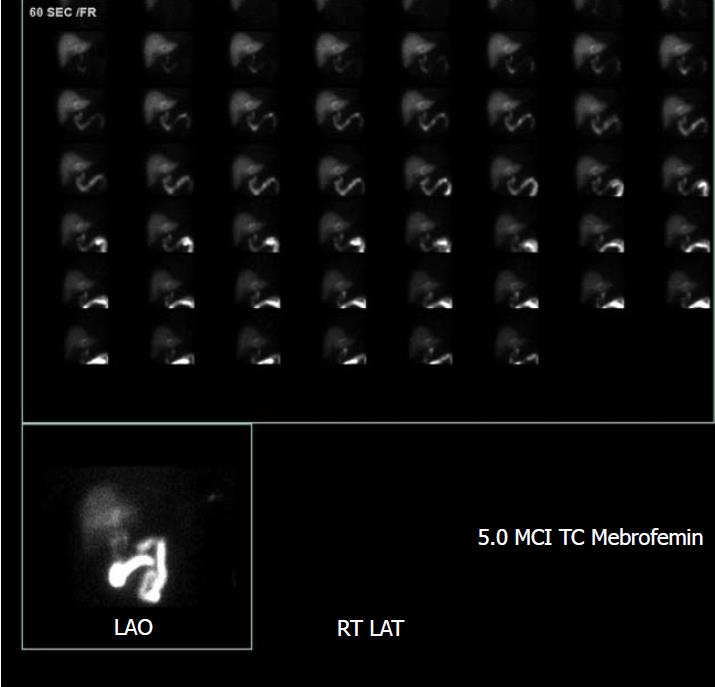
The patient was started on 3.375 g of Piperacillin/Tazobactam, every 6 h. The patient’s condition improved overall by hospital day two. He denied nausea, vomiting and abdominal pain. Given his reassuring vials, exam and symptomatology in the face of an abnormal ultrasound, a HIDA scan was performed which also suggested acute cholecystitis (Figure 2).
Soon after, the patient developed a low-grade fever of 100.4 F, with chills and rigors. A set of blood cultures was obtained, and general surgery was consulted. They recommended continuing IV antibiotics and preparation for surgery. Demerol (Meperidine) and Tylenol (acetaminophen) were added for fever and pain.
The patient thereafter underwent a laparoscopic cholecystectomy. The findings were significant for a severly gangrenous gallbladder, with copious purulent drainage from gallbladder, subhepatic abscess, and cholelithiasis, and this was confirmed in post-operative histopathology. The patient’s abdomen was irrigated copiously during the procedure. There were no perioperative complications.
On the next hospital day, the patient was feeling well without complaints including no abdominal pain, fevers, rigors or chills. Abdominal examination continued to be benign, and the surgical wound was well approximated. WBC trended down to normal limits. Alkaline phosphatase had down trended from 196 U/L on admission to as low as 127 U/L post-operatively. The patient’s diet was advanced to regular diabetic diet, which he tolerated well, and he was later discharged home.
The hyperglycemia seen in this patient was likely multifactorial, due to chronically poor compliance with diabetic medications and diet as well as acute infection. The patient’s HbA1c was 10.5% during this hospitalization and an endocrinologist was consulted who started the patient on an insulin regimen on discharge with regular follow ups.
Gangrenous cholecystitis.
Cholecystectomy.
Clinic follow up.
This case illustrates the sometimes difficult nature of identifying underlying diseases in patients with severe diabetes and associated complications. This patient had an elevated Alkaline Phosphatase and a mild leukocytosis in the setting of nausea and vomiting. This prompted further investigation in the form of medical imaging despite benign abdominal exam ultimately leading to diagnosis of acute cholecystitis with gangrenous gallbladder. This joins the ranks of three similar cases which have been reported in the literature, all with a history of DM[10-12].
Fagan et al[13] asserted that several variables, including WBC > 15000, history of diabetes, African American race, abnormal liver function tests (elevated ALT, AST, ALP, lipase levels) and pericholecystic fluid were associated with GC. An additional study found after multivariate analysis that in patients with cholecystitis those with history of diabetes and leukocytosis were significantly more likely to have a gangrenous gallbladder. Finally, Contini et al[7] have implicated that WBC count was the strongest predictor for presence of gangrene. The patient reported appears to support these findings given his history of diabetes as well as leukocytosis and elevated alkaline phosphatase on presentation.
It is important to recognize the natural history and life-threatening complications of GC. A gangrenous gallbladder almost invariably goes on to perforate and once gangrenous, the overall complication rate approaches 25%. Perforation can lead to abscess formation and peritonitis. Ultimately, mortality rates in GC are reported to be as high as 22%[7,14]. Accordingly, early diagnosis and surgical treatment is critical to mitigate serious complications including mortality in these patients[7,14].
The reason for the insidious nature of this patient’s presentation was presumably driven by his neuropathy. Neuropathy leads to a host of consequences including intrabdominal manifestations such as impaired gastric motility and inability to appreciate abdominal pain. While not fully understood, diabetics exhibit complex changes of metabolic, vascular and hormonal factors which both increase nerve fiber damage and diminish the body’s ability to repair these nerves[15,16]. Nerve damage from ischemia is thought to contribute to the development of diabetic neuropathy. In autopsies of patients with diabetic neuropathy thickened endoneuronal blood vessel walls and vascular occlusions are commonly found[17]. This is also supported by decreased endoneurinal oxygen tension measured in sural nerves of the patients with advanced polyneuropathy[18].
Impaired neuronal repair is also thought to contribute to diabetic neuropathy[19,20]. This may be due in part to a decrease of neurotrophic peptides which mediate nerve maintenance, repair and regeneration. Specifically, nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3, insulin-like growth factors, and vascular endothelial growth factor have all found to contribute to nerve health[20]. Moreover, insulin itself plays an important role as a neurotrophic factor to peripheral neurons; and the low insulin state present in type 1 diabetes may be particularly detrimental to overall nerve health[15,21].
In conclusion, this case illustrates the insidious presentation of a life-threatening surgical disease in a patient admitted for medical management of hyperglycemia. Gangrenous gallbladder is a serious complication of acute cholecystitis which was largely masked by a benign abdominal exam in a patient with severe diabetic neuropathy. This is similar to diabetic patients presenting with “silent” acute coronary syndrome. In conclusion, it is important that in patients with a history of poorly controlled diabetes, a higher index of suspicion should be raised for severe intraabdominal pathology even in the setting of a benign abdominal exam.
GC is a severe and potentially life-threatening complication of acute cholecystitis that occurs in up to 30% of cases. Risk factors of GC include male gender, age > 45 years, history of diabetes and heart disease. GC can be asymptomatic in patients with DM. Patients with leukocytosis and/or abnormal liver function tests with a history of DM should be considered for further work up, regardless of symptoms or physical examination.
CARE Checklist (2013) statement: The guidelines of the CARE Checklist (2013) have been adopted.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Avtanski D, Sahoo J, Saisho Y S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ
| 1. | Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease. Philadelphia, Pa: Saunders 2005; 1194-1195. |
| 2. | Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med. 2006;12:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 389] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 3. | Powers AC. Harrison’s Principles of Internal medicine. 18th ed. Chapter 344-Classification. New York: McGraw Hill 2012; . |
| 4. | Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, Atlanta, GA, 2011. Available from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. |
| 5. | Bingener J, Stefanidis D, Richards ML, Schwesinger WH, Sirinek KR. Early conversion for gangrenous cholecystitis: impact on outcome. Surg Endosc. 2005;19:1139-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 6. | Bennett GL, Rusinek H, Lisi V, Israel GM, Krinsky GA, Slywotzky CM, Megibow A. CT findings in acute gangrenous cholecystitis. AJR Am J Roentgenol. 2002;178:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 120] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 7. | Contini S, Corradi D, Busi N, Alessandri L, Pezzarossa A, Scarpignato C. Can gangrenous cholecystitis be prevented?: a plea against a “wait and see” attitude. J Clin Gastroenterol. 2004;38:710-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341:1906-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 708] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 9. | Chico A, Tomás A, Novials A. Silent myocardial ischemia is associated with autonomic neuropathy and other cardiovascular risk factors in type 1 and type 2 diabetic subjects, especially in those with microalbuminuria. Endocrine. 2005;27:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Chaudhry S, Hussain R, Rajasundaram R, Corless D. Gangrenous cholecystitis in an asymptomatic patient found during an elective laparoscopic cholecystectomy: a case report. J Med Case Rep. 2011;5:199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Nidimusili AJ, Alraies MC, Eisa N, Alraiyes AH, Shaheen K. Leukocytosis of unknown origin: gangrenous cholecystitis. Case Rep Med. 2013;2013:418014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Grant RL, Tie ML. False negative biliary scintigraphy in gangrenous cholecystitis. Australas Radiol. 2002;46:73-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Fagan SP, Awad SS, Rahwan K, Hira K, Aoki N, Itani KM, Berger DH. Prognostic factors for the development of gangrenous cholecystitis. Am J Surg. 2003;186:481-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Yamashita Y, Noritomi T, Matsuoka N, Sinya T, Sugi Y, Higa K, Kusumoto G, Nitahara K. [Surgical treatment of acute cholecystitis]. Masui. 2012;61:944-950; discussion 951-952. [PubMed] |
| 15. | Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: mechanisms to management. Pharmacol Ther. 2008;120:1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 498] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 16. | Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11:521-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 730] [Article Influence: 56.2] [Reference Citation Analysis (1)] |
| 17. | Feldman EL. Pathogenesis and prevention of diabetic polyneuropathy. Available from: https://www.uptodate.com/contents/pathogenesis-and-prevention-of-diabetic-polyneuropathy/print. |
| 18. | Newrick PG, Wilson AJ, Jakubowski J, Boulton AJ, Ward JD. Sural nerve oxygen tension in diabetes. Br Med J (Clin Res Ed). 1986;293:1053-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 193] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Kennedy JM, Zochodne DW. The regenerative deficit of peripheral nerves in experimental diabetes: its extent, timing and possible mechanisms. Brain. 2000;123:2118-2129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Kennedy JM, Zochodne DW. Impaired peripheral nerve regeneration in diabetes mellitus. J Peripher Nerv Syst. 2005;10:144-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 21. | Brussee V, Cunningham FA, Zochodne DW. Direct insulin signaling of neurons reverses diabetic neuropathy. Diabetes. 2004;53:1824-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |