Published online Nov 26, 2018. doi: 10.12998/wjcc.v6.i14.862
Peer-review started: September 5, 2018
First decision: October 4, 2018
Revised: October 15, 2018
Accepted: October 17, 2018
Article in press: October 16, 2018
Published online: November 26, 2018
Processing time: 83 Days and 1.2 Hours
Vasoactive intestinal peptide-producing tumors (VIPoma) usually originate in the pancreas and are characterized by diarrhea, hypokalemia, and achlorhydria (WDHA syndrome). In adults, nonpancreatic VIPoma is very rare. Herein, we report an unusual case of VIP-producing pheochromocytoma marked by persistent shock, flushing, and watery diarrhea and high sensitivity to octreotide. A 53-year-old woman was hospitalized for sudden-onset hypertension with convulsions, which then rapidly evolved to persistent shock, flushing, and watery diarrhea. Abdominal computed tomography indicated a left adrenal mass, accompanied by bleeding; and marked elevations of both plasma catecholamine and VIP concentrations were documented via laboratory testing. Surprisingly, all clinical symptoms responded swiftly to octreotide treatment. Once surgically treated, hormonal levels normalized in this patient, and the clinical symptoms dissipated. Postoperative pathological and immunohistopathological studies confirmed a VIP-secreting pheochromocytoma with strong, diffuse positivity for somatostatin receptor type 2. During a 6-mo follow-up period, she seemed in good health and was symptom-free.
Core tip: Vasoactive intestinal peptide-producing tumors (VIPoma) usually originate in the pancreas. VIP-secreting pheochromocytoma is very rare and most of the related cases reported are characterized by diarrhea, hypokalemia, and gastric acid deficiency (WDHA syndrome). To our knowledge, this is the first reported instance of VIP-secreting pheochromocytoma marked by persistent shock flushing and diarrhea and high sensitivity to octreotide. This case helps to improve the understanding of the pathogenesis, biology, and behavior of VIPoma and pheochromocytoma.
- Citation: Hu X, Cao W, Zhao M. Octreotide reverses shock due to vasoactive intestinal peptide-secreting adrenal pheochromocytoma: A case report and review of literature. World J Clin Cases 2018; 6(14): 862-868
- URL: https://www.wjgnet.com/2307-8960/full/v6/i14/862.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i14.862
Vasoactive intestinal peptide-producing tumor (VIPoma) is an unusual neuroendocrine tumor that autonomously secretes VIP. In adult, almost all VIPomas (90%) originate from pancreatic tissues whereas the remaining 10% originate from extra-pancreatic tissues, such as the bronchus, colon, liver, and pheochromocytoma[1]. VIP-secreting pheochromocytoma is extremely rare and most of the related cases reported are characterized by diarrhea, hypokalemia, and gastric acid deficiency (WDHA syndrome)[2-4]. In this case, the patient harbored an exceedingly rare adrenal pheochromocytoma, which ultimately ruptured and bled. Its principal manifestations included persistent shock, flushing, and watery diarrhea in the aftermath of sudden-onset hypertension with convulsions. Laboratory diagnostics and immunohistochemical attributes indicated that this pheochromocytoma secreted both catecholamines (CATs) and VIP.
A 53-year-old woman was admitted to the local hospital after 1 d of convulsions leading to loss of consciousness. After the attack (approximately 20 min), the patient eventually became conscious. Six months prior, she reported having paroxysmal palpitation attacks (5-20 min each), which spontaneously subsided, and had suffered occasional headaches, without sweating or chest pain. Consequently, medical attention was never sought.
Computed tomography (CT) of the head showed no abnormalities; but this historically normotensive patient produced a blood pressure (BP) reading of 230/100 mmHg. Nicardipine hydrochloride (3 μg/kg per min; Astellas Pharma Tech Co, Ltd, Tokyo, Japan) was administered for BP control, which was achieved approximately 8 h later (160/90 mmHg), and the convulsions disappeared. However, treatment was withdrawn without rebound effect following a sudden drop in BP (nadir: 53/35 mmHg). She was then transferred to our facility.
At the time of admission, the patient was somnolent but could be aroused and appeared dispirited, evoking a Glasgow coma score of 9 (E, 2; M, 4; V, 3). She presented with watery diarrhea > 10 times/d (total volume, 800-1200 mL/24 h). At a height of 162 cm and a body weight of 82 kg, her baseline vital signs were as follows: BP, 68/44 mmHg; heart rate, 92 bpm; respiratory rate, 18 bpm; and temperature, 36.8 °C. There was flushing of the face and neck (Figure 1) and sensitivity to percussion in the region of the left kidney. Thyromegaly, rales (neither lung by auscultation), cardiac murmurs, abdominal tenderness, and palpable lumps were not observed.
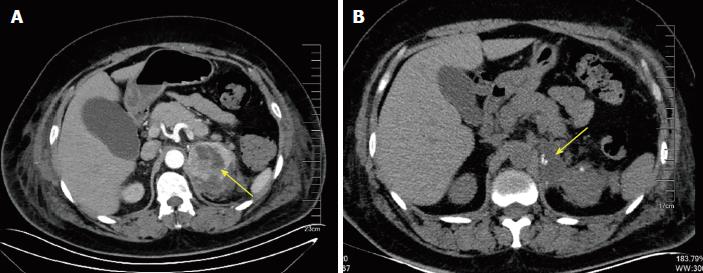
Laboratory tests showed that hematocrit and hemoglobin were within standard reference ranges and did not deviate significantly in several repeat attempts. Other results were as follows: glucose, 17.2 mmol/L (3.9-6.1); creatinine, 289.9 μmol/L (45-84); troponin I (TnI), 0.14 ng/mL (0.010-0.023); creatine kinase (CK), 230 ng/L (45-145); and CK-MB, 15 ng/L (2.0-7.2). A battery of biochemical tests, including liver function studies, blood gas analysis, blood coagulation indices, and electrolyte (K, Na, Cl, Ca, P, and Mg) levels, returned essentially normal results. The electrocardiogram showed T-wave inversion and slight ST-segment depression (0.1-0.2 mv) in leads V1-V6, II, III, and AVF. Coronary arteriography confirmed no coronary artery obstruction. By ultrasonic cardiography (UCG), the following parameters were determined: interventricular septal thickness, 10-12 mm; width of posterior left ventricular wall, 10 mm; left ventricular end-diastolic (156 mL) and end-systolic (69 mL) volumes; stroke volume, 87 mL; and ejection fraction, 56%. CT studies of the patient’s head and chest were not abnormal, but on the enhanced abdominal CT, a solitary mass of the left adrenal gland was identified, with signs of bleeding (Figure 2). In addition, blood and urinary CAT concentrations and urinary vanillylmandelic acid were significantly elevated (Table 1).
| Day 2 | Day 5 | Post-operation | Reference range | |
| Plasma | ||||
| Epinephrine (pg/mL) | 547 | 624 | 35 | < 130 |
| Norepinephrine (pg/mL) | 1683 | 1662 | 376 | 150–520 |
| Dopamine (pg/mL) | 214982 | 345 | 14 | < 30 |
| Vasoactive intestinal peptide (pg/mL) | 377 | 126 | < 10 | < 100 |
| Urine | ||||
| Metanephrine (mg/24 h) | 2.4 | 2.3 | 0.12 | 0.04–0.19 |
| Normetanephrine (mg/24 h) | 3.2 | 3.4 | 0.21 | 0.09–0.37 |
| Vanillylmandelic acid (mg/24 h) | 53.8 | 47.5 | 2.80 | 1.4–6.5 |
Treatment included copious intravenous fluid replacement (0.9% NaCl, 4000 mL/24 h), with potassium supplementation (KCl, 3-6 g/24 h), and an intravenous dopamine drip (12 μg/kg per min; Shanghai Fenge Pharmaceutical Co, Ltd, Shanghai, China) was initiated. The patient’s BP increased slightly in response, with systolic pressures still fluctuating from 80-100 mmHg. There was no mental improvement or resolution of facial and neck flushing, and despite complete solid/liquid fasting, the diarrhea persisted. Besides, we administered the patient with continuous intravenous insulin to keep the blood glucose around 10 mmol/L. We rechecked CK, CK-MB, TnI, and electrocardiogram every 6 h. The patient’s myocardial enzymes and TnI levels gradually returned to normal, and the ischemic manifestations on electrocardiogram were also significantly improved. This symptomology was not typical of pheochromocytoma. Given the array of hormones implicated in neuroendocrine tumors, significant elevation of plasma VIP (Table 1) was subsequently verified through additional diagnostics, whereas other substances [plasma pancreatic polypeptide, adrenocorticotropic hormone, somatostatin (SST), thyroid hormones, parathyroid hormone, calcitonin, adrenomedullin, and urine 5-hydroxy indoleacetic acid] remained normal.
To control diarrhea and facial flushing, the patient received intramuscular injections of octreotide (0.1 mg/8 h; Novartis International AG, Basel, Switzerland) on day 3 after admission, followed by a surprisingly rapid rise in BP. After 24 h, the dopamine drip was discontinued, and her BP had reached 123/75 mmHg. The patient’s mental state also cleared significantly, and she was more alert; the facial and neck flushing was relieved; and diarrhea was less frequent. On day 5 after admission, she was completely conscious, her facial and neck skin had returned to normal, and the diarrhea had stopped. At this point, she developed paroxysmal hypertension (peak BP: 190/100 mmHg), for which oral terazosin hydrochloride (2 mg/d; Abbott Laboratories, Chicago, IL, United States) was given.
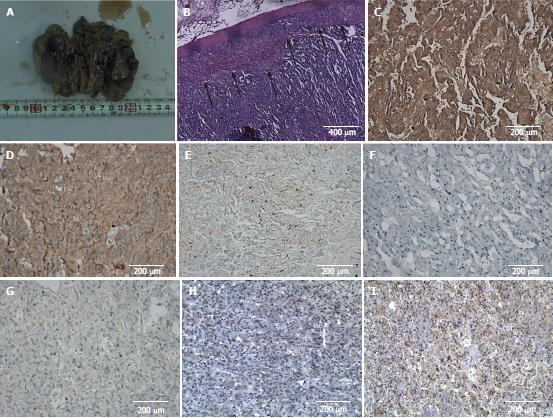
After 20 d, left laparoscopic adrenalectomy was performed. The mass of the left upper kidney and an encapsulated, posteriorly placed hematoma (due to rupture) were located. When dissecting this mass, the patient’s BP again climbed to 240/130 mmHg. No other abdominal tumors were discovered, and the right adrenal gland was morphologically normal. The resected mass measured 7 cm × 4.5 cm. On cut section, it was soft and yellow-brown, demonstrating two subcapsular clefts of 2-4 cm (Figure 3A). In histological sections, the tumor cells were nested or arranged in trabecular pattern, with variably sized, pleomorphic nuclei. Their cytoplasm was abundant, showing basophilic or amphophilic stippling. A compressed rim of normal adrenal cortex was retained at the tumor’s edge, and there was no obvious intervening septum (Figure 3B).
Immunohistochemical staining properties were as follows: Syn (+), CgA (+), S-100 (+), CK (-), and KI67 (< 5% +) (Figure 3C-G). On this basis, the histopathological diagnosis was adrenal pheochromocytoma. We then pursued immunohistochemical staining for VIP and SST receptor 2 (SSTR2), confirming 60%-70% cytoplasmic VIP positivity of pheochromocytoma cells. No positively stained ganglioneuroma component was evident (Figure 3H). Nearly all tumor cell membranes demonstrated SSTR2 positivity (Figure 3I).
After surgery, the patient discontinued octreotide and terazosin hydrochloride and recovered uneventfully from surgery. Her BP and heart rate returned to normal levels, as did various hormonal concentrations; and symptoms such as headache, palpitation, chest pain, facial flushing, and diarrhea were no longer problematic. During a 6-mo follow-up period, she seemed in good health and was symptom-free.
VIPoma syndrome of watery diarrhea, hypokalemia and achlorhydria was first described by Verner et al[5] in 1958, and has been considered to be due to excessive secretion of VIP. In adults, this syndrome is most commonly associated with pancreatic islet cell tumors, but is rarely caused by non-pancreatic tumors, such as bronchogenic carcinoma, medullary thyroid carcinoma, retroperitoneal histiocytoma, and adrenal pheochromocytoma[6]. In an investigation of 62 VIPoma patients, 52 (84%) had pancreatic tumors and 10 (16%) had ganglioneuroblastomas. Of the 10 patients with ganglioneuroblastomas, seven were children[7]. The first description of a nonpancreatic tumor producing VIP was a retroperitoneal ganglioneuroma reported by Fausa et al[8] in 1973. Loehry et al[9] first reported an WDHA syndrome caused by a pheochromocytoma in 1975. VIP is a 28-amino acid peptide that may stimulate the production of intestinal cyclic adenosine monophosphate, leading to massive intestinal secretion of water and electrolytes[6,7]. VIP also inhibits gastric acid secretion, promotes hepatic glycogenolysis and dilates peripheral systemic blood vessels. Clinical presentations of VIPoma commonly include secretory diarrhea, hypokalemia, hypochlorhydria, flushing, hyperglycemia, and metabolic acidosis[8]. Although this patient had obvious diarrhea, there was no apparent hypokalemia, which may be related to the relatively short onset time and electrolyte supplementation. Most patients with WDHA syndrome have suffered several months or years before seeking medical treatment, so their conditions are dire. Accordingly, we suspected that VIP was produced but only a small amount was secreted, as reported in the literature[9]. Rupture and bleeding may in fact have initiated a massive release of VIP into the circulation, igniting the patient’s progression of symptoms.
As we mentioned above, VIP is also a superactive vasodilatory substance capable of producing a generalized peripheral vascular effect[10,11]. Although published reports have yet to link hypotension or shock with VIP-producing pheochromocytomas, most of the affected patients show no hypertensive manifestations[12-14], implying that VIP may effectively antagonize vasoconstrictive CAT activity due to strong action of vasodilation. In this case, octreotide treatment not only significantly improved diarrhea and flushing symptoms, but also corrected situation of shock synchronously. However, octreotide had little impact on plasma CAT concentration, corroborating previous reports[15,16], which indicate that excessive VIP release may be the most important reason causing shock. On the other hand, the cardiotoxic effects of CATs are chronicled in a number of publications, having linked CAT excess with acute myocarditis and cardiogenic shock[17,18]. This is the most common cause of shock due to pheochromocytoma. In this case, however, UCG showed that left ventricular contractility was within normal range, which is different from most of patients with pheochromocytoma-induced shock. Although single echocardiography cannot completely exclude the possibility of cardiogenic shock, this result still indicates that cardiotoxic effects of CATs may not be the main cause of shock. Unfortunately, the patient refused invasive hemodynamic so that we cannot prove our presuming.
Octreotide is a synthetic long-acting SST analogue that effectively inhibits release of VIP from tumors and is approved by the FDA for treatment of WDHA[19]. Its biologic effects are exerted largely through binding with SSTRs on target cell membranes. SSTRs are G protein-coupled receptors of five subtypes (SSTR1-5). SSTR2 is expressed in > 80% of neuroendocrine tumors and is the subtype with the strongest affinity for octreotide[20]. Although some VIP-secreting pheochromocytomas are poorly responsive to octreotide due to lack of SSTR expression[4,12], this patient was promptly restored to near-normal VIP levels through such treatment, with substantial abatement of diarrhea and flushing. Immunohistochemistry later confirmed strong, diffuse SSTR2 positivity (Figure 3I) of tumor cells and this may be the reason why octreotide plays a magical role in the present case. Despite the significant efficacy of octreotide, the definitive treatment for VIPoma is surgery[21-25]. In the cases in which complete surgical resection is either unsuccessful or not feasible, octreotide is an important pharmacotherapeutic approach to controlling symptoms in VIPoma patients[26,27]. However, the long-term administration of octreotide may result in the development of resistance, sometimes extremely high doses of octreotide is necessary for continuous effects[28,29]. In patients with poor efficacy of somatostatin, interferon-α can be combined with octreotide to improve clinical symptoms and promote tumor regression[30,31].
In summary, we report an extremely rare case of VIP-secreting pheochromocytoma marked by persistent shock, flushing, and diarrhea and high sensitivity to octreotide. This case reminds us that the diversity of hormones secreted by neuroendocrine tumor gives rise to clinically complex patient scenarios and a sudden overdose of hormonal substances, when the tumor ruptures, may be fatal to the patient. Therefore, comprehensive hormone testing may be useful for early diagnosis and effective treatment, especially when the patient is in crisis due to unknown reasons.
In adults, vasoactive intestinal peptide-producing tumors (VIPoma) is most commonly originates from pancreatic islet cell tumors, but is rarely caused by pheochromocytoma. We report a VIP-secreting pheochromocytoma case marked by persistent shock, flushing, and diarrhea and high sensitivity to octreotide.
VIP-secreting pheochromocytoma.
Acute myocardial infarction and cardiogenic shock induced by pheochromocytoma.
Pheochromocytoma and VIPoma.
Left adrenal pheochromocytoma.
VIP-secreting pheochromocytoma.
Surgery after octreotide improved clinical symptoms.
It is the first reported instance of VIP-secreting pheochromocytoma marked by persistent shock flushing and diarrhea and high sensitivity to octreotide.
This case reminds us that the diversity of hormones secreted by neuroendocrine tumor gives rise to clinically complex patient scenarios, and a sudden overdose of hormonal substances, when the tumor ruptures, may be fatal to the patient. Comprehensive hormone testing may be useful for early diagnosis and effective treatment, especially when the patient is in crisis due to unknown reasons.
CARE Checklist (2013) statement: The manuscript was prepared and revised according to the CARE checklist.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ganea D, Rostoff P, Sahoo J S- Editor: Wang JL L- Editor: Wang TQ E- Editor: Song H
| 1. | Krejs GJ. VIPoma syndrome. Am J Med. 1987;82:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 67] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Smith SL, Slappy AL, Fox TP, Scolapio JS. Pheochromocytoma producing vasoactive intestinal peptide. Mayo Clin Proc. 2002;77:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Leibowitz-Amit R, Mete O, Asa SL, Ezzat S, Joshua AM. Malignant pheochromocytoma secreting vasoactive intestinal peptide and response to sunitinib: a case report and literature review. Endocr Pract. 2014;20:e145-e150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Jiang J, Zhang L, Wu Z, Ai Z, Hou Y, Lu Z, Gao X. A rare case of watery diarrhea, hypokalemia and achlorhydria syndrome caused by pheochromocytoma. BMC Cancer. 2014;14:553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Verner JV, Morrison AB. Islet cell tumor and a syndrome of refractory watery diarrhea and hypokalemia. Am J Med. 1958;25:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 329] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Dimitriadis GK, Weickert MO, Randeva HS, Kaltsas G, Grossman A. Medical management of secretory syndromes related to gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. 2016;23:R423-R436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Long RG, Bryant MG, Mitchell SJ, Adrian TE, Polak JM, Bloom SR. Clinicopathological study of pancreatic and ganglioneuroblastoma tumours secreting vasoactive intestinal polypeptide (vipomas). Br Med J (Clin Res Ed). 1981;282:1767-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 92] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Fausa O, Fretheim B, Elgjo K, Semb L, Gjone E. Intractable watery diarrhoea, hypokalaemia, and achlorhydria associated with non-pancreatic retroperitoneal neurogenous tumour containing vasoactive intestinal peptide (V.I.P.). Scand J Gastroenterol. 1973;8:713-717. [PubMed] |
| 9. | Loehry CA, Kingham JG, Whorwell PJ. Watery diarrhoea and hypokalaemia associated with a phaeochromocytoma. Postgrad Med J. 1975;51:416-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Schwartz CJ, Kimberg DV, Sheerin HE, Field M, Said SI. Vasoactive intestinal peptide stimulation of adenylate cyclase and active electrolyte secretion in intestinal mucosa. J Clin Invest. 1974;54:536-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 255] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Sackel SG, Manson JE, Harawi SJ, Burakoff R. Watery diarrhea syndrome due to an adrenal pheochromocytoma secreting vasoactive intestinal polypeptide. Dig Dis Sci. 1985;30:1201-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Deng G, Jin L. The effects of vasoactive intestinal peptide in neurodegenerative disorders. Neurol Res. 2017;39:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Sparagana M, Feldman JM, Molnar Z. An unusual pheochromocytoma associated with an androgen secreting adrenocortical adenoma. Evaluation of its polypeptide hormone, catecholamine, and enzyme characteristics. Cancer. 1987;60:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169:1217-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1142] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 15. | Said SI, Mutt V. Potent peripheral and splanchnic vasodilator peptide from normal gut. Nature. 1970;225:863-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 241] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Quarles Van Ufford-Mannesse P, Castro Cabezas M, Vroom TM, Van Gils A, Lips CJ, Niermeijer P. A patient with neurofibromatosis type 1 and watery diarrhoea syndrome due to a VIP-producing adrenal phaeochromocytoma. J Intern Med. 1999;246:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Ende K, Henkel B, Brodhun M, Salomon C, Lauten P, Conrad E, Seifert M, Stier A, Scharf JG. A 45-year-old female with hypokalemic rhabdomyolysis due to VIP-producing composite pheochromocytoma. Z Gastroenterol. 2012;50:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Ikuta S, Yasui C, Kawanaka M, Aihara T, Yoshie H, Yanagi H, Mitsunobu M, Sugihara A, Yamanaka N. Watery diarrhea, hypokalemia and achlorhydria syndrome due to an adrenal pheochromocytoma. World J Gastroenterol. 2007;13:4649-4652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Plouin PF, Bertherat J, Chatellier G, Billaud E, Azizi M, Grouzmann E, Epelbaum J. Short-term effects of octreotide on blood pressure and plasma catecholamines and neuropeptide Y levels in patients with phaeochromocytoma: a placebo-controlled trial. Clin Endocrinol (Oxf). 1995;42:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Lamarre-Cliche M, Gimenez-Roqueplo AP, Billaud E, Baudin E, Luton JP, Plouin PF. Effects of slow-release octreotide on urinary metanephrine excretion and plasma chromogranin A and catecholamine levels in patients with malignant or recurrent phaeochromocytoma. Clin Endocrinol (Oxf). 2002;57:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Iio K, Sakurai S, Kato T, Nishiyama S, Hata T, Mawatari E, Suzuki C, Takekoshi K, Higuchi K, Aizawa T. Endomyocardial biopsy in a patient with hemorrhagic pheochromocytoma presenting as inverted Takotsubo cardiomyopathy. Heart Vessels. 2013;28:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Wu XM, Chen JJ, Wu CK, Lin LY, Tseng CD. Pheochromocytoma presenting as acute myocarditis with cardiogenic shock in two cases. Intern Med. 2008;47:2151-2155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Dunne MJ, Elton R, Fletcher T, Hofker P, Shui J. Sandostatin® and Gastroenteropancreatic Endocrine Tumors - Therapeutic Characteristics. Sandostatin® in the Treatment of Gastroenteropancreatic Endocrine Tumors. Berlin: Springer 1989; . [DOI] [Full Text] |
| 24. | Papotti M, Bongiovanni M, Volante M, Allìa E, Landolfi S, Helboe L, Schindler M, Cole SL, Bussolati G. Expression of somatostatin receptor types 1-5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. A correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virchows Arch. 2002;440:461-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 230] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Belei OA, Heredea ER, Boeriu E, Marcovici TM, Cerbu S, Mărginean O, Iacob ER, Iacob D, Motoc AGM, Boia ES. Verner-Morrison syndrome. Literature review. Rom J Morphol Embryol. 2017;58:371-376. [PubMed] |
| 26. | Krejs GJ. Effect of somatostatin infusion on VIP-induced transport changes in the human jejunum. Peptides. 1984;5:271-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Dueno MI, Bai JC, Santangelo WC, Krejs GJ. Effect of somatostatin analog on water and electrolyte transport and transit time in human small bowel. Dig Dis Sci. 1987;32:1092-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Nguyen HN, Backes B, Lammert F, Wildberger J, Winograd R, Busch N, Rieband H, Matern S. Long-term survival after diagnosis of hepatic metastatic VIPoma: report of two cases with disparate courses and review of therapeutic options. Dig Dis Sci. 1999;44:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Lamberts SW, Pieters GF, Metselaar HJ, Ong GL, Tan HS, Reubi JC. Development of resistance to a long-acting somatostatin analogue during treatment of two patients with metastatic endocrine pancreatic tumours. Acta Endocrinol (Copenh). 1988;119:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Oberg K. Interferon-alpha versus somatostatin or the combination of both in gastro-enteropancreatic tumours. Digestion. 1996;57 Suppl 1:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Faiss S, Pape UF, Böhmig M, Dörffel Y, Mansmann U, Golder W, Riecken EO, Wiedenmann B; International Lanreotide and Interferon Alfa Study Group. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors--the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21:2689-2696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 336] [Article Influence: 15.3] [Reference Citation Analysis (0)] |