Published online Nov 26, 2018. doi: 10.12998/wjcc.v6.i14.716
Peer-review started: May 29, 2018
First decision: July 17, 2018
Revised: September 14, 2018
Accepted: October 23, 2018
Article in press: October 22, 2018
Published online: November 26, 2018
Processing time: 181 Days and 15.9 Hours
Liver metastasis (LM) is one of the major causes of death in patients with colorectal cancer (CRC). Approximately 60% of CRC patients develop LM during the course of their illness. About 85% of these patients have unresectable disease at the time of presentation. Surgical resection is currently the only curative treatment for patients with colorectal LM (CRLM). In recent years, with the help of modern multimodality therapy including systemic chemotherapy, radiation therapy, and surgery, the outcomes of CRLM treatment have significantly improved. This article summarizes the current status of surgical treatment of CRLM including evaluation of resectability, treatment for resectable LM, conversion therapy and liver transplantation for unresectable cases, liver resection for recurrent CRLM and elderly patients, and surgery for concomitant hepatic and extra-hepatic metastatic disease (EHMD). We believe that with the help of modern multimodality therapy, an aggressive oncosurgical approach should be implemented as it has the possibility of achieving a cure, even when EHMD is present in patients with CRLM.
Core tip: Surgical resection has become the standard curative treatment for patients with resectable colorectal liver metastases (CRLM). In recent years, with the help of modern multimodality therapy, the outcomes of surgical treatment have significantly improved. The current study summarizes the current status of surgical treatment of CRLM, including evaluation of resectability, treatment for resectable liver metastases, conversion therapy and liver transplantation for unresectable cases, liver resection for recurrent CRLM and elderly patients, and surgery for concomitant hepatic and extra-hepatic metastatic disease.
- Citation: Xu F, Tang B, Jin TQ, Dai CL. Current status of surgical treatment of colorectal liver metastases. World J Clin Cases 2018; 6(14): 716-734
- URL: https://www.wjgnet.com/2307-8960/full/v6/i14/716.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i14.716
Colorectal cancer (CRC) is a major health burden with a worldwide estimate of 1.4 million new cases annually, resulting in approximately 694000 deaths[1]. The liver is the most common metastatic target organ for CRC. It has been estimated that as many as 25% of patients with CRC have synchronous liver metastases (LM), and about 60% of patients are found with metachronous LM on follow-up examinations[2,3]. LM is one of the major causes of death in patients with CRC[4].
Surgery for colorectal LM (CRLM) is increasingly being used as a part of multimodality treatment as it considerably improves the overall survival (OS)[5]. Unfortunately, only about 20% of CRLM patients have resectable cancer[6]. With the advent of highly effective chemotherapy and medical management, and advances in the surgical techniques of liver resection (LR), the pool of resectable patients with CRLM has expanded; metastatic lesions that were previously deemed terminal or nonsurgical are now being considered for surgical resection. According to recent reports, the 5-year survival of CRLM patients receiving surgery and neoadjuvant therapy has increased to up to 50%[7]. However, more than 70% of patients with CRLM after LR develop recurrence in the remnant liver[7,8]. Therefore, surgical resection for resectable CRLM is still a controversial and evolving topic within the realm of surgical oncology. This article aims to summarize the current status of surgical treatment of CRLM.
Resectable CRLM has no definite definition, and the criteria for “resectable” disease have evolved with the development of newer surgical techniques and technology. The traditional resectable criteria of CRLM first proposed by Ekberg et al[9] in 1986 included fewer than four intrahepatic metastatic lesions, no extrahepatic metastatic disease (EHMD), and being able to achieve a resection margin (RM) of at least 1 cm. With the recent development of surgical techniques, including liver three-dimensional reconstruction imaging technology, portal vein embolization (PVE), and associated liver partition and portal venous ligation (PVL) for staged hepatectomy (ALPPS), indications of LR for metastatic lesions have gradually widened, although they still vary among different centers. In 2014, van Dam et al[10] have expanded the historical CRLM resection criteria to include patients with four or more LM that could be present in both the lobes, patients with centrally located tumors, and those with resectable extrahepatic disease. Patients with the extended indications have shown more major complications (33.1% vs 19.5%) as well as a shorter OS (41.4 mo vs 68.8 mo) and median disease-free survival (DFS) (10.2 mo vs 22.0 mo) compared with the traditional indication group. The 10-year DFS rates (cure rates) were 15.8% in the extended indication group and 35.5% in the traditional indication group because fewer patients with extended indications underwent R0 resection compared with patients with limited indications (77.5% vs 92.9%)[10]. In 2015, a study conducted by Viganò et al[11] showed that hepatectomy was also safe in selected patients with eight or more LM, although these patients had a shorter 5-year OS (20.1% vs 44.2%) and recurrence-free survival (13.6% vs 28.7%) compared with patients with fewer than eight LM. It was gratifying to see that patients with eight or more LM, who had no risk factors including extrahepatic disease, no response to chemotherapy, and primary rectal cancer, had a similar 5-year OS to patients with fewer than eight LM (44% vs 44.2%)[11]. In 2017, Allard et al[12] evaluated the long-term outcomes after liver surgery for patients with 10 or more LM. The results showed that the 5-year OS of these patients was 30%. Patients who underwent a macroscopically complete (R0/R1) resection had 3- and 5-year OS rates of 61% and 39%, respectively, compared to 29% and 5% for patients with R2/no resection (P < 0.0001)[12]. Therefore, the number and even the size of LM lesions are no longer contraindications of resection, as long as all visible lesions can be resected with a tumor-free margin and sufficient remnant liver. The current criteria for resectability of CRLM are as follows: any tumor number, any tumor distribution in the liver, stable or resectable EHMD (excluding portal lymphadenopathy), functional liver remnant > 20% of the total liver volume, venous involvement amenable to venous resection or reconstruction, and a tumor-free margin[5]. We have summarized the current recommended criteria in comparison to traditional resectability criteria for CRLM in Table 1.
| Item | Traditional criteria | Current criteria |
| EHMD | No EHMD | Stable or resectable EHMD (excluding portal lymphadenopathy) |
| LM number | Fewer than 4 lesions | No limit |
| LM distribution | Unilateral | No limit |
| Vascular invasion | No involvement | Amenable to venous resection or reconstruction |
| Resection margin width | More than 1 cm | Beyond 1 mm with a tumor-free margin |
| % of FLR of total liver volume | > 20% | > 20% for normal liver and slight chemotherapy-associated liver dysfunction; |
| > 30%–40% for severe chemotherapy-associated liver disease |
However, the criteria of resectability may vary even amongst the most experienced hepatic surgeons due to their different surgical experiences and techniques. We suggest that the determination of CRLM resectability should be based on oncological principles and technical feasibility as outlined in the Hepato-Pancreato-Biliary Expert Consensus Statement 2012[13]. If the patients have favorable factors such as maximal tumor size < 40 mm, age < 60 years, preoperative imaging using magnetic resonance imaging (MRI) and adjuvant chemotherapy, and the surgeons are confident to obtain a negative margin resection (R0) and sufficient remnant liver, the indication can be extended. If the patients have adverse prognostic factors associated with a very poor survival, such as no response to chemotherapy or unstable or unresectable extrahepatic disease, surgery may not be beneficial and the indications should be limited.
Sensitivity in detection of intrahepatic and extrahepatic metastases: The imaging methods useful for evaluating LM include trans-abdominal ultrasound (US), contrast-enhanced computed tomography (CT), contrast-enhanced MRI, and positron emission tomography (PET). Although US is a primitive preoperative imaging modality, US along with contrast-enhanced US (CEUS) has been widely used for detecting LM in clinical practice[14]. Nevertheless, CEUS has the advantages of real-time observation and shows a high specificity for characterizing focal liver lesions, comparable to CT and MRI[15].
A meta-analysis has shown that the sensitivities of CT, MRI, and PET/CT for the diagnosis of CRLM are 82.1%, 93.1%, and 74.1%, respectively; while the specificities are 73.5%, 87.3%, and 93.9%, respectively[16]. A review of the literature indicates that MRI, especially gadoxetate disodium (Gd)-enhanced MRI, is more sensitive than CT for detecting a liver lesion of less than 1 cm, particularly 5 mm[17]. From a cost-effectiveness point of view, contrast-enhanced MRI can be cost-effective, provided that it replaces contrast-enhanced CT and has an improved diagnostic accuracy[18]. However, neoadjuvant chemotherapy significantly decreases the sensitivity of CT and MRI[16]. Gd-enhanced and diffusion-weighted MRI in combination with contrast-enhanced 18F-fluorodeoxyglucose PET/CT will allow confident detection of LM, which disappear on post-neoadjuvant chemotherapy contrast-enhanced CT[19]. In clinical practice, despite having limited sensitivity, CT appears to be adequate for determining the resectability of CRLM for the majority of patients.
The sensitivity and specificity of PET/CT for the detection of LM are similar to those of CT[20]. Most recent studies recommend PET/CT for the detection of occult distant metastases[21,22]. It can detect 25% of extrahepatic lesions and avoid worthless surgery in approximately 20% of patients[23]. Therefore, PET/CT can significantly contribute to the staging of patients with CRLM[23,24]. PET/CT staging is associated with a significantly improved actual long-term survival, thus providing valuable prognostic information that can guide surgical and oncological treatments[24]. However, in a randomized controlled trial, the use of PET/CT did not significantly affect the surgical management, compared with CT alone[25]. These findings raise concerns about the role of PET/CT in this setting. In spite of conflicting results, PET-CT may be useful for the detection of extrahepatic disease, particularly in patients with recurrent disease or a high tumor load (multinodular and/or large metastases) or for whom difficult hepatic resections are planned[26].
Preoperative anatomical localization: CT, MRI, and PET/CT are the mainstay methods used to assess the resectability of liver lesions. Of them, CT is the most common modality for the initial diagnosis of CRLM and is usually adequate for treatment planning. Staging of a patient with CRC includes a CT scan of the chest, abdomen, and pelvis to evaluate for metastatic disease, as the majority of metastases are clinically silent. The currently established standard for planning liver surgery is contrast-enhanced CT, which as a rule enables appropriate resection planning, e.g., a precise identification and localization of primary and secondary liver tumors as well as the anatomical relationship to extrahepatic and/or intrahepatic vascular and biliary structures.
Intraoperative detection: Intraoperative US (IOUS) with and without contrast enhancement is recommended as a screening modality for detecting additional LM not seen on routine preoperative imaging. The use of IOUS and contrast-enhanced IOUS (CE-IOUS) improves decision-making by providing the most sensitive form of liver staging. The results of a study conducted by Arita et al[27] showed that during surgery, 25 additional nodules were newly identified using IOUS, among which 21 patients had histologically proven CRLM. Twenty-two additional nodules were newly identified using CE-IOUS, among which 17 nodules in 16 patients were histologically diagnosed as CRLM. The planned surgical procedure was modified based on the IOUS and CE-IOUS findings in 12 and 14 patients, respectively[27]. Laparoscopic US combined with laparoscopic real-time near-infrared fluorescence imaging can enhance the sensitivity of detection[28]. This may be particularly important in the era of laparoscopic resections, in which surgeons lose the opportunity to palpate the liver.
Post-hepatectomy liver failure (PHLF) is a significant cause of morbidity and mortality. Independent predictors of PHLF can be categorized into three main categories: Patient-related, liver-related, and surgery-related factors[29]. The most crucial patient-related factors affecting the outcome of LR are the volume and function of the FLR. Therefore, preoperative evaluation of the FLR volume and estimation of the functional capacity of the liver should be performed before major LR in patients with a normal liver. Moreover, this evaluation should be conducted even when performing minor LRs in patients with diseased livers.
Several studies have reported on the impact of FLR volumetric analysis on the outcomes of LR[30,31]. Generally speaking, the FLR size should be at least 20% for patients with normal livers and those who have received chemotherapy for no more than 12 wk[32]; however, considering that there is significant chemotherapy-associated steatohepatitis or sinusoidal obstruction in those receiving preoperative chemotherapy for more than a 12-wk duration or more than eight cycles, this cut-off value should be increased to 30%–40% in order to avoid postoperative liver failure[32,33]. The reason for this increase is that the risks of major complications, liver failure, and mortality increase to 47%, 20%, and 13%, respectively, if the FLR is < 20% of the total volume[34]. Preoperative evaluation of the FLR by virtual segmental volumetry using three-dimensional CT has been found to be superior to that estimated using standard equations[30,31,35].
Liver function can be assessed by biochemical tests, the indocyanine green (ICG) clearance test, and 99mTc-mebrofenin hepatobiliary scintigraphy (HBS). The ICG clearance test is primarily used to assess the hepatic function in patients with primary liver cancer and drug-induced liver injury with good accuracy[36-38]. However, its accuracy to predict the extent of chemotherapy-associated liver injury is not good. A study by Wakiya et al[38] has found that the preoperative ICG retention rate at 15 min (ICGR15) did not strongly correlate with the pathological sinusoidal injury and steatohepatitis scores in CRLM patients. The sensitivity and specificity of the ICG test for detecting pathological liver injury were 47% and 75%, respectively[33]. In order to perform a safe radical LR, it is necessary to estimate the hepatic functional reserve of the chemotherapy-associated liver based on a combination of several clinical indicators and not only rely on the ICG test. 99mTc-mebrofenin HBS is an effective method for determining liver function and can also provide information about the function of the segmental liver tissue; in addition, it has a good correlation with the ICGR15[39]. Moreover, it is a validated tool to assess the total and remnant liver function[40]. However, this method is not practical in clinical practice and has not been widely used.
In general, assessment of the FLR function should be conducted by combining the Child-Pugh score, FLR volume, and ICGR15 test results.
Optimal surgical management of patients with synchronous CRLM is still controversial. There are three main types of surgical strategies for synchronous CRLM patients[41]. The first type involves removal of the primary colorectal tumor, followed by chemotherapy and about 3–6 mo later with resection of LM as the final step (classic or bowel-first). The second type is synchronous resection of the primary tumor and LM in the same surgical procedure (combined). The third approach, commonly termed as the reverse or liver-first approach, involves LM resection as the first step, followed by chemo(radio)therapy, and removal of the primary tumor as the last step. The classical approach and the liver-first approach are both two-stage surgical procedures. In 2018, data from 1830 patients who lived in the United Kingdom showed that the percentages of patients who underwent the classical approach, the simultaneous approach, and the liver-first approach were 71.1%, 14.8%, and 14.2%, respectively[42].
The main advantages of the classical approach are avoidance of bowel-related complications (large bowel obstruction, bleeding, perforation, etc.) from the primary tumor and prevention of disease progression from the primary lesion. On the other hand, the main advantages of the liver-first approach are that it is possible to treat the metastatic disease before the resectable CRLM become unresectable and, as the liver is not exposed to chemoradiation, less FLR is required and fewer liver-related postoperative complications are caused. Hence, at most centers, the patients treated by the liver-first approach have more and larger LM compared to patients planned for the classical approach, and the primary tumor has no obvious obstruction, bleeding, or asymptomatic evidence[43,44]. A study of 623 patients with synchronous CRLM, of which 377 were treated by the classical approach and 246 by the liver-first approach, revealed that patients chosen for the classical approach more often had T4 primary tumors (23% vs 14%) and node-positive disease (70% vs 61%). The liver-first approach group had a higher liver tumor burden score (4.1 vs 3.6). Yet, no difference was seen in the 5-year OS between the two groups (54% vs 49%)[45].
However, these staged surgical treatments have some shortcomings. First, patients need a second surgery, thereby increasing the length of hospital stay and health care costs[46]. Second, the median time from the first to the second operation varies between 4.7–7 mo for patients treated using the classical approach and 2–9 mo for patients treated using the liver-first approach[45,47]. Another problem is that 16.3%–35% of liver-first and bowel-first patients fail to proceed to the second operation due to postoperative complications or disease progression[43,47]. The advocates of staged surgical treatment suggest that the progression of LM or the primary tumor after the first operation is characteristic of aggressive tumor biology; therefore, allowing a period of time to see if there is progression avoids performing extensive resections in those who will not benefit[48,49].
With improvements in LR techniques, the proportion of patients undergoing either a liver-first approach or a simultaneous approach has increased in recent years, from 26.8% in 2010 to 35.6% in 2015[42]. Several studies have demonstrated that simultaneous resection can be safely performed in appropriately selected patients[50-52]. A study of 1430 patients with synchronous CRLM has revealed that the combined procedure is equally safe compared to the staged procedure in patients undergoing complex operations, and it should be considered as the first strategy due to its advantages of reduced readmission within 30 d, faster recovery, shorter hospital stay, decreased hospital cost, and same rates of major events or anastomotic leak[51]. A study conducted by Silberhumer’s team also validated that there was no significant difference in the OS and DFS between the simultaneously resected and staged-resected patients, with 1-year survival rates of 90.5% and 92.6%, respectively, and 5-year survival rates of 38.5% and 38.9%, respectively[50]. However, Nanji et al[53] have argued that a selection bias resulted in better outcomes favoring the simultaneous approach because patients who underwent the simultaneous approach had fewer and smaller liver lesions and received less invasive resections. A meta-analysis and review recommended the following criteria to select patients for a simultaneous resection: Age < 70 years, LR of no more than three segments, colonic resection (especially a right-sided colectomy), and exclusion of coexisting severe conditions[54]. In short, simultaneous resection can be the recommended surgical approach in appropriately selected patients as it offers benefits to both the patients and the healthcare system.
Neoadjuvant chemotherapy (neoCTx) can control systemic disease, eliminate micro-metastatic disease, and even downsize metastatic liver lesions and the primary tumor. A study of 62 patients who underwent neoCTx, despite having resectable disease, found that 5 patients had progressive disease, 22 had stable disease, and 35 had partial response according to the RECIST criteria[55]. Among the patients with partial response, 29 had histopathologic downstaging[55]. Although its use remains controversial, several groups have found a positive survival benefit in synchronous CRLM patients with neoCTx[55]. A propensity score matching analysis of 149 patients showed that the 3-year DFS rate was significantly higher in patients with neoCTx than in those without (34.2% vs 16.8%, respectively)[56]. Furthermore, patients with partial response to neoCTx had better survival rates than those with stable or progressive disease[55]. Analysis of a single-institution prospective database including 1211 patients showed that the actual 10-year survival rate after resection of CRLM was 24%, with a 20% cure rate[57]. The authors suggested that preoperative strategies such as neoCTx would improve the actual 10-year survival and cure rates of patients with both a high clinical risk score and extrahepatic disease[57]. However, a two-center study over a 22-year period showed that neither the OS nor recurrence rates were improved using neoCTx in patients with solitary CRLM who underwent curative LR[58]. NeoCTx seems to be more beneficial for resectable patients with risk factors associated with an unfavorable prognosis.
Neoadjuvant bevacizumab-based chemotherapy has been found to be associated with a better OS in patients who underwent LR of synchronous CRLM, especially in patients treated by the classical approach[59]. However, the new EPOC randomized controlled trial for resectable CRLM showed that the progression-free survival was significantly shorter in the chemotherapy plus cetuximab group than in the chemotherapy alone group (14.1 mo vs 20.5 mo)[60]. Therefore, it is still unclear whether targeted therapy with cetuximab or bevacizumab should be offered with chemotherapy in the preoperative setting for resectable patients.
In summary, there are no significant differences in the outcomes between these three approaches in patients with synchronous CRLM[42]. The current evidence is insufficient to decide upon the optimal strategy for a given patient with synchronous CRLM. The timing of the resection and the type of surgical approach should be based on the patient characteristics and the protocols followed by individual centers. Individualized treatment should be offered by a multidisciplinary team after discussing the risks and benefits of each approach with the patient. We prefer concurrent surgery for eligible patients. Otherwise, we choose the classical approach or the liver-first approach based on the severity of the primary tumor and LM. In patients treated with neoCTx, there are chances of chemotherapy-related liver injury after receiving chemotherapeutic drugs. Hence, LR should be scheduled at 4–6 wk after the last day of conventional chemotherapy or 7–8 wk after the last day of bevacizumab-based chemotherapy[55,59].
There are several factors affecting the prognosis after curative hepatectomy for CRLM, which include RM, size, number and location of LM, synchronous LM, stage of the primary colorectal tumor, peritoneal dissemination and so on[61,62]. Among these factors, only the RM is under the surgeon’s direct control and can be modified to achieve optimal outcomes. To date, there is no consensus on the universal definition of a ‘‘positive’’ RM for CRLM. A positive RM is one of the risk factors for intrahepatic recurrence after curative resection of LM and is independently associated with the OS[63]. The results of a study on 2368 CRLM patients showed that all margin widths, including submillimeter margins, correlated with a prolonged OS, compared with an R1 resection, and that a tumor-free RM width > 1 cm had the longest median OS[63]. In the case of multiple LM, the R1 margin status was also associated with a worse OS among patients with a positive margin associated with the largest CRLM lesion[64]. A recent meta-analysis demonstrated that an R0 resection with a margin width > 1 cm was associated with both an improved DFS and OS, compared with an R0 resection with narrower margins, and that the RM (> 1 mm vs < 1 mm) was significantly associated with an improved OS at all time points[65]. These findings suggest that a LR with a negative RM should be performed whenever possible. Traditionally, a RM of at least 10 mm is considered the gold standard[66]. However, the controversy over the prognostic role of hepatic RM width continues.
Numerous studies have found that patients with subcentimeter or submillimeter margin widths do not have worse survival rates, suggesting the wider use of parenchymal-sparing hepatectomy (PSH) for CRLM in order to preserve the FLR[67-70]. In PSH, the aim is to obtain an oncologic resection with minimal tumor-free margins so as to preserve as much of the liver parenchyma as possible. Advocates of PSH argue that even a 1-mm tumor-free margin is sufficient in patients with CRLM[68]. Another systematic review reveals that the safety profile and oncologic outcomes of PSH are similar to those of anatomic LR for CRLM[70]. Hence, PSH may be considered an appropriate surgical approach in patients with CRLM.
Some researchers believe that it is tumor biology, not the surgical approach, that determines the prognosis[63,71]. The prolonged OS observed with submillimeter margins is not because the submillimeter R0 margin allows patients to survive longer; instead, submillimeter margin clearance is more likely to be achieved only when patients have good tumor biology[63]. The R0 resection rate and survival rate were higher among patients with CRLM having a fibrous capsule around the liver lesions than in those without it[72]. The KRAS mutation status also impacts the effect of the margin status on survival. A tumor-free margin provided a survival benefit to only patients with wild-type KRAS tumors, and the margin width was not found to be a prognostic factor in those with a KRAS mutant gene, in whom the OS with an R0 margin was similar to that in those with microscopically positive margins[67,71].
With the progress in modern chemotherapy, the prognostic influence of the RM status on survival has been studied but continues to remain controversial. A study of 466 CRLM patients showed that neoCTx did not influence the 5-year OS and DFS rates of the R0 group, but it had a positive influence on the R1 group[73]. Additionally, the OS and DFS rates were similar between R1 and R0 resections in patients treated with neoCTx[73]. Another research study also reported that the 5-year OS was not significantly associated with the margin status in bevacizumab-treated patients (46.8% vs 33% after R0 vs R1 resection, P = 0.081), in whom the 5-year survival rate was slightly worse (presumably reflecting more advanced disease) than among patients treated with cytotoxic agents alone[74]. Moreover, a prospective study of 334 patients with solitary CRLM showed that neoCTx did not influence survival, in either the entire patient group or in the subgroups with a positive or negative RM, but the patients treated with neoCTx and having a positive RM had a poorer survival than those with a negative RM[75]. The clear benefit for chemotherapy has been demonstrated in the adjuvant setting by several studies in which postoperative chemotherapy was found to be protective from recurrence regardless of the RM status[69,73,75,76]. Patients with a postoperative performance status > 2, who did not receive adjuvant chemotherapy, had a decreased progression-free survival and OS after LR for CRLM[77].
In summary, a wide RM (> 1 cm) should be attempted whenever possible. LR should not be precluded if narrower margins are anticipated in patients with multiple lesions or when resection borders are limited due to major vascular-biliary structures, since a submillimeter tumor-free margin may also improve survival.
Ablative techniques were initially used in patients with unresectable CRLM. In 2015, an international panel of ablation experts proposed that percutaneous ablation is suitable for patients with technically inoperable but limited liver disease and for those with limited liver reserve or co-morbidities that render them inoperable[78]. However, due to their safety, tolerability, repeatability, and less invasiveness, these techniques have been used to treat resectable CRLM patients[79]. A study of 53 resectable CRLM patients treated by LR or radiofrequency ablation (RFA) showed that the 1-, 3-, and 5-year cumulative survival rates in the RFA group were not significantly different compared to those of the LR group (85.7% vs 87.5%, 38.1% vs 53.1%, and 14.2% vs 31.3%, respectively), but the 1-, 3- and 5-year recurrence-free survival rates in the RFA group were significantly less than those in the LR group (76.1% vs 90.6%, 23.8% vs 56.3%, and 4.8% vs 28.1%, respectively)[80]. A meta-analysis also pointed out that compared to patients treated by LR, the recurrence rate with RFA was higher than that of surgery[81]. Although the median survival time with microwave ablation and RFA in the treatment of CRLM was similar, the local recurrence rate with RFA was significantly higher than that of microwave ablation[82,83]. A study by Mulier et al[84] further disclosed that the local recurrence rate of open RFA was equivalent to that of LR for tumors < 3 cm; but for larger tumors, the local recurrence rate was higher.
Ablation techniques are also often used in combination with hepatectomy, and the approach is usually named as combined intra-operative ablation and resection (CARe). The use of CARe is especially suitable for patients with multiple LM. With CARe, the small lesions are ablated and the large lesions are resected, with the aim of preserving as much liver parenchyma as possible. The prognosis of patients treated with CARe has been found to be comparable to that of patients treated by LR alone[85,86]. In addition, retaining as much liver volume as possible helps to perform a salvage hepatectomy, if required, which increases the survival rate after recurrence of LM[87]. Additionally, ablation is also a suitable alternative to hepatic resection for isolated hepatic recurrence after surgery for CRLM, and it is associated with a better OS compared with systemic chemotherapy alone; therefore, ablation should be considered for patients with resectable liver recurrence who are unfit or unwilling to accept LR[88].
Aliyev et al[89] found that in comparison with the LR group, RFA patients have a higher American Society of Anesthesiologists (ASA) score (3.0 vs 2.6, respectively, P = 0.002), a more frequent incidence of cardiopulmonary comorbidities (60% vs 38%, respectively, P = 0.045), and tumors located deeper in the liver parenchyma (39% vs 12%). Although ablation seemed to be associated with a shorter progression-free survival, post-procedure morbidity was significantly lower with ablation. A meta-analysis pointed out that patients treated with RFA had a shorter hospital stay and fewer complications, compared to those treated by LR[81]. The incidence of complications with microwave ablation and RFA in the treatment of CRLM was similar[82,83].
In brief, ablation is only suitable for selected patients with LM of less than 3 cm, tumors located deep in the liver parenchyma, patients with a high ASA score, or cardiopulmonary comorbidities. Furthermore, the heat-sink effect should be taken into consideration while treating LM located near vessels as the size of the ablation zone is affected by the flow rate and the distance from the vessels[90].
Laparoscopic LR (LLR), characterized by “less invasiveness,” is becoming increasingly popular for the treatment of primary and metastatic liver malignancies. The Oslo-Comet randomized controlled trial compared laparoscopic and open LR for CRLM and revealed that LLR was associated with a significantly lower postoperative complication rate (19% vs 31%, P = 0.021), a shorter hospital stay (56 h vs 96 h, P < 0.001), and a higher cost-efficiency; whereas there were no differences in the blood loss, operative time, resection margins, or 90-d mortality[91]. A recent meta-analysis demonstrated that a limited number (two or fewer) of metastases located in the left lateral segments are more suitable for LLR[92]. Moreover, the initial LLR for CRLM was associated with less inflammation, surgical stress, and postoperative adhesion, allowing a higher chance of repeated hepatectomies if recurrence occurred[93]. Concerning the technical difficulties and narrow operative field exposure, LLR for a major hepatectomy was adopted less frequently, but it was only attempted by a few specialized centers with a high volume of patients[94].
The da Vinci surgical system, also known as robot-assisted LR, is believed to overcome the disadvantages of a laparoscopy[95]. Robot-assisted LR is performed through a series of flexible mechanical arms, allowing more degrees of freedom, which can effectively avoid the “fulcrum effect” caused by rigid laparoscopic instruments. What’s more, the robotic approach makes the surgical procedure more precise by providing three-dimensional vision and avoiding hand tremors. Therefore, robot-assisted LR can be used in narrow spaces or curved transections, and it is particularly suitable for the handling of metastases located in the posterior-superior segments[96]. Standard laparoscopy or robot-assisted LR for minor LRs can be performed with favorable perioperative and long-term outcomes. Nevertheless, the robotic approach offers more benefits for a major hepatectomy and challenging cases[97].
Some patients are initially considered to have unresectable CRLM due to the size, number, and location of the LM and other poor prognostic factors. However, it should be noted that the definition of unresectable CRLM is not widely recognized at this moment. In 2013, Takahashi and colleagues proposed the definition of unresectability as follows: multiple bilobar LM that require resection of more than 70% of the nontumorous liver for removal of all tumors leading to an inadequate FLR, tumors invading all three hepatic veins, tumors invading both the left and right branches of the hepatic artery or portal vein, and extrahepatic metastasis other than resectable pulmonary metastasis[98]. As previously mentioned, CRLM occupying bilateral liver lobes or an inadequate tumor-free FLR is a challenge. The metastatic disease is often considered unresectable if it is not possible to radically excise all of the lesions while preserving at least two contiguous segments with an adequate FLR volume, blood flow, and biliary drainage[99]. Tumor shrinkage and FLR hypertrophy are the two most widely used approaches for converting unresectable CRLM to resectable disease.
With the availability of effective chemotherapy regimens and the development of innovative surgical techniques, an increasing number of patients whose disease is initially considered unresectable may find that their disease has become resectable following treatment. This process is known as conversion therapy. There are several methods to convert unresectable disease to a resectable state.
Preoperative PVE is the most widely used method for inducing atrophy of the liver segments to be resected and hypertrophy of the FLR, which can convert unresectable cancer into resectable cancer[100-103]. PVE is mainly used in patients when the preoperative FLR is < 25% of the total liver volume[103]. It takes about 4–6 wk following PVE for liver hypertrophy to occur. Recently, Xiao et al[100] have presented a new strategy for terminal branch portal vein embolization after six cycles of neoadjuvant therapy, which increases the FLR and causes remarkable tumor shrinkage, thus making LR feasible in 2 wk. However, there is an ongoing controversy surrounding PVE regarding the short-term safety of PVE and its long-term oncological benefit. A systematic review including 539 patients treated by PVE showed that 30% of these patients did not undergo LR, mostly due to tumor progression (84%); the median OS time in patients with PVE and non-PVE was 38.9 mo and 45.6 mo, respectively; the median DFS time was 15.7 (PVE) and 21.4 (non-PVE) mo, respectively[104]. Hence, some researchers believe that PVE should be carefully used because the usage of PVE in bilobar CRLM patients can accelerate the progression of disease in the remnant liver[105,106]. In contrast, other recent studies discovered that tumor progression after PVE has not been shown to affect the OS, and PVE followed by hepatectomy has been shown to be a safe and feasible strategy for unresectable CRLM[102,103,107]. A propensity score matched study of major LR with or without preoperative PVE showed that the PVE group and non-PVE group achieved similar 5-year OS (16% vs 9%, P = 0.776) and 3-year progression-free survival rates (14% vs 14%, P = 0.866), but it was remarkable that the PVE group had more extensive disease in terms of the number and diameter of LM and more often had synchronous disease[103].
PVL is another method to increase the FLR volume. A systematic review and meta-analysis showed that no significant differences were found in the rates of FLR hypertrophy [43.2% (PVE) vs 38.5% (PVL), P = 0.39] or in post-intervention mortality and morbidity[108]. However, the numbers of cancelled hepatic resections due to inadequate hypertrophy were significantly lower after PVL. But at the same time, PVL is more invasive than PVE because this method needs to be performed by laparoscopy or laparotomy[108].
For patients with bilobar CRLM requiring a major hepatectomy, the two-stage hepatectomy with PVE/PVL is the only curative option. In stage 1, the small metastatic lesions in FLR are resected in combination with synchronous PVL or percutaneous PVE after the operation to stimulate the growth of the FLR. Once the FLR becomes adequate, in the second stage, extensive LR is performed. This two-stage hepatectomy reduces the risk of liver failure and increases the chances of remission. Levi Sandri et al[109] reported their 10-year experience of two-stage hepatectomy in 46 patients with CRLM, among which 38 patients underwent PVL and the other patients underwent PVE. They observed that the long-term OS was 52 mo from the time of the first liver surgery[109]. A recent study has compared two-stage hepatectomy with one-stage major hepatectomy plus contralateral LR or ablation, and the results are encouraging. The two-stage hepatectomy group had fewer postoperative major complications (14% vs 26%, P = 0.03) and less hepatic failure (6% vs 20%, P = 0.001). Moreover, the two-stage hepatectomy group achieved a higher 5-year OS rate (35% vs 24%, P = 0.016)[48]. Although the two-stage hepatectomy approach is well established, it has been reported that almost one-third of patients fail to receive the second surgery due to tumor progression after the first surgery or an insufficient FLR[110].
Hence, how to deal with tumors in the FLR is extremely important. First, the appropriate selection of patients who are unlikely to experience tumor progression is vital, and this requires further study of tumor biology and the tumor microenvironment[104,109]. Second, multidisciplinary treatment such as neoadjuvant chemotherapy and transarterial chemoembolization should be tried to slow down the tumor progression[101]. Third, several methods including partial resection and ablation can be used to remove or destroy the tumors[48].
In conclusion, we recommend PVE as the preferred strategy to increase the FLR volume because it is a minimally invasive procedure for patients who do not need a staged LR. But in patients undergoing a two-stage hepatectomy, PVL is an ideal option because it can be performed intraoperatively and can avoid the additional cost of postoperative PVE with comparable outcomes as PVE.
In order to overcome the long waiting period for FLR regeneration after PVE/PVL, a new concept of LR called ALPPS, which allows rapid liver growth, was first described in 2012[111]. This procedure mainly includes two stages. In stage 1, the right portal vein is ligated with the simultaneous splitting of the liver parenchyma, usually along the falciform ligament or Cantlie’s line, and resection of metastasis from the FLR. Stage 2 includes specimen removal after several days, once the target FLR is achieved. The median time interval between the two stages of ALPPS is typically 8–11 d, which is significantly shorter than that of other methods[111-113].
A multicenter randomized controlled trial showed that the resection rate was higher in the ALPPS arm compared with the two-stage hepatectomy arm (92% vs 57%), with no differences in complications (Clavien-Dindo ≥ 3a) (43% vs 43%), 90-d mortality (8.3% vs 6.1%), or R0 resection rate (77% vs 57%)[112]. However, a multicenter matched case–control study showed that the feasibility of ALPPS for CRLM was not significantly better than that of a two-stage hepatectomy, whereas the perioperative complications were obviously increased in the ALLPS group[113]. Another matched case–control study demonstrated that early oncologic outcomes of patients with advanced LM receiving ALPPS were not superior to the matched patients receiving systemic treatment with palliative intent[114]. A very recent meta-analysis also revealed that there was no difference in the final postoperative FLR between ALPPS and two-stage hepatectomy in patients with unresectable CRLM, but the morbidity and mortality rates were higher with ALPPS[115].
Recent studies have confirmed that ALPPS can be used as an alternative rescue procedure after unsuccessful PVE or two-stage hepatectomy; it has been shown to be safe and effective in those patients who failed to achieve FLR > 30%[112,116,117]. Rescue ALPPS can allow previously unresectable disease to become amenable to surgery. In addition, a KRAS mutation has been found to be an independent predictor of poor survival after ALPPS[118]. Therefore, although ALPPS may be a suitable approach for these patients, appropriate patient selection and proper preoperative counselling about the risks and benefits of the procedure are essential in order to achieve good outcomes. Liberal use of imaging studies and discussion with radiologists can help to obtain crucial preoperative and perioperative information, which may change the surgical plan and contribute to better oncologic outcomes[119].
Chemotherapeutic and targeted biological agents can be used in unresectable cases to achieve tumor shrinkage so as to allow potentially curative resection. FOLFOX (oxaliplatin, fluorouracil, and leucovorin) and FOLFIRI (irinotecan, leucovorin, and fluorouracil) are the two standard chemotherapy regimens for unresectable CRLM. According to recent data, approximately 24%–52% of unresectable CRLM patients could be treated by conversion hepatectomy after receiving first-line systemic chemotherapy or hepatic artery infusion[120-122]. After conversion hepatectomy, these patients could achieve survival rates similar to those of patients who underwent an LR initially, with a predicted 5-year survival rate with conversion hepatectomy of 63%–76%[120-122].
The combination of FOLFOX or FOLFIRI with other drugs and targeted therapy for unresectable disease has been tested in clinical trials. Among these combined regimens, anti-epidermal growth factor receptor (EGFR) antibodies such as cetuximab and panitumumab as well as anti-vascular endothelial growth factor receptor (VGFR) monoclonal antibodies such as bevacizumab are the main components that have been proven to increase the response rate and tumor shrinkage[123-125]. The mutant-type (mt) KRAS status predisposes a patient with CRLM to a worse recurrence-free survival and OS, possibly as a result of aggressive tumor biology[126]. The KRAS mutation status remains an important predictor of response to these therapies. In patients with wild-type (wt) KRAS unresectable CRLM, the use of an anti-EGFR or anti-VGFR monoclonal antibody combined with standard chemotherapy regimens (FOLFOX or FOLFIRI) is not only preferred for the conversion to potentially curative resection, but it also improves the response rate, progression-free survival, and OS[123-125,127]. The results of a phase II trial comparing the efficacy of panitumumab plus FOLFOX4 or FOLFIRI for unresectable wt-KRAS CRLM revealed that both the combined regimens achieved a high rate of early tumor shrinkage and offered a greater chance of curative resection[125]. In addition, the EREBUS cohort study performed to assess the effectiveness of cetuximab in wt-KRAS patients in real practice showed that the rate of CRLM resection was 27.2%, the 24-mo probability of CRLM resection was 33.6%, the median progression-free survival was 9.2 mo for the total cohort and 13.0 mo for resected patients, and the median OS was 23.0 mo for the total cohort and was not reached after 36 mo for those who were resected[123]. Moreover, bevacizumab is effective in both wt-KRAS and mt-KRAS patients[124,127-129]. A randomized clinical trial showed that among patients with untreated wt-KRAS metastatic colorectal cancer, there was no significant difference in the OS between the addition of cetuximab or bevacizumab to chemotherapy as the initial biological treatment[127]. Furthermore, a study by Hatano et al[129] involving patients who received mFOLFOX6 with either bevacizumab or cetuximab based on their KRAS status disclosed that the overall response rate was 64.7% (wt/mt, 77.3%/41.7%, P = 0.04) and the overall conversion hepatectomy rate was 67.6% (wt/mt, 77.2%/50.0%, P = 0.09).
Recently, an interesting discovery reported by several researchers was that the primary tumor location is associated with the oncological outcomes: a primary tumor located on the right side had a worse prognosis than a tumor located on the left side[130,131]. Therefore, cetuximab and panitumumab are only recommended for left-sided primary tumors with wt-KRAS CRLM. After resection of the downsized LM, routine adjuvant chemotherapy should be given to reduce the chances of tumor recurrence. The most preferred regimen for postoperative adjuvant chemotherapy is mFOLFOX6 for 3 mo after metastasectomy[132].
In addition, there are some predictors of long-term survival in patients receiving conversion chemotherapy followed by LR for CRLM. First, early-tumor shrinkage and the partial response rate by RECIST criteria are the most powerful predictors of a long survival. Moreover, early-tumor shrinkage > 30% after 8 wk of chemotherapy is significantly associated with the OS. The greater the depth of response, the longer the median duration of response and the higher the OS[133,134]. Second, a left-sided primary tumor resection prolonged the median OS; however, for colon cancer patients with right-sided tumors, resection showed no benefit[130,131]. Third, the patients with a carcinoembryonic antigen half-life after the third chemotherapeutic course of less than 20 d had a significantly better progression-free survival and OS[135]. Fourth, a favorable pathological tumor response was independently associated with the DFS[55]. These prognostic factors are helpful in selecting ideal candidates for this strategy and also can guide the clinical management of patients. We propose that the use of anti-EGFR or anti-VGFR monoclonal antibody combined with standard chemotherapy regimens (FOLFOX or FOLFIRI) should be the first choice for unresectable CRLM patients based on the KRAS mutant status.
Liver transplantation can be regarded as the “ultimate” LR and is now gaining increasing interest for unresectable CRLM[6]. Liver transplantation for LM in the early 1990s achieved very poor perioperative outcomes and was abandoned[136]. However, in the past two decades, with dramatic improvements in surgical techniques and neoadjuvant therapy, which includes irinotecan, oxaliplatin, cetuximab, and bevacizumab, the prognosis of appropriately selected patients who underwent liver transplantation has improved, with 5-year OS rates reportedly reaching more than 50%, which is comparable with chemotherapy and other treatments for unresectable disease[137-139]. Toso et al[138] published an encouraging result of 12 patients with unresectable CRLM who underwent liver transplantation. The OS rates were 83%, 62%, and 50% at 1, 3, and 5 years, respectively. Most importantly, five patients had no recurrence and were still alive during the follow-up period, thus showing that long-term DFS can also be achieved through liver transplantation[138]. However, liver transplantation continues to remain a controversial treatment for unresectable CRLM.
The most important limiting factor for further use of liver transplantation is the shortage of grafts available. Quite a few patients have died due to tumor progression while waiting for a proper donor liver. Recurrence and death are still common after liver transplantation. In addition, ethical issues also remain to be resolved. Accounting for these reasons, distributing deceased donor grafts to patients with CRLM does not seem appropriate (at this point) as it will most likely impact the lives of other patients on the waiting list. Therefore, defining the patient population that would benefit the most from liver transplantation is crucial. Selection strategies should be based on prognostic factors found to be favorable for survival: diameter of the largest CLM < 55 mm, time interval of > 2 years between colorectal and transplant operations, pre-liver transplantation carcinoembryonic antigen level < 80 ng/mL, and responsive or stable disease under chemotherapy[140]. Further studies are needed to refine the risk stratification and optimize patient selection. Fortunately, several trials are ongoing to further address the potential of liver transplantation for unresectable CRLM[137,141].
After the initial LR, the recurrence rate of CRLM has been estimated to be as high as 56.7%, with the most common site being the remnant liver[142]. There are several alternatives such as repeat hepatectomy, ablation, stereotactic body radiation therapy, transcatheter arterial chemoembolization, and systemic chemotherapy for the treatment of intrahepatic recurrent CRLM. Among these therapies, a repeat LR has been found to be feasible, effective, and potentially curative in some selected patients. The postoperative morbidity rate following the initial hepatectomy is not significantly different from that after a repeated hepatectomy, although repeated LR is associated with more perioperative risks due to dense adhesions, altered liver anatomy, and reduced liver remnant after the initial operation[143,144]. Moreover, postoperative complications after aggressive repeated hepatectomy for CRLM adversely affects the oncological outcomes[143].
Several studies have evaluated the long-term outcomes of repeated LR. A recent meta-analysis showed that compared with the initial LR, repeated LR has comparable postoperative outcomes and a similar long-term survival[145]. The 5-year survival rate was > 40% after repeated LR despite the DFS being lower than that of the initial hepatectomy[143,146,147]. Furthermore, redo-hepatectomy for single recurrent CRLM is as effective as primary surgical treatment for single CRLM. However, redo-hepatectomy for multiple recurrent CRLM is less effective than that for single recurrent CRLM[144]. Multiple CRLM, large tumor size, extrahepatic metastases, and short tumor-free interval predict significantly poor outcomes[144,148]. Therefore, only selected patients are suitable for repeated surgery.
We believe that the following criteria suggested by Luo et al[149], according to a systemic review and meta-analysis, can be used to select patients for repeated LR, which is associated with a significantly longer survival compared with other treatment therapies such as RFA, transarterial chemoembolization, radiation, and chemotherapy: (1) DFS after initial LR > 1 year; (2) solitary CRLM; (3) unilobar CRLM; (4) maximal size of CRLM at the second LR < 5 cm; (5) absence of extrahepatic disease during the second hepatic resection; and (6) R0 resection at the second hepatectomy[149].
Patients aged over 65 years are often considered elderly[150,151]. Several papers have assessed the impact of age on morbidity and mortality following hepatectomy for CRLM. Although postoperative morbidity and mortality are significantly higher in those with an advanced age, LR for CRLM seems justified in selected elderly patients[151-153]. In a study on elderly patients, a major hepatectomy was found to be safe and feasible in the selected octogenarian patients, with no significant differences in the perioperative outcomes, DFS, or OS[154]. Mäkelä et al[155] have reported that even in the oldest patients (age > 80 years), favorable long-term survival can be achieved by surgical resection. As there is a higher possibility of noncancer-related deaths in elderly patients during the follow-up period, a slightly lower long-term survival is acceptable. Therefore, an elderly age should not be considered a contraindication to hepatic resection of CRLM. The main concern is the higher rate of postoperative complications caused by co-morbidities such as cardiopulmonary and cerebrovascular diseases[156]. These pre-existing disorders are frequently related to an increased difficulty in giving anesthesia and conducting an operation, with the risk of postoperative death. Hence, the strict assessment of the preoperative general condition and the careful selection of elderly patients are the keys to achieve satisfactory short- and long-term outcomes. Recent research has advocated the use of cardiopulmonary exercise testing for preoperative evaluation and enhanced recovery after surgery as a part of postoperative management in elderly patients[157]. The authors showed that in appropriately selected patients, the postoperative outcomes were comparable to the younger counterparts[157].
Considering the potential advantage of a minimally invasive approach for elderly patients such as less trauma and faster recovery, laparoscopic and robot-assisted LR have been attempted. Martínez-Cecilia et al[150] expanded the use of LLR for elderly patients, and their results suggested that LLR offers equivalent oncological outcomes, with a reduction in both minor and major postoperative morbidities in those aged > 70 years. Even compared with laparoscopic RFA, LLR for elderly people was safe and tolerable, with similar perioperative outcomes[158]. It seems that LLR for CRLM in elderly patients is a promising treatment option. Therefore, further randomized controlled trials are required to determine the real benefits and risks associated with LLR.
In CRC patients, 23%–38% of them have or will develop EHMD[159,160]. In the early 1990s, EHMD was considered as one of the contraindications of CRLM resection because of its low 5-year survival rate[161]. A population-based study of 15133 CRC patients showed that patients with isolated lung metastases had better cancer-specific survival and OS as compared to patients with metastases to the liver, bone, and brain[162]. Therefore, limited EHMD such as pulmonary EHMD is now no longer a contraindication of LR and patients can receive a R0 resection as long as the FLR is sufficient so that the patient can tolerate the major surgery of both the liver and EHMD[57,163,164]. The cure rate reached 19% in patients who received potentially curative resection of both the liver and EHMD[164]; this rate is comparable to CRLM patients without EHMD who received hepatectomy[57]. The median OS of patients undergoing resection for CRLM in the setting of EHMD was 34.4 mo, with estimated 3-, 5-, and 10-year survival rates of 49%, 28%, and 10%, respectively, with the combined use of effective chemotherapy and surgery[163]. Therefore, complete resection of concomitant hepatic and EHMD significantly prolongs survival.
Some studies have hypothesized that the location of the EHMD affects the prognosis[162,163,165,166]. Patients with minimal liver disease and EHMD of the lungs had the best outcomes, while those with peritoneal and lymph node metastases were associated with the worst prognosis. In a population-based study, the results showed that the OS time for CRC patients with isolated liver, lung, bone, and brain metastases was 16, 20, 7, and 5 mo, respectively[162]. The 3- and 5-year OS rates were 58% and 26% for pulmonary EHMD, 37% and 17% for peritoneal EHMD, and 35% and 15% for lymph nodal metastases; the combined relative risk of death after 5 years was 1.49 for lung EHMD, 1.59 for peritoneal EHMD, and 1.70 for lymph nodal EHMD[165]. The results of a retrospective study further showed that the survival time of patients with perihepatic lymph node metastases was significantly shorter than that for those patients without it (recurrence-free survival: 5.3 mo vs 13.8 mo; OS: 20.5 mo vs 71.3 mo). The median OS was significantly longer in patients with para-aortic compared to hepatoduodenal ligament lymph node metastases (58.2 mo vs 15.5 mo). Patients with three or more perihepatic lymph node metastases had a significantly worse median OS than those with one or two (16.3 mo vs 25.4 mo)[166]. These findings suggest that there are marked differences in survival depending on which lymph nodes are involved.
The survival rates of patients who underwent resection were much higher than those of patients who only received chemotherapy[142,167]. However, unresectability of EHMD is a contraindication for curative LR, as it is extremely likely to result in a poor prognosis[163]. Thus, the resectability of EHMD should be evaluated before the surgical treatment of CRLM. Chua et al[168] considered that neoadjuvant chemotherapy could be used as a tool to assess the biological characteristics of a tumor. If a tumor has a positive response to chemotherapy, the patients may be selected for surgery of the liver and EHMD[168]. The scoring system also may be helpful to measure whether the EHMD should be resected. A predictive model was constructed by Adam et al[169] to select the appropriate candidates, and the five prognostic factors found were as follows: absence of isolated lung metastases, carcinoembryonic antigen level ≥ 10 ng/mL, CRLM ≥ 6 at the time of diagnosis, EHMD concomitant with CRLM recurrence, and the location of the primary tumor in the right colon[169]. The 5-year OS was 64% in the patients without all of these factors, whereas the presence of 4–5 factors reduced the 5-year OS to nil. They also built a risk scoring system in which three factors, LM size, EHMD site, and number of LM, were assigned 1 point each[169]. They found that the patients whose risk score was 3 had the worst outcomes, similar to those who received chemotherapy alone. The patients whose risk score was nil had the best outcome, similar to patients with resectable LM without EHMD. Even those at low risk can achieve a relatively long survival.
In summary, these recent studies provide some evidence that CRLM with concurrent EHMD can be resected to yield promising medium- and long-term survival; thus, it should no longer be considered an absolute contraindication to curative surgery in selected CRLM patients.
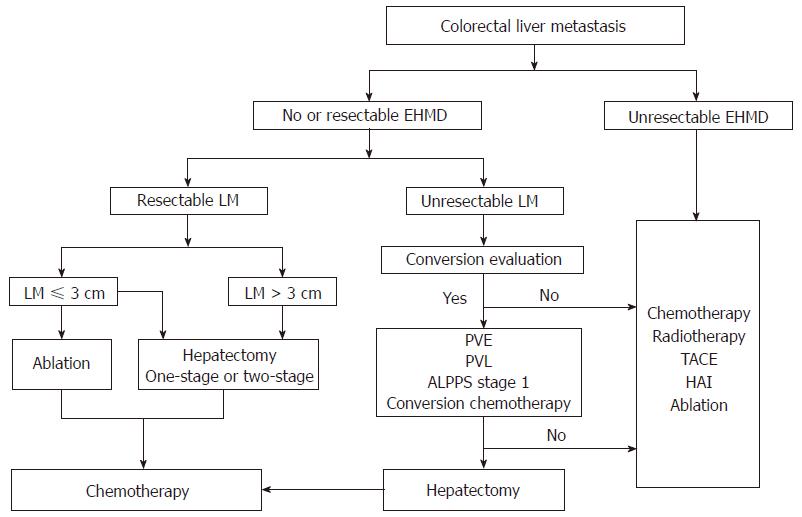
Treatment of patients with CRLM is still a major clinical challenge. Curative resection is the best treatment option to prolong the survival, but further work is needed to better identify patients who are likely to benefit the most from surgery. With the help of modern multimodality therapy such as effective systemic chemotherapy, an aggressive oncosurgical approach should be implemented as it has the possibility of achieving a cure even when EHMD is present in patients with CRLM. In some strictly selected patients, liver transplantation may be a potential treatment option. We propose a simple flow chart to help in planning out the treatment of patients with CRLM (Figure 1).
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Matsuda A, Le Bian AZ S- Editor: Wang JL L- Editor: Wang TQ E- Editor: Song H
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20511] [Article Influence: 2051.1] [Reference Citation Analysis (20)] |
| 2. | Kemeny N. The management of resectable and unresectable liver metastases from colorectal cancer. Curr Opin Oncol. 2010;22:364-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Lupinacci RM, Andraus W, De Paiva Haddad LB, Carneiro D’ Albuquerque LA, Herman P. Simultaneous laparoscopic resection of primary colorectal cancer and associated liver metastases: a systematic review. Tech Coloproctol. 2014;18:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11573] [Cited by in RCA: 13157] [Article Influence: 1879.6] [Reference Citation Analysis (4)] |
| 5. | Chakedis J, Squires MH, Beal EW, Hughes T, Lewis H, Paredes A, Al-Mansour M, Sun S, Cloyd JM, Pawlik TM. Update on current problems in colorectal liver metastasis. Curr Probl Surg. 2017;54:554-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Line PD, Hagness M, Dueland S. The Potential Role of Liver Transplantation as a Treatment Option in Colorectal Liver Metastases. Can J Gastroenterol Hepatol. 2018;2018:8547940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Hof J, Wertenbroek MW, Peeters PM, Widder J, Sieders E, de Jong KP. Outcomes after resection and/or radiofrequency ablation for recurrence after treatment of colorectal liver metastases. Br J Surg. 2016;103:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Omichi K, Shindoh J, Cloyd JM, Mizuno T, Chun YS, Conrad C, Aloia TA, Tzeng CD, Vauthey JN. Liver resection is justified for patients with bilateral multiple colorectal liver metastases: A propensity-score-matched analysis. Eur J Surg Oncol. 2018;44:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Ekberg H, Tranberg KG, Andersson R, Lundstedt C, Hägerstrand I, Ranstam J, Bengmark S. Determinants of survival in liver resection for colorectal secondaries. Br J Surg. 1986;73:727-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 338] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | van Dam RM, Lodewick TM, van den Broek MA, de Jong MC, Greve JW, Jansen RL, Bemelmans MH, Neumann UP, Olde Damink SW, Dejong CH. Outcomes of extended versus limited indications for patients undergoing a liver resection for colorectal cancer liver metastases. HPB (Oxford). 2014;16:550-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Viganò L, Capussotti L, Majno P, Toso C, Ferrero A, De Rosa G, Rubbia-Brandt L, Mentha G. Liver resection in patients with eight or more colorectal liver metastases. Br J Surg. 2015;102:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Allard MA, Adam R, Giuliante F, Lapointe R, Hubert C, Ijzermans JNM, Mirza DF, Elias D, Laurent C, Gruenberger T. Long-term outcomes of patients with 10 or more colorectal liver metastases. Br J Cancer. 2017;117:604-611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN; Americas Hepato-Pancreato-Biliary Association; Society of Surgical Oncology; Society for Surgery of the Alimentary Tract. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford). 2013;15:91-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 14. | Ellebæk SB, Fristrup CW, Mortensen MB. Intraoperative Ultrasound as a Screening Modality for the Detection of Liver Metastases during Resection of Primary Colorectal Cancer - A Systematic Review. Ultrasound Int Open. 2017;3:E60-E68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013;34:11-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 16. | Choi SH, Kim SY, Park SH, Kim KW, Lee JY, Lee SS, Lee MG. Diagnostic performance of CT, gadoxetate disodium-enhanced MRI, and PET/CT for the diagnosis of colorectal liver metastasis: Systematic review and meta-analysis. J Magn Reson Imaging. 2018;47:1237-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Ko Y, Kim J, Park JK, Kim H, Cho JY, Kang SB, Ahn S, Lee KJ, Lee KH. Limited detection of small (≤ 10 mm) colorectal liver metastasis at preoperative CT in patients undergoing liver resection. PLoS One. 2017;12:e0189797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Saing S, Haywood P, Duncan JK, Ma N, Cameron AL, Goodall S. Cost-effective imaging for resectability of liver lesions in colorectal cancer: an economic decision model. ANZ J Surg. 2018;88:E507-E511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Dunet V, Halkic N, Prior JO, Anaye A, Meuli RA, Sempoux C, Denys A, Schmidt S. Detection and Viability of Colorectal Liver Metastases After Neoadjuvant Chemotherapy: A Multiparametric PET/CT-MRI Study. Clin Nucl Med. 2017;42:258-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Expert Panel on Gastrointestinal Imaging, Fowler KJ, Kaur H, Cash BD, Feig BW, Gage KL, Garcia EM, Hara AK, Herman JM, Kim DH, Lambert DL, Levy AD, Peterson CM, Scheirey CD, Small W Jr, Smith MP, Lalani T, Carucci LR. ACR Appropriateness Criteria® Pretreatment Staging of Colorectal Cancer. J Am Coll Radiol. 2017;14:S234-S244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 21. | Shim JR, Lee SD, Han SS, Lee SJ, Lee DE, Kim SK, Kim SH, Park SJ, Oh JH. Prognostic significance of 18F-FDG PET/CT in patients with colorectal cancer liver metastases after hepatectomy. Eur J Surg Oncol. 2018;44:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Hall NC, Ruutiainen AT. Colorectal Cancer: Imaging Conundrums. Surg Oncol Clin N Am. 2018;27:289-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Viganò L, Lopci E, Costa G, Rodari M, Poretti D, Pedicini V, Solbiati L, Chiti A, Torzilli G. Positron Emission Tomography-Computed Tomography for Patients with Recurrent Colorectal Liver Metastases: Impact on Restaging and Treatment Planning. Ann Surg Oncol. 2017;24:1029-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Abbadi RA, Sadat U, Jah A, Praseedom RK, Jamieson NV, Cheow HK, Whitley S, Ford HE, Wilson CB, Harper SJ. Improved long-term survival after resection of colorectal liver metastases following staging with FDG positron emission tomography. J Surg Oncol. 2014;110:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Moulton CA, Gu CS, Law CH, Tandan VR, Hart R, Quan D, Fairfull Smith RJ, Jalink DW, Husien M, Serrano PE. Effect of PET before liver resection on surgical management for colorectal adenocarcinoma metastases: a randomized clinical trial. JAMA. 2014;311:1863-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 26. | Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L, Sobrero A, Teh C, Tejpar S, Van Cutsem E, Vauthey JN, Påhlman L; of the EGOSLIM (Expert Group on OncoSurgery management of LIver Metastases) group. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev. 2015;41:729-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 396] [Article Influence: 39.6] [Reference Citation Analysis (2)] |
| 27. | Arita J, Ono Y, Takahashi M, Inoue Y, Takahashi Y, Matsueda K, Saiura A. Routine Preoperative Liver-specific Magnetic Resonance Imaging Does Not Exclude the Necessity of Contrast-enhanced Intraoperative Ultrasound in Hepatic Resection for Colorectal Liver Metastasis. Ann Surg. 2015;262:1086-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 28. | Boogerd LS, Handgraaf HJ, Lam HD, Huurman VA, Farina-Sarasqueta A, Frangioni JV, van de Velde CJ, Braat AE, Vahrmeijer AL. Laparoscopic detection and resection of occult liver tumors of multiple cancer types using real-time near-infrared fluorescence guidance. Surg Endosc. 2017;31:952-961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Lafaro K, Buettner S, Maqsood H, Wagner D, Bagante F, Spolverato G, Xu L, Kamel I, Pawlik TM. Defining Post Hepatectomy Liver Insufficiency: Where do We stand? J Gastrointest Surg. 2015;19:2079-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 30. | Martel G, Cieslak KP, Huang R, van Lienden KP, Wiggers JK, Belblidia A, Dagenais M, Lapointe R, van Gulik TM, Vandenbroucke-Menu F. Comparison of techniques for volumetric analysis of the future liver remnant: implications for major hepatic resections. HPB (Oxford). 2015;17:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Pulitano C, Crawford M, Joseph D, Aldrighetti L, Sandroussi C. Preoperative assessment of postoperative liver function: the importance of residual liver volume. J Surg Oncol. 2014;110:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Shindoh J, Tzeng CW, Aloia TA, Curley SA, Zimmitti G, Wei SH, Huang SY, Mahvash A, Gupta S, Wallace MJ. Optimal future liver remnant in patients treated with extensive preoperative chemotherapy for colorectal liver metastases. Ann Surg Oncol. 2013;20:2493-2500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 33. | Takamoto T, Hashimoto T, Ichida A, Shimada K, Maruyama Y, Makuuchi M. Surgical Strategy Based on Indocyanine Green Test for Chemotherapy-Associated Liver Injury and Long-Term Outcome in Colorectal Liver Metastases. J Gastrointest Surg. 2018;22:1077-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Abdalla EK. Resection of colorectal liver metastases. J Gastrointest Surg. 2011;15:416-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Simpson AL, Geller DA, Hemming AW, Jarnagin WR, Clements LW, D’Angelica MI, Dumpuri P, Gönen M, Zendejas I, Miga MI. Liver planning software accurately predicts postoperative liver volume and measures early regeneration. J Am Coll Surg. 2014;219:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (2)] |
| 36. | Kanoria S, Robertson FP, Mehta NN, Fusai G, Sharma D, Davidson BR. Effect of Remote Ischaemic Preconditioning on Liver Injury in Patients Undergoing Major Hepatectomy for Colorectal Liver Metastasis: A Pilot Randomised Controlled Feasibility Trial. World J Surg. 2017;41:1322-1330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Krieger PM, Tamandl D, Herberger B, Faybik P, Fleischmann E, Maresch J, Gruenberger T. Evaluation of chemotherapy-associated liver injury in patients with colorectal cancer liver metastases using indocyanine green clearance testing. Ann Surg Oncol. 2011;18:1644-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Wakiya T, Kudo D, Toyoki Y, Ishido K, Kimura N, Narumi S, Kijima H, Hakamada K. Evaluation of the usefulness of the indocyanine green clearance test for chemotherapy-associated liver injury in patients with colorectal cancer liver metastasis. Ann Surg Oncol. 2014;21:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Erdogan D, Heijnen BH, Bennink RJ, Kok M, Dinant S, Straatsburg IH, Gouma DJ, van Gulik TM. Preoperative assessment of liver function: a comparison of 99mTc-Mebrofenin scintigraphy with indocyanine green clearance test. Liver Int. 2004;24:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Cieslak KP, Bennink RJ, de Graaf W, van Lienden KP, Besselink MG, Busch OR, Gouma DJ, van Gulik TM. Measurement of liver function using hepatobiliary scintigraphy improves risk assessment in patients undergoing major liver resection. HPB (Oxford). 2016;18:773-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 41. | Ihnát P, Vávra P, Zonča P. Treatment strategies for colorectal carcinoma with synchronous liver metastases: Which way to go? World J Gastroenterol. 2015;21:7014-7021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Vallance AE, van der Meulen J, Kuryba A, Charman SC, Botterill ID, Prasad KR, Hill J, Jayne DG, Walker K. The timing of liver resection in patients with colorectal cancer and synchronous liver metastases: a population-based study of current practice and survival. Colorectal Dis. 2018;20:486-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 43. | Sturesson C, Valdimarsson VT, Blomstrand E, Eriksson S, Nilsson JH, Syk I, Lindell G. Liver-first strategy for synchronous colorectal liver metastases - an intention-to-treat analysis. HPB (Oxford). 2017;19:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Esposito F, Lim C, Sa Cunha A, Pessaux P, Navarro F, Azoulay D; French Colorectal Liver Metastases Working Group, Association Française de Chirurgie (AFC). Primary Tumor Versus Liver-First Approach for Synchronous Colorectal Liver Metastases: An Association Française de Chirurgie (AFC) Multicenter-Based Study with Propensity Score Analysis. World J Surg. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Valdimarsson VT, Syk I, Lindell G, Norén A, Isaksson B, Sandström P, Rizell M, Ardnor B, Sturesson C. Outcomes of liver-first strategy and classical strategy for synchronous colorectal liver metastases in Sweden. HPB (Oxford). 2018;20:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Le Souder EB, Azin A, Hirpara DH, Walker R, Cleary S, Quereshy F. Considering the cost of a simultaneous versus staged approach to resection of colorectal cancer with synchronous liver metastases in a publicly funded healthcare model. J Surg Oncol. 2018;117:1376-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Welsh FK, Chandrakumaran K, John TG, Cresswell AB, Rees M. Propensity score-matched outcomes analysis of the liver-first approach for synchronous colorectal liver metastases. Br J Surg. 2016;103:600-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Mizuno T, Cloyd JM, Omichi K, Chun YS, Conrad C, Tzeng CD, Wei SH, Aloia TA, Vauthey JN. Two-Stage Hepatectomy vs One-Stage Major Hepatectomy with Contralateral Resection or Ablation for Advanced Bilobar Colorectal Liver Metastases. J Am Coll Surg. 2018;226:825-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Slesser AA, Simillis C, Goldin R, Brown G, Mudan S, Tekkis PP. A meta-analysis comparing simultaneous versus delayed resections in patients with synchronous colorectal liver metastases. Surg Oncol. 2013;22:36-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 50. | Silberhumer GR, Paty PB, Denton B, Guillem J, Gonen M, Araujo RLC, Nash GM, Temple LK, Allen PJ, DeMatteo RP. Long-term oncologic outcomes for simultaneous resection of synchronous metastatic liver and primary colorectal cancer. Surgery. 2016;160:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 51. | Abelson JS, Michelassi F, Sun T, Mao J, Milsom J, Samstein B, Sedrakyan A, Yeo HL. Simultaneous Resection for Synchronous Colorectal Liver Metastasis: the New Standard of Care? J Gastrointest Surg. 2017;21:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 52. | Gavriilidis P, Sutcliffe RP, Hodson J, Marudanayagam R, Isaac J, Azoulay D, Roberts KJ. Simultaneous versus delayed hepatectomy for synchronous colorectal liver metastases: a systematic review and meta-analysis. HPB (Oxford). 2018;20:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 53. | Nanji S, Mackillop WJ, Wei X, Booth CM. Simultaneous resection of primary colorectal cancer and synchronous liver metastases: a population-based study. Can J Surg. 2017;60:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 54. | Yin Z, Liu C, Chen Y, Bai Y, Shang C, Yin R, Yin D, Wang J. Timing of hepatectomy in resectable synchronous colorectal liver metastases (SCRLM): Simultaneous or delayed? Hepatology. 2013;57:2346-2357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 55. | Berardi G, De Man M, Laurent S, Smeets P, Tomassini F, Ariotti R, Hoorens A, van Dorpe J, Varin O, Geboes K. Radiologic and pathologic response to neoadjuvant chemotherapy predicts survival in patients undergoing the liver-first approach for synchronous colorectal liver metastases. Eur J Surg Oncol. 2018;44:1069-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Kim CW, Lee JL, Yoon YS, Park IJ, Lim SB, Yu CS, Kim TW, Kim JC. Resection after preoperative chemotherapy versus synchronous liver resection of colorectal cancer liver metastases: A propensity score matching analysis. Medicine (Baltimore). 2017;96:e6174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Creasy JM, Sadot E, Koerkamp BG, Chou JF, Gonen M, Kemeny NE, Balachandran VP, Kingham TP, DeMatteo RP, Allen PJ. Actual 10-year survival after hepatic resection of colorectal liver metastases: what factors preclude cure? Surgery. 2018;163:1238-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (1)] |
| 58. | Acciuffi S, Meyer F, Bauschke A, Settmacher U, Lippert H, Croner R, Altendorf-Hofmann A. Analysis of prognostic factors after resection of solitary liver metastasis in colorectal cancer: a 22-year bicentre study. J Cancer Res Clin Oncol. 2018;144:593-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Lim C, Doussot A, Osseis M, Esposito F, Salloum C, Calderaro J, Tournigand C, Azoulay D. Bevacizumab improves survival in patients with synchronous colorectal liver metastases provided the primary tumor is resected first. Clin Transl Oncol. 2018;20:1274-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Primrose J, Falk S, Finch-Jones M, Valle J, O’Reilly D, Siriwardena A, Hornbuckle J, Peterson M, Rees M, Iveson T. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15:601-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 310] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 61. | Kishiki T, Lapin B, Matsuoka H, Watanabe T, Takayasu K, Kojima K, Sugihara K, Masaki T. Optimal Surveillance Protocols After Curative Resection in Patients With Stage IV Colorectal Cancer: A Multicenter Retrospective Study. Dis Colon Rectum. 2018;61:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Memeo R, de Blasi V, Adam R, Goéré D, Piardi T, Lermite E, Turrini O, Navarro F, de’Angelis N, Cunha AS, Pessaux P; French Colorectal Liver Metastases Working Group, Association Française de Chirurgie (AFC). Margin Status is Still an Important Prognostic Factor in Hepatectomies for Colorectal Liver Metastases: A Propensity Score Matching Analysis. World J Surg. 2018;42:892-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Sadot E, Groot Koerkamp B, Leal JN, Shia J, Gonen M, Allen PJ, DeMatteo RP, Kingham TP, Kemeny N, Blumgart LH. Resection margin and survival in 2368 patients undergoing hepatic resection for metastatic colorectal cancer: surgical technique or biologic surrogate? Ann Surg. 2015;262:476-485; discussion 483-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 64. | Sasaki K, Margonis GA, Maitani K, Andreatos N, Wang J, Pikoulis E, He J, Wolfgang CL, Weiss M, Pawlik TM. The Prognostic Impact of Determining Resection Margin Status for Multiple Colorectal Metastases According to the Margin of the Largest Lesion. Ann Surg Oncol. 2017;24:2438-2446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Margonis GA, Sergentanis TN, Ntanasis-Stathopoulos I, Andreatos N, Tzanninis IG, Sasaki K, Psaltopoulou T, Wang J, Buettner S, Papalois ΑE, He J, Wolfgang CL, Pawlik TM, Weiss MJ. Impact of Surgical Margin Width on Recurrence and Overall Survival Following R0 Hepatic Resection of Colorectal Metastases: A Systematic Review and Meta-analysis. Ann Surg. 2018;267:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 66. | Dhir M, Lyden ER, Wang A, Smith LM, Ullrich F, Are C. Influence of margins on overall survival after hepatic resection for colorectal metastasis: a meta-analysis. Ann Surg. 2011;254:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 67. | Margonis GA, Sasaki K, Andreatos N, Kim Y, Merath K, Wagner D, Wilson A, Buettner S, Amini N, Antoniou E. KRAS Mutation Status Dictates Optimal Surgical Margin Width in Patients Undergoing Resection of Colorectal Liver Metastases. Ann Surg Oncol. 2017;24:264-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 68. | Hamady ZZ, Lodge JP, Welsh FK, Toogood GJ, White A, John T, Rees M. One-millimeter cancer-free margin is curative for colorectal liver metastases: a propensity score case-match approach. Ann Surg. 2014;259:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 69. | Truant S, Séquier C, Leteurtre E, Boleslawski E, Elamrani M, Huet G, Duhamel A, Hebbar M, Pruvot FR. Tumour biology of colorectal liver metastasis is a more important factor in survival than surgical margin clearance in the era of modern chemotherapy regimens. HPB (Oxford). 2015;17:176-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 70. | Moris D, Ronnekleiv-Kelly S, Rahnemai-Azar AA, Felekouras E, Dillhoff M, Schmidt C, Pawlik TM. Parenchymal-Sparing Versus Anatomic Liver Resection for Colorectal Liver Metastases: a Systematic Review. J Gastrointest Surg. 2017;21:1076-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 71. | Margonis GA, Sasaki K, Kim Y, Samaha M, Buettner S, Amini N, Antoniou E, Pawlik TM. Tumor Biology Rather Than Surgical Technique Dictates Prognosis in Colorectal Cancer Liver Metastases. J Gastrointest Surg. 2016;20:1821-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 72. | Brunner SM, Kesselring R, Rubner C, Martin M, Jeiter T, Boerner T, Ruemmele P, Schlitt HJ, Fichtner-Feigl S. Prognosis according to histochemical analysis of liver metastases removed at liver resection. Br J Surg. 2014;101:1681-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 73. | Laurent C, Adam JP, Denost Q, Smith D, Saric J, Chiche L. Significance of R1 Resection for Advanced Colorectal Liver Metastases in the Era of Modern Effective Chemotherapy. World J Surg. 2016;40:1191-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 74. | Sasaki K, Margonis GA, Andreatos N, Wilson A, Weiss M, Wolfgang C, Sergentanis TN, Polychronidis G, He J, Pawlik TM. Prognostic impact of margin status in liver resections for colorectal metastases after bevacizumab. Br J Surg. 2017;104:926-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Makowiec F, Bronsert P, Klock A, Hopt UT, Neeff HP. Prognostic influence of hepatic margin after resection of colorectal liver metastasis: role of modern preoperative chemotherapy. Int J Colorectal Dis. 2018;33:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Wang Y, Wang ZQ, Wang FH, Yuan YF, Li BK, Ding PR, Chen G, Wu XJ, Lu ZH, Pan ZZ. The Role of Adjuvant Chemotherapy for Colorectal Liver Metastasectomy after Pre-Operative Chemotherapy: Is the Treatment Worthwhile? J Cancer. 2017;8:1179-1186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Strandberg Holka P, Eriksson S, Eberhard J, Bergenfeldt M, Lindell G, Sturesson C. Significance of poor performance status after resection of colorectal liver metastases. World J Surg Oncol. 2018;16:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 78. | Gillams A, Goldberg N, Ahmed M, Bale R, Breen D, Callstrom M, Chen MH, Choi BI, de Baere T, Dupuy D. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontières meeting 2013. Eur Radiol. 2015;25:3438-3454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 225] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 79. | Ruers T, Punt C, Van Coevorden F, Pierie JP, Borel-Rinkes I, Ledermann JA, Poston G, Bechstein W, Lentz MA, Mauer M, Van Cutsem E, Lutz MP, Nordlinger B; EORTC Gastro-Intestinal Tract Cancer Group, Arbeitsgruppe Lebermetastasen und—tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO) and the National Cancer Research Institute Colorectal Clinical Study Group (NCRI CCSG). Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol. 2012;23:2619-2626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 312] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 80. | He N, Jin QN, Wang D, Yang YM, Liu YL, Wang GB, Tao KX. Radiofrequency ablation vs. hepatic resection for resectable colorectal liver metastases. J Huazhong Univ Sci Technolog Med Sci. 2016;36:514-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 81. | van Amerongen MJ, Jenniskens SFM, van den Boezem PB, Fütterer JJ, de Wilt JHW. Radiofrequency ablation compared to surgical resection for curative treatment of patients with colorectal liver metastases - a meta-analysis. HPB (Oxford). 2017;19:749-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 82. | Correa-Gallego C, Fong Y, Gonen M, D’Angelica MI, Allen PJ, DeMatteo RP, Jarnagin WR, Kingham TP. A retrospective comparison of microwave ablation vs. radiofrequency ablation for colorectal cancer hepatic metastases. Ann Surg Oncol. 2014;21:4278-4283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 83. | Yang B, Li Y. A comparative study of laparoscopic microwave ablation with laparoscopic radiofrequency ablation for colorectal liver metastasis. J BUON. 2017;22:667-672. [PubMed] |
| 84. | Mulier S, Ruers T, Jamart J, Michel L, Marchal G, Ni Y. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? An update. Dig Surg. 2008;25:445-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 85. | Imai K, Allard MA, Castro Benitez C, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Baba H, Adam R. Long-term outcomes of radiofrequency ablation combined with hepatectomy compared with hepatectomy alone for colorectal liver metastases. Br J Surg. 2017;104:570-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 86. | Evrard S, Poston G, Kissmeyer-Nielsen P, Diallo A, Desolneux G, Brouste V, Lalet C, Mortensen F, Stättner S, Fenwick S. Combined ablation and resection (CARe) as an effective parenchymal sparing treatment for extensive colorectal liver metastases. PLoS One. 2014;9:e114404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 87. | Mise Y, Aloia TA, Brudvik KW, Schwarz L, Vauthey JN, Conrad C. Parenchymal-sparing Hepatectomy in Colorectal Liver Metastasis Improves Salvageability and Survival. Ann Surg. 2016;263:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 88. | Dupré A, Jones RP, Diaz-Nieto R, Fenwick SW, Poston GJ, Malik HZ. Curative-intent treatment of recurrent colorectal liver metastases: A comparison between ablation and resection. Eur J Surg Oncol. 2017;43:1901-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 89. | Aliyev S, Agcaoglu O, Aksoy E, Taskin HE, Vogt D, Fung J, Siperstein A, Berber E. Efficacy of laparoscopic radiofrequency ablation for the treatment of patients with small solitary colorectal liver metastasis. Surgery. 2013;154:556-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 90. | Ringe KI, Lutat C, Rieder C, Schenk A, Wacker F, Raatschen HJ. Experimental Evaluation of the Heat Sink Effect in Hepatic Microwave Ablation. PLoS One. 2015;10:e0134301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 91. | Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, Kazaryan AM, Kristiansen R, Fagerland MW, Hausken J, Tønnessen TI, Abildgaard A, Barkhatov L, Yaqub S, Røsok BI, Bjørnbeth BA, Andersen MH, Flatmark K, Aas E, Edwin B. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg. 2018;267:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 480] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 92. | Xie SM, Xiong JJ, Liu XT, Chen HY, Iglesia-García D, Altaf K, Bharucha S, Huang W, Nunes QM, Szatmary P. Laparoscopic Versus Open Liver Resection for Colorectal Liver Metastases: A Comprehensive Systematic Review and Meta-analysis. Sci Rep. 2017;7:1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 93. | Montalti R, Berardi G, Laurent S, Sebastiani S, Ferdinande L, Libbrecht LJ, Smeets P, Brescia A, Rogiers X, de Hemptinne B. Laparoscopic liver resection compared to open approach in patients with colorectal liver metastases improves further resectability: Oncological outcomes of a case-control matched-pairs analysis. Eur J Surg Oncol. 2014;40:536-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 94. | Di Fabio F, Barkhatov L, Bonadio I, Dimovska E, Fretland ÅA, Pearce NW, Troisi RI, Edwin B, Abu Hilal M. The impact of laparoscopic versus open colorectal cancer surgery on subsequent laparoscopic resection of liver metastases: A multicenter study. Surgery. 2015;157:1046-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 95. | Efanov M, Alikhanov R, Tsvirkun V, Kazakov I, Melekhina O, Kim P, Vankovich A, Grendal K, Berelavichus S, Khatkov I. Comparative analysis of learning curve in complex robot-assisted and laparoscopic liver resection. HPB (Oxford). 2017;19:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 96. | Nota CLMA, Molenaar IQ, van Hillegersberg R, Borel Rinkes IHM, Hagendoorn J. Robotic liver resection including the posterosuperior segments: initial experience. J Surg Res. 2016;206:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 97. | Wang WH, Kuo KK, Wang SN, Lee KT. Oncological and surgical result of hepatoma after robot surgery. Surg Endosc. 2018;32:3918-3924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 98. | Takahashi S, Konishi M, Kinoshita T, Gotohda N, Kato Y, Saito N, Sugito M, Yoshino T. Predictors for early recurrence after hepatectomy for initially unresectable colorectal liver metastasis. J Gastrointest Surg. 2013;17:939-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Charnsangavej C, Clary B, Fong Y, Grothey A, Pawlik TM, Choti MA. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1261-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 233] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 100. | Xiao N, Yu K, Yu S, Wu J, Wang J, Shan S, Zheng S, Wang L, Wang J, Peng S. The paradigm of tumor shrinkage and rapid liver remnant hypertrophy for conversion of initially unresectable colorectal liver metastasis: a case report and literature review. World J Surg Oncol. 2017;15:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 101. | Simoneau E, Alanazi R, Alshenaifi J, Molla N, Aljiffry M, Medkhali A, Boucher LM, Asselah J, Metrakos P, Hassanain M. Neoadjuvant chemotherapy does not impair liver regeneration following hepatectomy or portal vein embolization for colorectal cancer liver metastases. J Surg Oncol. 2016;113:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | Cassinotto C, Dohan A, Gallix B, Simoneau E, Boucher LM, Metrakos P, Cabrera T, Torres C, Muchantef K, Valenti DA. Portal Vein Embolization in the Setting of Staged Hepatectomy with Preservation of Segment IV ± I Only for Bilobar Colorectal Liver Metastases: Safety, Efficacy, and Clinical Outcomes. J Vasc Interv Radiol. 2017;28:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 103. | Huiskens J, Olthof PB, van der Stok EP, Bais T, van Lienden KP, Moelker A, Krumeich J, Roumen RM, Grünhagen DJ, Punt CJA. Does portal vein embolization prior to liver resection influence the oncological outcomes - A propensity score matched comparison. Eur J Surg Oncol. 2018;44:108-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 104. | Ironside N, Bell R, Bartlett A, McCall J, Powell J, Pandanaboyana S. Systematic review of perioperative and survival outcomes of liver resections with and without preoperative portal vein embolization for colorectal metastases. HPB (Oxford). 2017;19:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 105. | Elias D, De Baere T, Roche A, Mducreux , Leclere J, Lasser P. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg. 1999;86:784-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 240] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 106. | Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, Ohta K, Yamaguchi T, Matsubara T, Takahashi T. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 247] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 107. | Yamashita S, Sakamoto Y, Yamamoto S, Takemura N, Omichi K, Shinkawa H, Mori K, Kaneko J, Akamatsu N, Arita J. Efficacy of Preoperative Portal Vein Embolization Among Patients with Hepatocellular Carcinoma, Biliary Tract Cancer, and Colorectal Liver Metastases: A Comparative Study Based on Single-Center Experience of 319 Cases. Ann Surg Oncol. 2017;24:1557-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 108. | Isfordink CJ, Samim M, Braat MNGJA, Almalki AM, Hagendoorn J, Borel Rinkes IHM, Molenaar IQ. Portal vein ligation versus portal vein embolization for induction of hypertrophy of the future liver remnant: A systematic review and meta-analysis. Surg Oncol. 2017;26:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 109. | Levi Sandri GB, Santoro R, Vennarecci G, Lepiane P, Colasanti M, Ettorre GM. Two-stage hepatectomy, a 10 years experience. Updates Surg. 2015;67:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 110. | Lam VW, Laurence JM, Johnston E, Hollands MJ, Pleass HC, Richardson AJ. A systematic review of two-stage hepatectomy in patients with initially unresectable colorectal liver metastases. HPB (Oxford). 2013;15:483-491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 111. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 932] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 112. | Sandström P, Røsok BI, Sparrelid E, Larsen PN, Larsson AL, Lindell G, Schultz NA, Bjørnbeth BA, Isaksson B, Rizell M. ALPPS Improves Resectability Compared With Conventional Two-stage Hepatectomy in Patients With Advanced Colorectal Liver Metastasis: Results From a Scandinavian Multicenter Randomized Controlled Trial (LIGRO Trial). Ann Surg. 2018;267:833-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 227] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 113. | Ratti F, Schadde E, Masetti M, Massani M, Zanello M, Serenari M, Cipriani F, Bonariol L, Bassi N, Aldrighetti L. Strategies to Increase the Resectability of Patients with Colorectal Liver Metastases: A Multi-center Case-Match Analysis of ALPPS and Conventional Two-Stage Hepatectomy. Ann Surg Oncol. 2015;22:1933-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 114. | Olthof PB, Huiskens J, Wicherts DA, Huespe PE, Ardiles V, Robles-Campos R, Adam R, Linecker M, Clavien PA, Koopman M. Survival after associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) for advanced colorectal liver metastases: A case-matched comparison with palliative systemic therapy. Surgery. 2017;161:909-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 115. | Moris D, Ronnekleiv-Kelly S, Kostakis ID, Tsilimigras DI, Beal EW, Papalampros A, Dimitroulis D, Felekouras E, Pawlik TM. Operative Results and Oncologic Outcomes of Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS) Versus Two-Stage Hepatectomy (TSH) in Patients with Unresectable Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. World J Surg. 2018;42:806-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 116. | Maulat C, Philis A, Charriere B, Mokrane FZ, Guimbaud R, Otal P, Suc B, Muscari F. Rescue associating liver partition and portal vein ligation for staged hepatectomy after portal embolization: Our experience and literature review. World J Clin Oncol. 2017;8:351-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 117. | Sparrelid E, Gilg S, Brismar TB, Lundell L, Isaksson B. Rescue ALPPS is efficient and safe after failed portal vein occlusion in patients with colorectal liver metastases. Langenbecks Arch Surg. 2017;402:69-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 118. | Serenari M, Alvarez FA, Ardiles V, de Santibañes M, Pekolj J, de Santibañes E. The ALPPS Approach for Colorectal Liver Metastases: Impact of KRAS Mutation Status in Survival. Dig Surg. 2018;35:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 119. | Zattar-Ramos LC, Bezerra RO, Siqueira LT, Marques DT, Menezes MR, Herman P, Machado MA, Cerri GG, Leite CC. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) in colorectal liver metastasis: the radiologist’s perspective. Abdom Radiol (NY). 2016;41:2150-2160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 120. | Maeda Y, Shinohara T, Nagatsu A, Futakawa N, Hamada T. Long-Term Outcomes of Conversion Hepatectomy for Initially Unresectable Colorectal Liver Metastases. Ann Surg Oncol. 2016;23 Suppl 2:S242-S248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 121. | Nozawa H, Ishihara S, Kawai K, Hata K, Kiyomatsu T, Tanaka T, Nishikawa T, Otani K, Yasuda K, Sasaki K. Conversion to Resection in Patients Receiving Systemic Chemotherapy for Unresectable and/or Metastatic Colorectal Cancer-Predictive Factors and Prognosis. Clin Colorectal Cancer. 2018;17:e91-e97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 122. | Pak LM, Kemeny NE, Capanu M, Chou JF, Boucher T, Cercek A, Balachandran VP, Kingham TP, Allen PJ, DeMatteo RP. Prospective phase II trial of combination hepatic artery infusion and systemic chemotherapy for unresectable colorectal liver metastases: Long term results and curative potential. J Surg Oncol. 2018;117:634-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 123. | Rouyer M, François E, Cunha AS, Monnereau A, Noize P, Robinson P, Droz-Perroteau C, Le Monies de Sagazan A, Jové J, Lassalle R. Effectiveness of Cetuximab as First-Line Therapy for Patients With Wild-Type KRAS and Unresectable Metastatic Colorectal Cancer in Real-Life Practice: Results of the EREBUS Cohort. Clin Colorectal Cancer. 2018;17:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 124. | Tomasello G, Petrelli F, Ghidini M, Russo A, Passalacqua R, Barni S. FOLFOXIRI Plus Bevacizumab as Conversion Therapy for Patients With Initially Unresectable Metastatic Colorectal Cancer: A Systematic Review and Pooled Analysis. JAMA Oncol. 2017;3:e170278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (1)] |
| 125. | Carrato A, Abad A, Massuti B, Grávalos C, Escudero P, Longo-Muñoz F, Manzano JL, Gómez A, Safont MJ, Gallego J, García-Paredes B, Pericay C, Dueñas R, Rivera F, Losa F, Valladares-Ayerbes M, González E, Aranda E; Spanish Cooperative Group for the Treatment of Digestive Tumours (TTD). First-line panitumumab plus FOLFOX4 or FOLFIRI in colorectal cancer with multiple or unresectable liver metastases: A randomised, phase II trial (PLANET-TTD). Eur J Cancer. 2017;81:191-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 126. | Andreatos N, Ronnekleiv-Kelly S, Margonis GA, Sasaki K, Gani F, Amini N, Wilson A, Pawlik TM. From bench to bedside: Clinical implications of KRAS status in patients with colorectal liver metastasis. Surg Oncol. 2016;25:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 127. | Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, Schrag D, Greene C, O’Neil BH, Atkins JN. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA. 2017;317:2392-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 680] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 128. | Gruenberger T, Bridgewater J, Chau I, García Alfonso P, Rivoire M, Mudan S, Lasserre S, Hermann F, Waterkamp D, Adam R. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26:702-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 250] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 129. | Hatano E, Okuno M, Nakamura K, Ishii T, Seo S, Taura K, Yasuchika K, Yazawa T, Zaima M, Kanazawa A. Conversion to complete resection with mFOLFOX6 with bevacizumab or cetuximab based on K-ras status for unresectable colorectal liver metastasis (BECK study). J Hepatobiliary Pancreat Sci. 2015;22:634-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 130. | Boeckx N, Koukakis R, Op de Beeck K, Rolfo C, Van Camp G, Siena S, Tabernero J, Douillard JY, André T, Peeters M. Primary tumor sidedness has an impact on prognosis and treatment outcome in metastatic colorectal cancer: results from two randomized first-line panitumumab studies. Ann Oncol. 2017;28:1862-1868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 131. | Peeters M, Price T, Taieb J, Geissler M, Rivera F, Canon JL, Pentheroudakis G, Koukakis R, Burdon P, Siena S. Relationships between tumour response and primary tumour location, and predictors of long-term survival, in patients with RAS wild-type metastatic colorectal cancer receiving first-line panitumumab therapy: retrospective analyses of the PRIME and PEAK clinical trials. Br J Cancer. 2018;119:303-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 132. | Brandi G, De Lorenzo S, Nannini M, Curti S, Ottone M, Dall’Olio FG, Barbera MA, Pantaleo MA, Biasco G. Adjuvant chemotherapy for resected colorectal cancer metastases: Literature review and meta-analysis. World J Gastroenterol. 2016;22:519-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 133. | Taieb J, Rivera F, Siena S, Karthaus M, Valladares-Ayerbes M, Gallego J, Geissler M, Koukakis R, Demonty G, Peeters M. Exploratory analyses assessing the impact of early tumour shrinkage and depth of response on survival outcomes in patients with RAS wild-type metastatic colorectal cancer receiving treatment in three randomised panitumumab trials. J Cancer Res Clin Oncol. 2018;144:321-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 134. | Rivera F, Karthaus M, Hecht JR, Sevilla I, Forget F, Fasola G, Canon JL, Guan X, Demonty G, Schwartzberg LS. Final analysis of the randomised PEAK trial: overall survival and tumour responses during first-line treatment with mFOLFOX6 plus either panitumumab or bevacizumab in patients with metastatic colorectal carcinoma. Int J Colorectal Dis. 2017;32:1179-1190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 135. | Nagai Y, Beppu T, Sakamoto Y, Miyamoto Y, Hayashi H, Nitta H, Imai K, Masuda T, Okabe H, Hirashima K. Carcinoembryonic antigen half-life is an early predictor of therapeutic effects in induction chemotherapy for liver metastases from colorectal cancer. Anticancer Res. 2014;34:5529-5535. [PubMed] |
| 136. | Ravaioli M, Ercolani G, Neri F, Cescon M, Stacchini G, Del Gaudio M, Cucchetti A, Pinna AD. Liver transplantation for hepatic tumors: a systematic review. World J Gastroenterol. 2014;20:5345-5352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 137. | Gorgen A, Muaddi H, Zhang W, McGilvray I, Gallinger S, Sapisochin G. The New Era of Transplant Oncology: Liver Transplantation for Nonresectable Colorectal Cancer Liver Metastases. Can J Gastroenterol Hepatol. 2018;2018:9531925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 138. | Toso C, Pinto Marques H, Andres A, Castro Sousa F, Adam R, Kalil A, Clavien PA, Furtado E, Barroso E, Bismuth H; Compagnons Hépato-Biliaires Group. Liver transplantation for colorectal liver metastasis: Survival without recurrence can be achieved. Liver Transpl. 2017;23:1073-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 139. | Dueland S, Guren TK, Hagness M, Glimelius B, Line PD, Pfeiffer P, Foss A, Tveit KM. Chemotherapy or liver transplantation for nonresectable liver metastases from colorectal cancer? Ann Surg. 2015;261:956-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 140. | Hagness M, Foss A, Line PD, Scholz T, Jørgensen PF, Fosby B, Boberg KM, Mathisen O, Gladhaug IP, Egge TS. Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann Surg. 2013;257:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 281] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 141. | Moris D, Tsilimigras DI, Chakedis J, Beal EW, Felekouras E, Vernadakis S, Schizas D, Fung JJ, Pawlik TM. Liver transplantation for unresectable colorectal liver metastases: A systematic review. J Surg Oncol. 2017;116:288-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 142. | de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 590] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 143. | Fukami Y, Kaneoka Y, Maeda A, Takayama Y, Onoe S. Postoperative complications following aggressive repeat hepatectomy for colorectal liver metastasis have adverse oncological outcomes. Surg Today. 2017;47:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 144. | Neal CP, Nana GR, Jones M, Cairns V, Ngu W, Isherwood J, Dennison AR, Garcea G. Repeat hepatectomy is independently associated with favorable long-term outcome in patients with colorectal liver metastases. Cancer Med. 2017;6:331-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 145. | Wurster EF, Tenckhoff S, Probst P, Jensen K, Dölger E, Knebel P, Diener MK, Büchler MW, Ulrich A. A systematic review and meta-analysis of the utility of repeated versus single hepatic resection for colorectal cancer liver metastases. HPB (Oxford). 2017;19:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 146. | Heise D, Bayings W, Tuinhof A, Eickhoff R, Kroh A, Ulmer F, Dejong CHC, Neumann U, Binnebösel M. Long-term outcome and quality of life after initial and repeat resection of colorectal liver metastasis: A retrospective analysis. Int J Surg. 2017;48:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 147. | Oba M, Hasegawa K, Shindoh J, Yamashita S, Sakamoto Y, Makuuchi M, Kokudo N. Survival benefit of repeat resection of successive recurrences after the initial hepatic resection for colorectal liver metastases. Surgery. 2016;159:632-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 148. | Lee H, Choi SH, Cho YB, Yun SH, Kim HC, Lee WY, Heo JS, Choi DW, Jung KU, Chun HK. Repeat hepatic resection in patients with colorectal liver metastases. World J Gastroenterol. 2015;21:2124-2130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 149. | Luo LX, Yu ZY, Huang JW, Wu H. Selecting patients for a second hepatectomy for colorectal metastases: an systemic review and meta-analysis. Eur J Surg Oncol. 2014;40:1036-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 150. | Martínez-Cecilia D, Cipriani F, Vishal S, Ratti F, Tranchart H, Barkhatov L, Tomassini F, Montalti R, Halls M, Troisi RI. Laparoscopic Versus Open Liver Resection for Colorectal Metastases in Elderly and Octogenarian Patients: A Multicenter Propensity Score Based Analysis of Short- and Long-term Outcomes. Ann Surg. 2017;265:1192-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 151. | Nardo B, Serafini S, Ruggiero M, Grande R, Fugetto F, Zullo A, Novello M, Rizzuto A, Bonaiuto E, Vaccarisi S. Liver resection for metastases from colorectal cancer in very elderly patients: New surgical horizons. Int J Surg. 2016;33 Suppl 1:S135-S141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 152. | van Tuil T, Dhaif AA, Te Riele WW, van Ramshorst B, van Santvoort HC. Systematic Review and Meta-Analysis of Liver Resection for Colorectal Metastases in Elderly Patients. Dig Surg. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 153. | Leal JN, Sadot E, Gonen M, Lichtman S, Kingham TP, Allen PJ, DeMatteo RP, Jarnagin WR, D’Angelica MI. Operative morbidity and survival following hepatectomy for colorectal liver metastasis in octogenarians: a contemporary case matched series. HPB (Oxford). 2017;19:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 154. | De Blasi V, Memeo R, Adam R, Goéré D, Cherqui D, Regimbeau JM, Rivoire M, Perotto LO, Navarro F, Sa Cunha A. Major Hepatectomy for Colorectal Liver Metastases in Patients Aged Over 80: A Propensity Score Matching Analysis. Dig Surg. 2018;35:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 155. | Mäkelä JT, Klintrup KH, Rautio TT. Mortality and Survival after Surgical Treatment of Colorectal Cancer in Patients Aged over 80 Years. Gastrointest Tumors. 2017;4:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 156. | Xie M, Zhu J, He X, Yang Z, Chen X, Lan P, Lian L. Liver Metastasis from Colorectal Cancer in the Elderly: Is Surgery Justified? Dig Dis Sci. 2015;60:3525-3535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 157. | Tufo A, Dunne DFJ, Manu N, Joshi H, Lacasia C, Jones L, Malik HZ, Poston GJ, Fenwick SW. Hepatectomy for octogenarians with colorectal liver metastasis in the era of enhanced recovery. Eur J Surg Oncol. 2018;44:1040-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 158. | Yazici P, Akyuz M, Yigitbas H, Dural C, Okoh A, Aydin N, Berber E. A comparison of perioperative outcomes in elderly patients with malignant liver tumors undergoing laparoscopic liver resection versus radiofrequency ablation. Surg Endosc. 2017;31:1269-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 159. | Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 1009] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 160. | van der Geest LG, Lam-Boer J, Koopman M, Verhoef C, Elferink MA, de Wilt JH. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis. 2015;32:457-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 374] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 161. | Scheele J, Altendorf-Hofmann A. Resection of colorectal liver metastases. Langenbecks Arch Surg. 1999;384:313-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 162. | Luo D, Liu Q, Yu W, Ma Y, Zhu J, Lian P, Cai S, Li Q, Li X. Prognostic value of distant metastasis sites and surgery in stage IV colorectal cancer: a population-based study. Int J Colorectal Dis. 2018;33:1241-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 163. | Leung U, Gönen M, Allen PJ, Kingham TP, DeMatteo RP, Jarnagin WR, D'Angelica MI. Colorectal Cancer Liver Metastases and Concurrent Extrahepatic Disease Treated With Resection. Ann Surg. 2017;265:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 164. | Imai K, Castro Benitez C, Allard MA, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Bismuth H, Baba H, Adam R. Potential of a cure in patients with colorectal liver metastases and concomitant extrahepatic disease. J Surg Oncol. 2017;115:488-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 165. | Hadden WJ, de Reuver PR, Brown K, Mittal A, Samra JS, Hugh TJ. Resection of colorectal liver metastases and extra-hepatic disease: a systematic review and proportional meta-analysis of survival outcomes. HPB (Oxford). 2016;18:209-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 166. | Okuno M, Goumard C, Mizuno T, Kopetz S, Omichi K, Tzeng CD, Chun YS, Lee JE, Vauthey JN, Conrad C. Prognostic impact of perihepatic lymph node metastases in patients with resectable colorectal liver metastases. Br J Surg. 2018;105:1200-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 167. | Ferrarotto R, Pathak P, Maru D, Agarwal A, Overman M, Hoff PM, Kopetz S. Durable complete responses in metastatic colorectal cancer treated with chemotherapy alone. Clin Colorectal Cancer. 2011;10:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 168. | Chua TC, Saxena A, Liauw W, Chu F, Morris DL. Hepatectomy and resection of concomitant extrahepatic disease for colorectal liver metastases--a systematic review. Eur J Cancer. 2012;48:1757-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 169. | Adam R, de Haas RJ, Wicherts DA, Vibert E, Salloum C, Azoulay D, Castaing D. Concomitant extrahepatic disease in patients with colorectal liver metastases: when is there a place for surgery? Ann Surg. 2011;253:349-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |