Published online Nov 6, 2018. doi: 10.12998/wjcc.v6.i13.703
Peer-review started: July 30, 2018
First decision: August 31, 2018
Revised: September 20, 2018
Accepted: October 11, 2018
Article in press: October 12, 2018
Published online: November 6, 2018
Processing time: 99 Days and 20.7 Hours
Pyoderma gangrenosum (PG) is an uncommon ulcerative cutaneous condition of an unknown etiology and is often associated with immune diseases. However, PG rarely shows visceral involvement, especially in the kidney. A 20-year-old female presented with pedal edema and skin ulceration of both lower limbs. The skin lesion began as an erythematous plaque and then became a blister. She also complained of abdominal distension and a decreasing urine volume. Laboratory data showed high proteinuria, hypoalbuminemia and hyperlipidemia. Her skin and kidney were biopsied. The pathological results indicated PG and immunoglobulin A (IgA) nephropathy. The patient was finally cured with prednisolone in combination with cyclosporine A (CsA).
Core tip: This is the first report of successfully treated pyoderma gangrenosum (PG) occurring concurrently with immunoglobulin A (IgA) nephropathy. Both are immune-mediated disorders and should be paid attention to.
- Citation: Li XL, Ma ZG, Huang WH, Chai EQ, Hao YF. Successful treatment of pyoderma gangrenosum with concomitant immunoglobulin A nephropathy: A case report and review of literature. World J Clin Cases 2018; 6(13): 703-706
- URL: https://www.wjgnet.com/2307-8960/full/v6/i13/703.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i13.703
Pyoderma gangrenosum (PG) is an uncommon, ulcerative, cutaneous condition of an unknown cause, with an estimated annual incidence of 3-10 cases per million in the population[1]. PG is usually associated with systemic diseases such as inflammatory bowel disease, rheumatoid arthritis, seronegative arthritis, and autoimmune hepatitis and hematologic disorders such as paraproteinemia (especially immunoglobulin A paraproteinemia) and neutrophil malignancies[2], most of which exhibit mucocutaneous involvement. PG with visceral (especially renal) involvement is rare. Here, we report, to the best of our knowledge, the first case of a patient with PG in combination with immunoglobulin A (IgA) nephropathy, who was successfully treated with a glucocorticoid in combination with cyclosporine A (CsA).
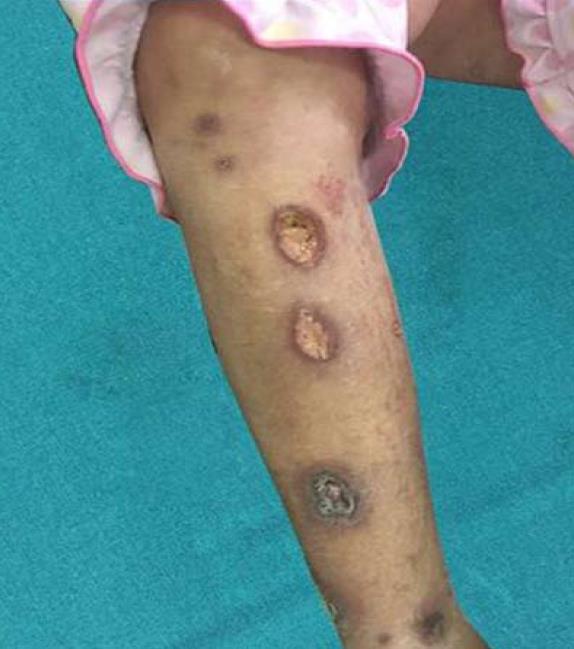
A 20-year-old female presented with swelling and ulceration of both lower limbs, which lasted for 1 wk. The skin lesion began as an erythematous plaque and then became a blister. In spite of antibiotic treatment and wound care, the lesion progressed for 1 wk as a painful ulceration of 3-5 cm in diameter, with a violaceous border and purulent or sanguineous exudate at the base (Figure 1). Additionally, she reported mucopurulent bloody stool and severe abdominal heaviness, but no fever, weight loss, arthralgia or other signs or symptoms of systemic illness.
The laboratory workup revealed moderate anemia (87 g/L), slightly increased C-reactive protein (33.8 mg/L) and ESR (27 mmol/L) levels, and negativity for autoantibodies, rheumatoid factor and antistreptolysin O. Additionally, high proteinuria (13 g/24 h), hypoalbuminemia (16 g/L) and hyperlipidemia were observed. Stool tests showed pyocytes and red blood cells, but no bacterial cultures were obtained. Abdominal ultrasound indicated massive ascites. The skin lesions were cultured for bacteria and Mycobacterium tuberculosis, but the results were negative.
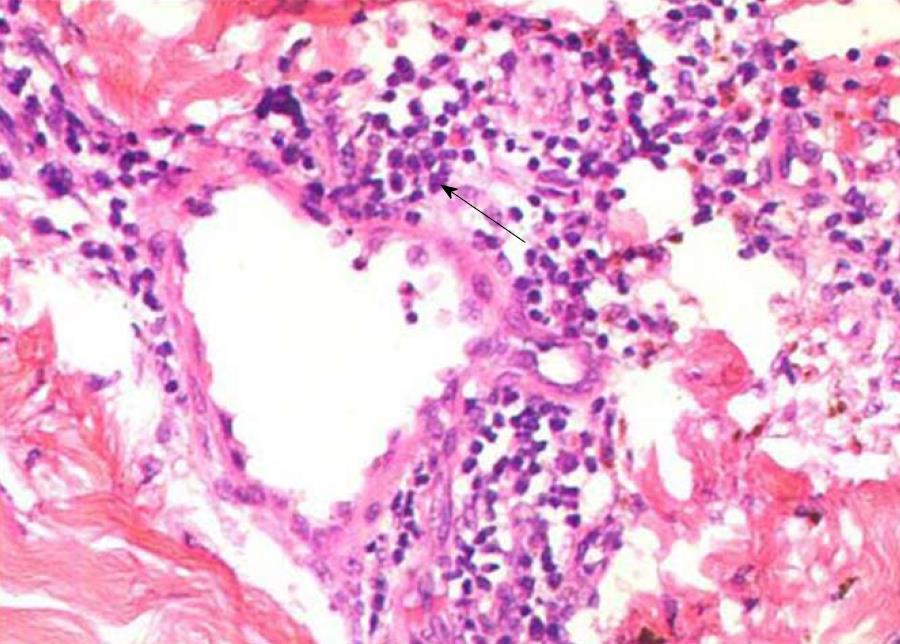
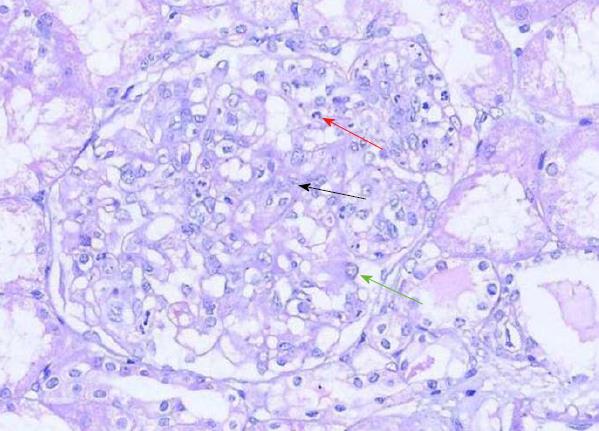
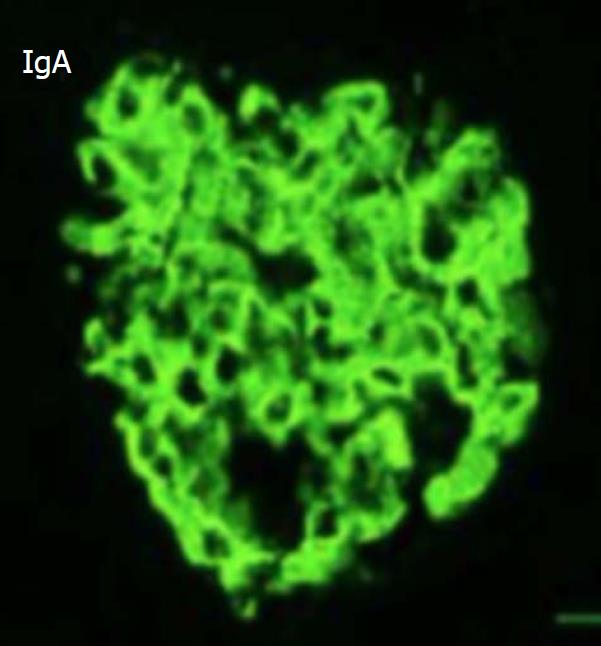
The edges of the lesions were biopsied. The histological results showed massive small lymphocytes arranged around blood vessels throughout the dermis (Figure 2). In addition, renal biopsy was performed. Light microscopy showed moderate enlargement of the mesangial area caused by an increase mesangial cells and the matrix as well as diffuse proliferation and degeneration of endothelial cells, infiltrated with neutrophils (Figure 3). Immunofluorescence analysis showed deposition of IgA and complement 3 in the mesangial area (Figure 4).
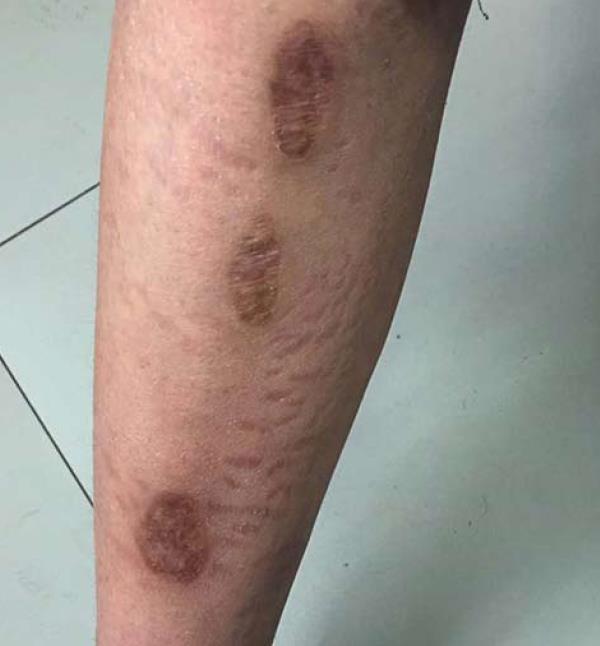
Prednisolone at 1 mg/kg plus cyclophosphamide at 0.6 mg/2 wk were prescribed. After 2 wk, the stool had returned to normal, and the skin lesions had improved. However, proteinuria, oliguria, and ascites were not alleviated after 2 mo of treatment. Thereafter, prednisolone was tapered off at 10% of the dosage every 10 d until the dose reached 5 mg, and cyclophosphamide was replaced by CsA 3 mg/(kg∙d) (75 mg b.i.d.). After 2 wk, urine output increased to normal. Other renal symptoms were also gradually alleviated. In month 4, urine protein disappeared, and CsA was then tapered off at 25 mg every 2 mo. One year later, all indices were normal, with only pigmentation remaining in the skin lesions (Figure 5).
PG was first described by Brocq in 1916 and further characterized by Perry et al[3]. It can affect an individual at any age but usually occurs between the ages of 20 and 50 years, with female predominance[4,5]. Skin lesions typically appear on the lower limbs but may also be observed on the upper extremities, head, and neck and even the genitals. It is diagnosed clinically, with no specific laboratory tests. The diagnosis is mainly based on the criteria proposed by Su et al[6] in 2004, including two major criteria: (1) rapid progression of a painful, necrolytic cutaneous ulcer with an irregular, violaceous, and undermined border, and (2) exclusion of other causes of cutaneous ulceration; at least two minor criteria must also be present: (1) a history suggestive of pathergy or a clinical finding of cribriform scarring, (2) concomitant systemic diseases, (3) histopathologic findings of sterile dermal neutrophilia, ± mixed inflammation ± lymphocytic vasculitis, and (4) a rapid response to systemic steroid treatment[6]. Of note, the histopathologic findings are not specific and may vary with the biopsy site and duration of the disease[7]. Thus, the diagnosis of PG is established based on characteristic clinical features, a good response to treatment and exclusion of infections (by bacteria, fungi, and typical or atypical mycobacteria), neoplastic disorders, and vasculitic disorders by biopsy and culture.
The etiology of PG remains unknown. However, 50% of cases are associated with systemic diseases, which are most commonly autoimmune disorders, suggesting that dysregulation of the immune system plays a role in disease pathogenesis. IgA nephropathy also involves immune-mediated inflammation of the glomeruli of the kidney and is characterized by deposition of the IgA antibody in the glomerular mesangium. However, PG and IgA nephropathy have not previously been concomitantly found; this is the first case report involving these two diseases.
The first-line modality for both diseases is systemic corticosteroids. The mainstay of treatment is long-term immunosuppression, often with a high dose of corticosteroids [prednisolone 0.5-2 mg/(kg∙d)] or a low dose of CsA [3-6 mg/(kg∙d)]. In the present case, we initially prescribed prednisolone at 1 mg/kg daily in combination with cyclophosphamide at 0.6 mg/2 wk. However, the kidneys and skin did not show a parallel response. The skin lesion improved rapidly, whereas the kidney-related symptoms showed no remission until cyclophosphamide was replaced by CsA.
The possible immunological link between the two disease entities remains unclear. Physicians should always bear in mind the possibility of a diagnosis of IgA nephropathy in PG patients. We also wish to present this unusual case in the hope that it will provide a valuable contribution to the treatment of the disease and to the literature.
To our knowledge, this is the first report of successfully treated PG occurring concurrently with IgA nephropathy. Both are immune-mediated disorders and can be cured with prednisolone in combination with CsA.
A 20-year-old female with pyoderma gangrenosum (PG) with concomitant immunoglobulin A (IgA) nephropathy was successfully treated.
According to the laboratory results and clinical manifestations, nephric syndrome and PG were diagnosed.
Skin infections should be excluded.
High proteinuria (13 g/24 h), hypoalbuminemia (16 g/L) and hyperlipidemia suggested nephrotic syndrome.
Abdominal ultrasound indicated normally sized kidneys and massive ascites.
Renal biopsy showed IgA nephrology, with stronger staining (3+) for IgA and C3 in the mesangial area, and skin biopsy indicated massive small lymphocytes arranged around blood vessels throughout the dermis, suggesting vasculitis.
Prednisolone at 1 mg/kg plus cyclophosphamide at 0.6 mg/2wk, followed by prednisone at 5 mg/kg plus cyclosporine A (CsA) at 3 mg/(kg∙d) (75 mg b.i.d.).
There are no reports of coexistence of PG and IgA nephropathy. This is the first reported case of PG with concomitant IgA nephropathy to be successfully treated.
PG and IgA nephropathy are both autoimmune diseases.
Low-dosage prednisone (5 mg/kg) plus CsA may be helpful in patients with PG concomitant with IgA nephropathy.
We wish to thank Dr. Ma for his help in the diagnosis and treatment of the patient.
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Choi MR, Ekpenyong CEE, Nechifor G S- Editor: Ma RY L- Editor: A E- Editor: Wu YXJ
| 1. | Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13:191-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 282] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 2. | Alavi A, French LE, Davis MD, Brassard A, Kirsner RS. Pyoderma Gangrenosum: An Update on Pathophysiology, Diagnosis and Treatment. Am J Clin Dermatol. 2017;18:355-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 185] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 3. | Perry HO, Brunsting LA. Pyoderma gangrenosum; a clinical study of nineteen cases. AMA Arch Derm. 1957;75:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 103] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Gameiro A, Pereira N, Cardoso JC, Gonçalo M. Pyoderma gangrenosum: challenges and solutions. Clin Cosmet Investig Dermatol. 2015;8:285-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Brown TS, Marshall GS, Callen JP. Cavitating pulmonary infiltrate in an adolescent with pyoderma gangrenosum: a rarely recognized extracutaneous manifestation of a neutrophilic dermatosis. J Am Acad Dermatol. 2000;43:108-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Su WP, Davis MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 359] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 7. | Callen JP, Jackson JM. Pyoderma gangrenosum: an update. Rheum Dis Clin North Am. 2007;33:787-802, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |