Published online Nov 6, 2018. doi: 10.12998/wjcc.v6.i13.675
Peer-review started: July 31, 2018
First decision: August 20, 2018
Revised: August 23, 2018
Accepted: August 28, 2018
Article in press: August 28, 2018
Published online: November 6, 2018
Processing time: 99 Days and 4 Hours
Placenta previa is the main cause of bleeding throughout pregnancy, and it is associated with serious complications, such as infection, that lead to a poor prognosis. Gynecological sonography is recommended as the first-line examination technique for the surveillance and determination of vaginal bleeding and for early intervention. We report the case of a patient with gradually expanded hypoechoic lesion and extremely high serum α-fetoprotein level during her third trimester, and discuss their potential relationship in evaluating the progression of placental necrosis.
Core tip: Placental necrosis with extremely high maternal serum α-fetoprotein (AFP) is rare. We reported a 23-year-old female patient with central placenta previa suffered from repeated vaginal bleeding. Follow-up ultrasonography revealed a gradually enlarging hypoecho between the amniotic sac and the uterine myometrium. Until 32 wk of gestation, laboratory results showed extremely elevated maternal serum AFP. Both intraoperative exploration of the placenta and histological examination demonstrated the hypoechoic area was necrotic tissue. To our knowledge, this is the first report of a rare case of extreme AFP level in placental necrosis. Clinicians should consider the combination usage of quantitative ultrasound imaging and AFP as a practical tool for assessing placental lesions.
- Citation: Yu MY, Xi L, Zhang JX, Zhang SC. Possible connection between elevated serum α-fetoprotein and placental necrosis during pregnancy: A case report and review of literature. World J Clin Cases 2018; 6(13): 675-678
- URL: https://www.wjgnet.com/2307-8960/full/v6/i13/675.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i13.675
Placenta previa refers to a clinical situation in which the lower edge of the placenta reaches and covers the internal orifice of the uterus, and its bulk position is lower than that of the fetal presentation[1]. During the third trimester, both irregular contractions and enlargement of the lower segment of the uterus cause a separation of the uterine wall and the placenta leading to sudden and repeated abdominal pain and vaginal bleeding[2]. α-fetoprotein (AFP) is currently used to predict the quality of the fetus. Its elevation in amniotic fluid may indicate the possibility of anencephalus or neural tube defects[3]. Moreover, the presence of incipient abortion or stillborn fetus is associated with the sudden upregulated AFP in maternal serum, which could reach 380-500 ng/mL.
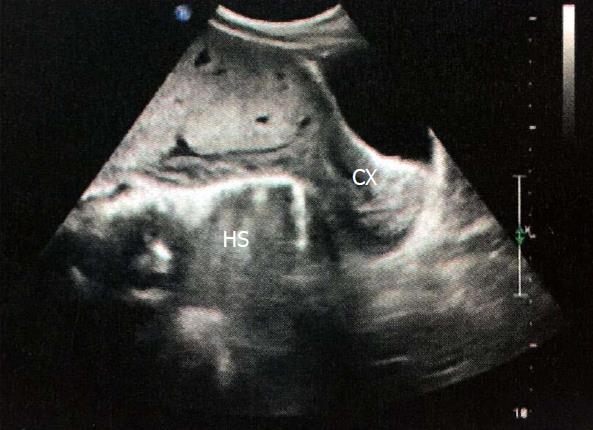
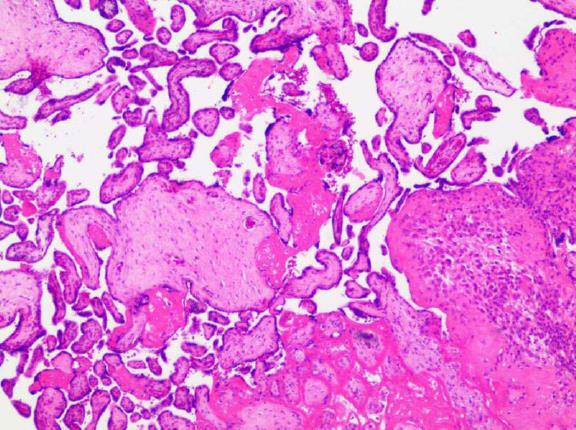
A 23-year-old female patient at 14 wk of gestation was admitted for vaginal bleeding. This was the patient’s first pregnancy with no medical history of miscarriage or abortion. Gynecological sonography showed that the lower margin of the placenta completely covered the cervix, and there was a 3.1 cm × 1.5 cm hypoechoic area between the amniotic sac and the uterine myometrium that had no significant blood flow signal. A clinical diagnosis of central placenta previa combined with a risk of preterm labor was promptly made. Three weeks later when the patient was discharged from the hospital, repeated gynecological sonography was performed and showed that the hypoechoic area had enlarged to 6.0 cm × 2.4 cm. She received outpatient follow-up at 19 wk of gestation, and the results revealed that hypoechoic area measured approximately 5.5 cm × 1.5 cm × 4.7 cm. No intervention protocol was carried out at that time. She was readmitted to our hospital at 32 wk of gestation on February 27, 2015 for a sudden volume of vaginal bleeding without significant abdominal pain. Considering that she had a previous history of central placenta previa (very likely with hematoma) and intermittent bleeding, and was in her third trimester when massive hemorrhage might occur at any time, a comprehensive examination including gynecological sonography, blood/urine testing, blood coagulation test, electrocardiogram, and fetal heart rate monitoring were performed. As shown in Figure 1, ultrasound examination revealed a heterogeneous echo measuring 4.2 cm × 3.5 cm. Blood tests are detailed in Table 1. Notably, her serum AFP level was extremely elevated at 1032 ng/mL. One day later, doctors performed a caesarean surgery. After the baby was delivered, doctors examined the placenta and found an abnormal area with black appearance between the placenta and the uterine myometrium. It was then confirmed to be necrosis tissue by histopathological examination (Figure 2), and its location was approximate to the hypoechoic area indicated in the pre-operation ultrasound examination. Before she was discharged from the hospital, we performed another blood test and the result showed that her serum AFP level had returned to baseline. As for the baby, its weight was 1550 g. Apgar was 9 at the first minute and was 10 at the tenth minute. It was soon admitted to the NICU for further treatment. We also followed up with the patient and her baby until this article was written. Her serum AFP level remained normal. Her baby had asthma since ten months and suffered from herpangina at one year old. The baby also had mild anemia (99 g/L hemoglobin).
| Name | Items | Result | Reference range |
| Blood routine examination | WBC | 13.79 × 109/L | 3.50-9.50 × 109/L |
| LY | 0.84 × 109/L | 1.10-3.20 × 109/L | |
| MO | 0.43 × 109/L | 0.10-0.60 × 109/L | |
| NE | 12.50 × 109/L | 1.80-6.30 × 109/L | |
| EO | 0.00 × 109/L | 0.02-0.52 × 109/L | |
| BA | 0.02 × 109/L | 0.00-0.06 × 109/L | |
| RBC | 2.98 × 1012/L | 3.80-5.10 × 1012/L | |
| HGB | 90 g/L | 115-150 g/L | |
| PLT | 212 × 109/L | 125-350 × 109/L | |
| CRP | 12.00 mg/L | 0-8 mg/L | |
| Coagulation function tests | PT | 11.80 s | 11 ± 3 s |
| INR | 0.98 | - | |
| APTT | 19.10 s | 24.5 ± 10 s | |
| FIB | 2.44 g/L | 2.0-4.0 g/L | |
| TT | 15.90 s | 18 ± 3 s | |
| D-D | 1.08 mg/L | < 0.55 mg/L | |
| Biochemistry examination | HbA1c | 4.90% | 4.0-6.4 % |
| ALT | 15.7 U/L | 7-40 U/L | |
| AST | 20.3 U/L | 13-35 U/L | |
| Thyroid function tests | FT3 | 2.94 pmol/L | 3.10-6.80 pmol/L |
| TSH | 1.09 mIU/L | 0.27-4.20 mIU/L | |
| FT4 | 14.23 pmol/L | 12.00-22.00 pmol/L | |
| Tumor markers | AFP | 1032.00 ng/mL | < 20.0 ng/mL |
| CEA | 0.43 ng/mL | < 4.7 ng/mL | |
| CA125 | 168.20 U/mL | < 35.0 U/mL | |
| CA199 | 26.47 U/mL | < 27.0 U/mL | |
| NSE | 17.05 ng/mL | < 16.3 ng/mL |
We reported a case of central placenta previa accompanied by intermittent bleeding. Follow-up gynecological sonography showed a gradual enlarging and subsequently stable hypoechoic area between the placenta and the uterine wall. Prenatal testing of peripheral blood revealed elevated levels of C-reactive protein (CRP) and neutrophils, and a severely increased serum AFP level without a history of hepatitis, miscarriage, or abortion. Later during a caesarean surgery, necrosis of the placenta was confirmed.
The placenta is the exclusive source of oxygen and nutrients for the fetus. Diffusion to and from the maternal circulatory system is essential for maintaining these life-sustaining functions of the placenta. The basic mechanism of placental abruption is vascular damage caused by a spasm or sclerosis of small spiral arteries followed by hematoma formation between the placenta and the bottom of the decidua and finally placental separation from the uterus[4]. One report suggests that maternal viral infection, such as HBV and HIV infection, may increase the necrotic rate of placental trophoblastic cells[5]. If the separated area is small, bleeding quickly stops. Most patients have no clinical symptoms or are unaware of the bleeding. Only clots that remain on the maternal surface of the placenta are often discovered on a postpartum examination. However, if the separated area is large enough to cause coagulation failure, a hematoma will form in the posterior aspect of the placenta and progressively expand followed by tissue necrosis, as was the case in our study.
Maternal serum AFP is synthesized from fetal hepatocytes and the yolk sac. It enters maternal circulation through the placenta. The placenta serves as a barrier. But when placental necrosis happens, the separation of placenta and uterus leads to barrier leak, which will increase the amount of AFP delivered from the fetus to the mother. Studies have demonstrated that maternal serum AFP is clinically elevated in cases of a morbidly adherent placenta, and it is a secondary indicator of placenta previa[6-8]. However, until now there have been no reports emphasizing its relationship with the degree of placental damage (such as hematoma necrosis). This is the first report of a rare case of extreme AFP level in placental necrosis. Although color Doppler flow imaging (CDFI) widely used in gynecological sonography can distinguish a blood flow inside a hematoma or the uterine myometrium[9], it only evaluates ongoing lesions, not already established lesions.
An insufficient placental blood supply may lead to ischemia-reperfusion damage and fetal growth restriction[10]. Therefore, intervention should be promptly performed when the size of placental necrosis reaches a tipping point that is very likely to induce irreversible injuries to the fetus and the mother. The combination of quantitative ultrasound imaging and maternal-fetal interface biochemical markers (such as AFP in our case) is a valuable assessing tool for this situation.
A 23-year-old female patient at 32 wk of gestation was admitted for vaginal bleeding.
Central placenta previa with repeated intermittent vaginal bleeding.
Laboratory diagnosis
Laboratory investigations showed moderately elevated neutrophils and C-reactive protein as well as extremely elevated α-fetoprotein (AFP) (1032 ng/mL).
Ultrasonography revealed a heterogeneous and gradually enlarging hypoechoic area (reached 4.2 cm × 3.5 cm before labor) between the amniotic sac and the uterine myometrium.
After caesarean, histological examination of placenta demonstrated the hypoechoic area was necrotic tissue.
A caesarean surgery with placental exploration was performed.
This is the first report of a rare case of placental necrosis with extremely elevated serum AFP.
The combination of quantitative ultrasound imaging and AFP is valuable in assessing maternal-fetal interface lesion during pregnancy.
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): D, D
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Khajehei M, Zhang X S- Editor: Ji FF L- Editor: Filipodia E- Editor: Tan WW
| 1. | Silver RM. Abnormal Placentation: Placenta Previa, Vasa Previa, and Placenta Accreta. Obstet Gynecol. 2015;126:654-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 290] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 2. | Jung EJ, Cho HJ, Byun JM, Jeong DH, Lee KB, Sung MS, Kim KT, Kim YN. Placental pathologic changes and perinatal outcomes in placenta previa. Placenta. 2018;63:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Krantz DA, Hallahan TW, Sherwin JE. Screening for open neural tube defects. Clin Lab Med. 2010;30:721-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Stepan H, Geipel A, Schwarz F, Krämer T, Wessel N, Faber R. Circulatory soluble endoglin and its predictive value for preeclampsia in second-trimester pregnancies with abnormal uterine perfusion. Am J Obstet Gynecol. 2008;198:175.e1-175.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Liu Y, Zhang J, Zhang R, Li S, Kuang J, Chen M, Liu X. [Relationship between the immunohistopathological changes of hepatitis B virus carrier mothers’ placentas and fetal hepatitis B virus infection]. Zhonghua Fuchanke Zazhi. 2002;37:278-280. [PubMed] |
| 6. | Lyell DJ, Faucett AM, Baer RJ, Blumenfeld YJ, Druzin ML, El-Sayed YY, Shaw GM, Currier RJ, Jelliffe-Pawlowski LL. Maternal serum markers, characteristics and morbidly adherent placenta in women with previa. J Perinatol. 2015;35:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Kelly RB, Nyberg DA, Mack LA, Fitzsimmons J, Uhrich S. Sonography of placental abnormalities and oligohydramnios in women with elevated alpha-fetoprotein levels: comparison with control subjects. AJR Am J Roentgenol. 1989;153:815-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Gagnon A, Wilson RD; Society of Obstetricians and Gynaecologists of Canada Genetics Committee. Obstetrical complications associated with abnormal maternal serum markers analytes. J Obstet Gynaecol Can. 2008;30:918-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Cali G, Forlani F, Foti F, Minneci G, Manzoli L, Flacco ME, Buca D, Liberati M, Scambia G, D’Antonio F. Diagnostic accuracy of first-trimester ultrasound in detecting abnormally invasive placenta in high-risk women with placenta previa. Ultrasound Obstet Gynecol. 2018;52:258-264. [PubMed] [DOI] [Full Text] |
| 10. | Thaete LG, Qu XW, Neerhof MG, Hirsch E, Jilling T. Fetal Growth Restriction Induced by Transient Uterine Ischemia-Reperfusion: Differential Responses in Different Mouse Strains. Reprod Sci. 2018;25:1083-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |