Published online Nov 6, 2018. doi: 10.12998/wjcc.v6.i13.611
Peer-review started: July 23, 2018
First decision: August 25, 2018
Revised: September 3, 2018
Accepted: October 9, 2018
Article in press: October 9, 2018
Published online: November 6, 2018
Processing time: 107 Days and 19.6 Hours
To evaluate the long-term outcome of an acute hemodynamic response-guided protocol in which acute responders to intravenous propranolol received traditional nonselective beta-blockers (NSBBs) and acute nonresponders received carvedilol.
Retrospective review of a protocol for primary prophylaxis of variceal bleeding guided by the acute hemodynamic response to intravenous propranolol. Fifty-two acute responders treated with traditional NSBB (i.e. propranolol or nadolol) were compared with 24 acute nonresponders receiving carvedilol. A second hemodynamic study was performed in 27 and 13 patients, respectively. The primary endpoint was development of first or further decompensation. Secondary endpoints included death from any cause, association between acute and chronic hemodynamic response, and baseline clinical and laboratory variables related to the acute hemodynamic response.
Acute responders and acute nonresponders presented similar 1, 2, and 3-year probabilities of first decompensation (NSBB: 0%, 13.7%, 26.1% vs carvedilol: 0%, 20%, 20%, P = 0.968) or further decompensation (21.2%, 26.1%, 40.9% vs 21.2%, 50.0%, 50.0%, P = 0.525). A previous episode of hepatic encephalopathy was the only independent predictor of decompensation [hazard ratio (95% confidence interval): 8.03 (2.76-23.37)]. Mortality rates were similar in acute responders and acute nonresponders with compensated (P = 0.428) or decompensated cirrhosis (P = 0.429). No clinical, laboratory, endoscopic or hemodynamic parameter predicted the acute hemodynamic response. In patients receiving traditional NSBB, the acute and chronic changes of hepatic venous pressure gradient were correlated (r = 0.59, P = 0.001). Up to 69.2% of acute nonresponders gained chronic response with carvedilol.
Early identification and treatment with carvedilol of acute nonresponders to intravenous propranolol improves the clinical outcome of this high-risk group of patients, probably due to its greater effects for reducing portal pressure.
Core tip: In patients with cirrhosis treated with traditional nonselective beta-blockers (NSBBs) (i.e. propranolol and nadolol), the lack of acute hemodynamic response to intravenous propranolol has been consistently associated with a higher risk of decompensation and death. Moreover, carvedilol is more effective than traditional NSBB in reducing portal pressure. In the present study, we evaluated for the first time the clinical impact of an acute hemodynamic response-guided protocol for the primary prophylaxis of variceal bleeding in which acute hemodynamic responders were treated with traditional NSBB and acute nonresponders with carvedilol. Importantly, the risk of decompensation and survival were similar in both groups, strongly suggesting that carvedilol improved the long-term outcome of acute nonresponders.
- Citation: Fortea JI, Puente Á, Ruiz P, Ezcurra I, Vaquero J, Cuadrado A, Arias-Loste MT, Cabezas J, Llerena S, Iruzubieta P, Rodríguez-Lope C, Huelin P, Casafont F, Fábrega E, Crespo J. Impact of an acute hemodynamic response-guided protocol for primary prophylaxis of variceal bleeding. World J Clin Cases 2018; 6(13): 611-623
- URL: https://www.wjgnet.com/2307-8960/full/v6/i13/611.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i13.611
The natural history of cirrhosis is marked by the clinical manifestations of portal hypertension, the most important being variceal bleeding, ascites, spontaneous bacterial peritonitis and hepatic encephalopathy. Their absence or presence defines the two main prognostic stages of liver cirrhosis: compensated and decompensated cirrhosis[1]. Current guidelines emphasize that the goal of treatment in the former is to prevent the development of any type of complication (i.e. first decompensation), whereas in the latter the objective should be the prevention of an additional complication (i.e. further decompensation) and the improvement of survival[1,2]. Studies in primary and secondary prophylaxis of variceal bleeding have shown that these goals can be achieved by decreasing portal pressure, assessed by the hepatic venous pressure gradient (HVPG), to < 12 mmHg or 20% from baseline after chronic treatment with nonselective beta-blockers (NSBBs)[3-5]. In the setting of primary prophylaxis, a lower decrease of at least 10% is also clinically relevant and is a better cutoff to define hemodynamic response[6,7].
Traditional NSBBs (i.e. propranolol and nadolol) and carvedilol are valid first-line treatments in patients starting primary prophylaxis of variceal bleeding[1]. Although no clinical trial has adequately compared their efficacy head-to-head, several randomized controlled trials[8,9] and a meta-analysis have shown that carvedilol is more effective in reducing HVPG[10]. These enhanced effects on portal pressure reduction are due to a fall in both intrahepatic and portal-collateral resistance through its intrinsic anti-α-1-adrenergic activity[11]. Confirmation of the chronic hemodynamic response to NSBB requires measuring the HVPG at baseline and after chronic treatment with NSBB[1]. The acute hemodynamic test [i.e. HVPG response after 20 min of the intravenous (i.v.) injection of 0.15 mg/kg propranolol], however, has been proposed as a valid and more cost-effective alternative to separate HVPG procedures[1,2].
Supporting this notion, recent studies in patients treated with traditional beta-blockers showed that the risk of decompensation was lower in those who had an acute response than in those who were acute nonresponders[6,7,12]. The acute test also predicted the chronic hemodynamic response, thereby enabling the earlier identification of nonresponders who might benefit from a treatment adjustment. Despite the potential advantages, the role of the acute hemodynamic response to guide therapy has never been assessed in the setting of primary prophylaxis of variceal bleeding and only scarcely in other conditions[13,14].
Based on the greater efficacy of carvedilol for reducing HVPG and the potential utility of the acute hemodynamic response to guide therapy, we implemented a protocol for primary prophylaxis of variceal bleeding in our institution in which acute responders were treated with traditional NSBB and acute nonresponders with carvedilol. The aim of the present study was to compare the risk of first or further decompensation of cirrhosis in each group since the implementation of the protocol in 2012.
We retrospectively reviewed all the hemodynamic studies performed in our Gastroenterology and Hepatology Department between February 2012 and January 2017. Potential candidates were those referred for a baseline hemodynamic study before the initiation of primary prophylaxis of variceal bleeding. The inclusion criteria were as follows: definitive diagnosis of cirrhosis (based on histology or by unequivocal clinical and radiological findings), baseline HVPG values ≥ 12 mmHg, presence of gastroesophageal varices without any previous episode of variceal bleeding, and evaluation of the acute HVPG response to i.v. propranolol. Patients were excluded if they had contraindication to NSBB, splanchnic venous thrombosis, history of surgery for portal hypertension (including transjugular intrahepatic portosystemic shunt), congestive liver, acute-on-chronic liver failure, liver transplantation or hepatocellular carcinoma at stages C or D of the Barcelona-Clinic Liver Cancer staging system. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) as reflected in a priori approval by the Clinical Research Ethics Committee of Cantabria. A waiver of informed consent was provided since the study was considered a retrospective review.
Hemodynamic studies were performed as previously described[15]. Briefly, after an overnight fast a catheter introducer was placed under local anesthesia in the right internal jugular vein using the Seldinger technique and was used to advance a 7-F balloon-tipped catheter into the right hepatic vein and a Swan-Ganz catheter into the pulmonary artery under fluoroscopic guidance. The occluded position was confirmed by the absence of reflux after injection of contrast medium. Free hepatic venous pressure was measured in the right hepatic vein close to the inferior vena cava. Portal pressure gradient was measured as the HVPG, which is the difference between the wedged and free hepatic venous pressures. All intravascular pressure measurements were performed in triplicate using a previously calibrated, highly sensitive transducer, with external zero at the mid-axillary line. A permanent recording of tracings was obtained. Electrocardiography, arterial pressure, heart rate, and oxygen saturation were monitored noninvasively throughout the study with an automatic monitor. After completing baseline hemodynamic measurements, a single intravenous bolus of propranolol was administered (0.15 mg/kg) over 5 min. Twenty minutes later, the HVPG response was assessed as previously described[6,12].
Acute or chronic hemodynamic response was defined as a decrease in HVPG to < 12 mmHg or as a ≥ 10% reduction in HVPG from baseline, as recommended by the Baveno VI consensus[2].
According to our institutional protocol, acute responders were treated with propranolol or nadolol (i.e. traditional NSBBs) and nonresponders with carvedilol. After the baseline hemodynamic study, propranolol (20 mg b.i.d.), nadolol (20 mg q.d.) or carvedilol (6.25 mg q.d.) were given orally. If tolerated, the dose was subsequently increased until the resting heart rate descended to 55 beats/min, systolic pressure decreased below 90 mmHg, or the maximum dose was reached (160 mg b.i.d. for propranolol, 160 mg q.d. for nadolol, and 6.25 mg b.i.d. for carvedilol). In patients with concomitant arterial hypertension, carvedilol could be increased up to 12.5 mg b.i.d.
Patients were followed-up according to the standardized protocols of our unit. Briefly, they were attended in the outpatient clinic within 1 mo after the performance of the baseline hemodynamic study, and every 3-6 mo thereafter. Medical history, laboratory values, imaging tests and treatment compliance (including abstinence from alcohol) were recorded in each visit. Follow-up data were collected until July 2017, death or liver transplantation.
The primary endpoint was development of first or further decompensation of cirrhosis. Decompensation was defined when gastrointestinal bleeding owing to portal hypertension, ascites, hepatorenal syndrome, spontaneous bacterial peritonitis, or hepatic encephalopathy occurred. Bleeding from esophagogastric varices or portal hypertensive gastropathy was defined according to Baveno VI criteria[2]. Ascites was defined as de novo in patients who had never been diagnosed with ascites before or as worsening of preexisting ascites in patients requiring a sustained increase in diuretic dose or large-volume paracentesis. In all cases, it was confirmed by ultrasound and/or paracentesis. Spontaneous bacterial peritonitis was defined following current guidelines[16] and hepatic encephalopathy was diagnosed on clinical basis.
Secondary endpoints included death from any cause, association between acute and chronic hemodynamic response, and baseline clinical and laboratory variables related to the acute hemodynamic response.
Quantitative variables were expressed as mean ± standard deviation (SD) and qualitative variables as proportions. Comparisons between groups were performed with unpaired Student’s t-test, Mann-Whitney test or the Fisher’s exact test as appropriate. The correlation between acute and chronic changes in HVPG was estimated by the Pearson correlation coefficient, whereas the number of patients correctly and incorrectly classified by the acute HVPG response with respect to the chronic response was compared with the McNemar’s test. The adjusted association with the acute hemodynamic response was evaluated by logistic regression analysis introducing variables that were considered related (P < 0.1) in a univariate analysis or clinically significant regardless of the P value. The strength of the association of each variable with the acute response was estimated by the odds ratio (OR) with its 95% confidence interval (CI). The actuarial probabilities in patients treated with traditional NSBB and those treated with carvedilol were calculated according to the Kaplan-Meier method and compared using the log-rank test.
Per protocol analysis was performed, as patients not treated with medical therapy according to our institutional protocol were excluded from the analysis. Follow-up was censored at the date of the analyzed event, liver transplantation or death. Patients undergoing liver transplantation were censored as alive, and patients lost to follow-up were censored as free of the analyzed event the day of the last visit. The adjusted association with the risk of reaching the endpoint was investigated with the Cox proportional hazards regression analysis, by introducing covariates that were related (P < 0.1) in univariate analysis or that were considered clinically significant regardless of the P value. The contribution of each variable to the risk of reaching the endpoint was estimated by the hazard ratio (HR) with its 95%CI. P < 0.05 was considered statistically significant. The maximum number of variables included in the multivariable analysis was 1 per 5-10 outcomes. Statistical analysis was performed with IBM SPSS Statistics v22.0 for Mac (IBM Corp., Armonk, NY, United States) and GraphPad Prism v6.00 for Mac OS X (GraphPad Software, San Diego, CA, United States).
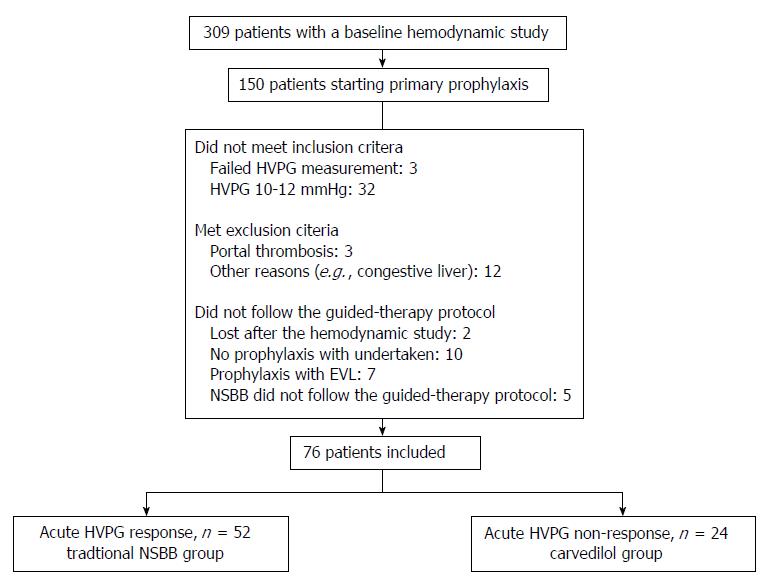
Four hundred and thirty-eight hemodynamic studies were performed in 309 patients during the study period. The hemodynamic study was performed in the context of evaluation of primary prophylaxis of variceal bleeding in 150 patients. Seventy-four of these patients were not included in the study because they did not fulfill inclusion criteria (n = 35), they presented exclusion criteria (n = 15), or they did not follow the guided-therapy protocol for diverse reasons (n = 24) (see flowchart in Figure 1). Of the 76 patients that were valid for the analysis, 52 patients (68.4%) had an acute hemodynamic response to i.v. propranolol and received traditional NSBB for primary prophylaxis, and 24 patients (31.6%) did not have an acute hemodynamic response to i.v. propranolol and received carvedilol. Mean duration of follow-up was similar in both groups (traditional NSBB: 21.8 ± 13.1 mo vs carvedilol: 24.1 ± 14.9 mo; P = 0.51).
There were no clinical, laboratory, endoscopic, or hemodynamic variables capable of predicting the acute hemodynamic response to i.v. propranolol, neither in the univariate analysis (Table 1) nor in a multivariable analysis, including variables occasionally related with the acute hemodynamic response to i.v. propranolol in prior studies[17-19]. In particular, the acute hemodynamic response was not associated with the etiology of liver disease (alcoholic vs nonalcoholic) [OR (95%CI): 0.84 (0.25-2.79); P = 0.780], bilirubin [OR (95%CI): 0.81 (0.63-1.06); P = 0.123], albumin [OR (95%CI): 0.71 (0.28-1.83); P = 0.476], or baseline HVPG [OR (95%CI): 1.05 (0.91-1.21); P = 0.534] in our study. Acute hemodynamic response to propranolol was based on a ≥ 10% reduction in HVPG from baseline in 96% of the patients and/or on a decrease in HVPG to < 12 mmHg in 23.1% (Table 1). The acute hemodynamic response was associated with a decrease of mean arterial pressure (MAP) that did not occur in nonresponders (% change MAP: -5.6% ± 12.2% vs 2.7% ± 9.7%, P < 0.008) (Table 1).
| Variable1 | Acute responders, n = 52 | Acute nonresponders, n = 24 | P value |
| Age in yr | 57.8 ± 10.2 | 57.1 ± 8.7 | 0.764 |
| Sex (male) | 40 (76.9) | 19 (79.2) | 1 |
| Body mass index | 28.6 ± 5.0 | 28.5 ± 4.8 | 0.918 |
| Associated diseases2 | 34 (65.4) | 16 (66.7) | 1 |
| Regular medication | |||
| Statins | 3 (5.8) | 3 (12.5) | 0.373 |
| Metformin | 10 (19.2) | 5 (20.8) | 1 |
| Antiplatelet agent | 7 (13.5) | 1 (4.2) | 0.423 |
| Anticoagulation | 2 (3.8) | 0 (0) | 1 |
| Etiology of liver disease3 | 0.971 | ||
| Alcohol | 34 (65.4) | 17 (70.8) | 0.794 |
| Hepatitis C | 4 (7.7) | 2 (8.3) | 1 |
| Alcohol + hepatitis C | 4 (7.7) | 2 (8.3) | 1 |
| Other | 10 (19.2) | 3 (12.6) | 0.744 |
| Active alcoholism | |||
| At first hemodynamic study | 11 (27.5) | 4 (20.0) | 0.753 |
| During follow-up | 3 (7.9) | 2 (10.5) | 1 |
| Active hepatitis C | |||
| At first hemodynamic study | 8 (100) | 4 (100) | 1 |
| During follow-up | 4 (50) | 2 (50.0) | 1 |
| Esophageal varices | 49 (94.2) | 21 (87.5) | 0.310 |
| Small | 3 (6.1) | 3 (14.3) | 0.355 |
| Large | 46 (93.9) | 18 (85.7) | |
| Gastric varices | 3 (5.8) | 3 (12.5) | 0.373 |
| Red signs | 10 (20.0) | 3 (13.6) | 0.742 |
| Hemoglobin in g/dL | 12.7 ± 2.1 | 12.7 ± 2.2 | 0.994 |
| Platelet count as × 103/μL | 102 ± 45 | 122 ± 53 | 0.100 |
| Prothrombin time as INR | 1.36 ± 0.24 | 1.39 ± 0.26 | 0.685 |
| Bilirubin in mg/dL3 | 1.7 ± 1.1 | 2.8 ± 4.5 | 0.235 |
| Albumin in g/dL3 | 3.6 ± 0.6 | 3.6 ± 0.6 | 0.878 |
| Creatinine in mg/dL | 0.72 ± 0.24 | 0.73 ± 0.24 | 0.900 |
| Sodium in mEq/L | 139 ± 3 | 138 ± 4 | 0.108 |
| Hyponatremia (< 135) | 3 (6.0) | 5 (20.8) | 0.103 |
| Ascites | 31 (59.6) | 14 (58.3) | 1 |
| Refractory ascites | 1 (1.9) | 1 (4.2) | 0.535 |
| Hepatic encephalopathy | 8 (15.4) | 4 (16.7) | 1 |
| Spontaneous bacterial peritonitis | 4 (7.7) | 0 (0) | 0.301 |
| Hepatocellular carcinoma | 3 (5.8) | 1 (4.2) | 1 |
| No previous decompensation | 21 (40.4) | 9 (37.5) | 1 |
| MELD | 11.5 ± 3.2 | 12.3 ± 4.4 | 0.353 |
| Child-Pugh score | 6.5 ± 1.4 | 6.7 ± 1.6 | 0.560 |
| A/B/C, % | 58/40/2 | 50/42/8 | 0.388 |
| Propranolol dose in acute test in mg | 12.1 ± 2.4 | 12.6 ± 2.8 | 0.44 |
| Free hepatic venous pressure in mmHg | 10.8 ± 4.3 | 11.7 ± 4.7 | 0.386 |
| Change from baseline, % | +18.5 ± 23.5 | +3.4 ± 11.2 | < 0.001 |
| Wedged hepatic venous pressure in mmHg | 30.0 ± 5.4 | 30.4 ± 5.5 | 0.581 |
| Change from baseline, % | -6.6 ± 5.3 | -1.2 ± 6.3 | < 0.001 |
| Hepatic venous pressure gradient in mmHg3 | 18.8 ± 3.7 | 18.7 ± 3.7 | 0.854 |
| Change from baseline, % | -17.8 ± 7.7 | -3.9 ± 5.6 | < 0.001 |
| Decrease by > 10%, % | 96.2 | 0 | < 0.001 |
| Decrease to < 12 mmHg, % | 23.1 | 0 | 0.014 |
| Mean arterial pressure in mmHg | 99 ± 9 | 95 ± 11 | 0.145 |
| Change from baseline, % | -5.6 ± 12.2 | +2.7 ± 9.7 | 0.008 |
| Heart rate as bpm | 78 ± 13 | 81 ± 15 | 0.315 |
| Change from baseline, % | -18.8 ± 8.5 | -19.4 ± 7.3 | 0.779 |
| Right atrial pressure in mmHg | 7.0 ± 2.9 | 7.3 ± 3.8 | 0.712 |
| Change from baseline, % | +51.4 ± 41.1 | +45.1 ± 45.3 | 0.565 |
| Pulmonary arterial pressure in mmHg | 18.3 ± 4.9 | 17.8 ± 5.0 | 0.700 |
| Change from baseline, % | +18.6 ± 18.9 | +16.1 ± 14.9 | 0.606 |
| Pulmonary wedge pressure in mmHg | 11.8 ± 4.1 | 11.4 ± 5.0 | 0.711 |
| Change from baseline, % | +28.3 ± 37.2 | +38.4 ± 54.9 | 0.384 |
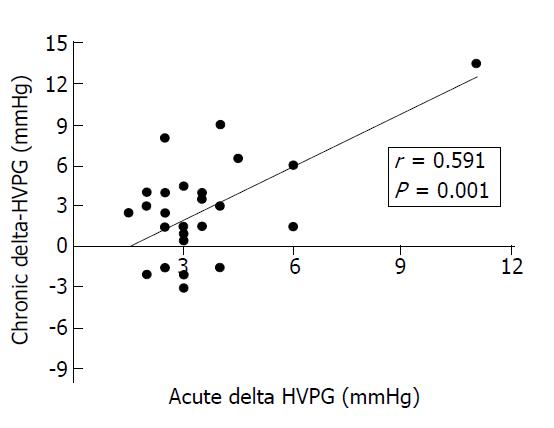
Twenty-seven patients (51.9%) in the traditional NSBB group and 13 (54.2%) in the carvedilol group had a second hemodynamic study performed after a mean ± SD duration of 26.3 ± 12.8 wk and 28.0 ± 18.8 wk, respectively. Among these patients, a chronic hemodynamic response was observed in 15 of 27 patients (55.6%) treated with traditional NSBB and in 9 of 13 patients (69.2%) treated with carvedilol (Fisher’s exact test, P = 0.50). The misclassification rate (i.e. chronic nonresponse with traditional NSBB or chronic response with carvedilol) was not significantly different between groups (McNemar´s test, P = 0.664). In patients receiving traditional NSBB, the magnitude of the chronic change of HVPG was correlated with that observed after acute i.v. propranolol in the initial study (r = 0.59, P = 0.001; Figure 2). Most clinical, laboratory, endoscopic and hemodynamic parameters at baseline were similar in chronic responders and chronic nonresponders in the traditional NSBB and carvedilol groups, except for the alcoholic etiology of liver disease in the traditional NSBB group [chronic response: 14/15 (93.3%) vs chronic nonresponse: 6/12 (50%), P = 0.024] (Table 2).
| Traditional NSBB | Carvedilol | |||||
| Variable1 | CR, n = 15 | CNR, n = 12 | P value | CR, n = 9 | CNR, n = 4 | P value |
| Age in yr | 58.9 ± 8.3 | 57.8 ± 10.3 | 0.766 | 59.2 ± 9.2 | 57.3 ± 6.8 | 0.685 |
| Sex (male) | 12 (80.0) | 9 (75.0) | 1 | 8 (88.9) | 3 (75.0) | 1 |
| Body mass index | 28.8 ± 2.1 | 29.1 ± 4.3 | 0.795 | 30.5 ± 5.9 | 28.0 ± 3.6 | 0.370 |
| Associated diseases2 | 12 (80.0) | 10 (83.3) | 1 | 7 (77.8) | 2 (50) | 0.530 |
| Regular medication | ||||||
| Statins | 2 (13.3) | 1 (8.3) | 1 | 2 (22.2) | 0 (0) | 1 |
| Metformin | 3 (20) | 2 (16.7) | 1 | 1 (11.1) | 2 (50.0) | 0.203 |
| Antiplatelet agent | 1 (6.7) | 3 (25) | 0.294 | 0 (0) | 0 (0) | 1 |
| Anticoagulation | 1 (6.7) | 1 (8.3) | 1 | 0 (0) | 0 (0) | 1 |
| Etiology of liver disease | 0.063 | 1 | ||||
| Alcohol | 14 (93.3) | 6 (50.0) | 0.024 | 8 (88.9) | 4 (100) | 1 |
| Hepatitis C | 0 (0) | 2 (16.7) | 0.188 | 0 (0) | 0 (0) | 1 |
| Alcohol + hepatitis C | 0 (0) | 2 (16.7) | 0.188 | 0 (0) | 0 (0) | 1 |
| Other | 1 (6.7) | 2 (16.6) | 0.569 | 1 (11.1) | 0 (0) | 1 |
| Active alcoholism | 0 (0) | 1 (9.1) | 0.440 | 1 (12.5) | 1 (25.0) | 1 |
| Active hepatitis C | 0 (0) | 0 (0) | 1 | 0 (0) | 0 (0) | 1 |
| Esophageal varices | 14 (93.3) | 11 (91.7) | 6 (66.7) | 4 (100) | ||
| Small, % | 0 | 0 | 1 | 33.3 | 0 | 0.467 |
| Large, % | 100 | 100 | 66.7 | 100 | ||
| Gastric varices | 1 (6.7) | 1 (8.3) | 1 | 3 (33.3) | 0 (0.0) | 0.497 |
| Red signs | 3 (20.0) | 1 (8.3) | 0.605 | 1 (12.5) | 1 (25.0) | 1 |
| Baseline MELD | 11.5 ± 2.9 | 11.0 ± 3.4 | 0.660 | 12.3 ± 6.3 | 12.8 ± 1.0 | 0.852 |
| Change from baseline, % | -4.1 ± 14.2 | 0.8 ± 16.8 | 0.426 | -0.8 ± 28.0 | -7.9 ± 14.5 | 0.565 |
| Baseline Child-Pugh score | 6.7 ± 1.4 | 6.1 ± 1.1 | 0.225 | 6.8 ± 2.0 | 7.0 ± 0.0 | 0.753 |
| Change from baseline, % | -3.0 ± 12.1 | 0.3 ± 9.9 | 0.465 | -7.2 ± 13.7 | 0.0 ± 11.6 | 0.363 |
| Baseline Child-Pugh class A/B/C, % | 53/47/0 | 42/58/0 | 1 | 67/11/22 | 0/100/0 | 0.010 |
| Change from baseline A/B/C, % | 67/33/0 | 67/33/0 | 1 | 67/33/0 | 75/25/0 | 0.266 |
| Hemoglobin in g/dL | 12.8 ± 2.1 | 14.0 ± 2.2 | 0.150 | 12.8 ± 2.0 | 14.5 ± 2.7 | 0.319 |
| Platelet count as × 103/μL | 107 ± 35 | 86 ± 27 | 0.102 | 91 ± 32 | 114 ± 41 | 0.367 |
| Prothrombin time as INR | 1.37 ± 0.18 | 1.32 ± 0.15 | 0.498 | 1.28 ± 0.21 | 1.35 ± 0.04 | 0.358 |
| Bilirubin in mg/dL | 1.3 ± 0.7 | 1.6 ± 0.8 | 0.349 | 1.57 ± 0.96 | 1.93 ± 1.19 | 0.619 |
| Albumin in g/dL | 3.5 ± 0.5 | 3.7 ± 0.4 | 0.397 | 3.9 ± 0.4 | 3.5 ± 0.4 | 0.080 |
| Creatinine in mg/dL | 0.72 ± 0.18 | 0.78 ± 0.16 | 0.415 | 0.71 ± 0.25 | 0.66 ± 0.05 | 0.590 |
| Sodium in mEq/L | 140 ± 2 | 140 ± 3 | 0.342 | 139 ± 2 | 139 ± 2 | 0.638 |
| Ascites | 9 (60.0) | 6 (50.0) | 0.707 | 5 (55.6) | 4 (100) | 0.228 |
| Hepatic encephalopathy | 4 (26.7) | 0 (0) | 0.106 | 0 (0) | 1 (25.0) | 0.308 |
| SBP | 3 (20.0) | 0 (0) | 0.231 | 0 (0) | 0 (0) | 1 |
| Hepatocellular carcinoma | 0 (0) | 0 (0) | 1 | 0 (0) | 0 (0) | 1 |
| Hemodynamic variables | ||||||
| Weeks between studies | 24.2 ± 12.3 | 29.1 ± 13.4 | 0.327 | 26.2 ± 14.0 | 31.9 ± 29.4 | 0.732 |
| Propranolol dose in mg | 136 ± 111 | 165 ± 123 | 0.677 | |||
| Nadolol dose in mg | 87 ± 47 | 95 ± 21 | 0.659 | |||
| Carvedilol dose in mg | 18.8 ± 12.5 | 14.1 ± 7.9 | 0.434 | |||
| FHVP in mmHg | 10.0 ± 2.7 | 11.2 ± 2.4 | 0.250 | 11.9 ± 3.9 | 11.0 ± 2.1 | 0.583 |
| Change from baseline, % | 36.4 ± 62.6 | -3.5 ± 30.4 | 0.054 | 10.4 ± 31.1 | 1.8 ± 8.4 | 0.458 |
| WHVP in mmHg | 29.6 ± 2.5 | 29.0 ± 2.9 | 0.622 | 28.5 ± 5.7 | 31.1 ± 5.1 | 0.437 |
| Change from baseline, % | -8.5 ± 15.9 | 0.7 ± 10.6 | 0.100 | -9.5 ± 9.5 | 5.8 ± 9.2 | 0.034 |
| HVPG in mmHg | 19.5 ± 2.9 | 17.9 (2.5) | 0.126 | 16.4 ± 2.5 | 20.1 ± 3.2 | 0.100 |
| Change from baseline, % | - 26.0 ± 12.5 | 5.7 ± 17.7 | < 0.0001 | -21.2 ± 12.8 | -7.6 ± 13.3 | 0.012 |
| Decrease by > 10% | 15 (100) | 0 (0) | < 0.0001 | 8 (88.9) | 0 (0) | 0.007 |
| Decrease < 12 mmHg | 3 (20) | 0 (0) | 0.231 | 4 (44.0) | 0 (0) | 0.228 |
| MAP in mmHg | 99 ± 9 | 98 ± 8 | 0.642 | 96 ± 12 | 97 ± 11 | 0.897 |
| Change from baseline, % | -5.6 ± 7.3 | 0.3 ± 12.1 | 0.192 | 6.6 ± 17.8 | -2.0 ± 7.7 | 0.273 |
| Heart rate as bpm | 77 ± 11 | 77 ± 16 | 0.902 | 82 ± 11 | 76 ± 9 | 0.311 |
| Change from baseline, % | -26.2 ± 12.5 | -19.8 ± 14.9 | 0.265 | -26.8 ± 10.6 | -17.1 ± 7.7 | 0.102 |
| Right atrial pressure in mmHg | 6.4 ± 2.1 | 7.4 ± 2.3 | 0.264 | 8.4 ± 5.0 | 7.0 ± 1.4 | 0.458 |
| Change from baseline, % | 74.2 ± 82.3 | 24.1 ± 66.5 | 0.100 | 35.5 ± 97.2 | 23.2 ± 27.0 | 0.733 |
| PAP in mmHg | 18.3 ± 4.2 | 17.9 ± 4.3 | 0.813 | 20.1 ± 4.8 | 17.5 ± 3.1 | 0.281 |
| Change from baseline, % | 35.3 ± 42.8 | 17.4 ± 27.6 | 0.222 | -4.5 ± 18.7 | 27.5 ± 43.9 | 0.242 |
| PWP in mmHg | 11.5 ± 3.3 | 11.8 ± 3.5 | 0.819 | 12.8 ± 5.3 | 12.0 ± 3.2 | 0.751 |
| Change from baseline, % | 50.9 ± 57.5 | 32.0 ± 61.2 | 0.417 | 5.5 ± 37.1 | 14.8 ± 10.8 | 0.506 |
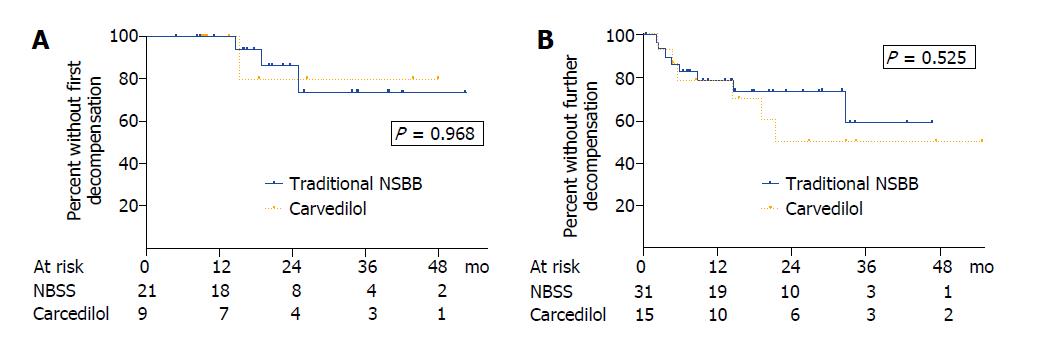
In patients with compensated cirrhosis, the actuarial probability of presenting their first decompensation at 1, 2 and 3 years was 0%, 13.7% and 26.1% in acute responders receiving traditional NSBB compared with 0%, 20% and 20% in acute nonresponders receiving carvedilol (P = 0.968) (Figure 3A). In patients with decompensated liver disease, the actuarial probability of presenting further hepatic decompensations at 1, 2 and 3 years was 21.2%, 26.1% and 40.9% in those receiving traditional NSBB compared with 21.2%, 50.0% and 50.0% in those receiving carvedilol (P = 0.525) (Figure 3B). No differences in the actuarial probability of presenting a decompensation were found either when patients with compensated and decompensated cirrhosis were pooled for analysis (P = 0.505) or when the 6 patients taking statins were excluded from the analysis (P = 0.319).
Twelve patients (23.1%) in the traditional NSBB group and 8 patients (33.3%) in the carvedilol group had a decompensation event during follow-up (P = 0.405), and most of them (n = 15, 75%) had decompensated liver disease at recruitment (Table 3). The type of decompensation was similar between groups, being the most common hepatic encephalopathy and ascites (Table 3). The actuarial probability of hepatic encephalopathy at 2 years was 12.7% and 26.8% (P = 0.358), whereas that of ascites was 11.1% and 23.8% (P = 0.362) in the traditional NSBB and carvedilol groups, respectively. The 2-year actuarial probability of variceal bleeding was 2.0% and 16.3%; this complication occurred in 2 patients in the traditional NSBB group and in 3 patients in the carvedilol group (P = 0.078).
| Variable1 | Traditional NSBB, n = 52 | Carvedilol, n = 24 | P value |
| Decompensation (global)2 | 12 (23.1) | 8 (33.3) | 0.405 |
| First decompensation | 3 (14.3) | 2 (22.2) | 0.622 |
| Further decompensation | 9 (29.0) | 6 (40.0) | 0.514 |
| Portal hypertension-related bleeding | 2 (3.8) | 3 (12.5) | 0.318 |
| Ascites | |||
| Overall | 7 (13.5) | 4 (16.7) | 0.734 |
| De novo ascites | 3 (5.8) | 1 (4.2) | 1 |
| Spontaneous bacterial peritonitis | 1 (1.9) | 2 (8.3) | 0.233 |
| Hepatorenal syndrome | 1 (1.9) | 1 (4.2) | 0.535 |
| Hepatic encephalopathy | |||
| Overall | 7 (13.5) | 5 (20.8) | 0.502 |
| De novo hepatic encephalopathy | 3 (5.8) | 4 (16.7) | 0.191 |
| Hepatocellular carcinoma (de novo) | 3 (6.1) | 0 (0) | 0.546 |
| Portal thrombosis | 5 (9.6) | 3 (12.5) | 0.702 |
| Nonselective beta-blocker | |||
| Propranolol dose, n/mg per day | 35 / 107.6 | ||
| Nadolol dose, n/mg per day | 17 / 83.5 | ||
| Carvedilol dose, n/mg per day | 24 / 9.2 | ||
| Chronic hemodynamic response | |||
| Change from baseline HVPG, % | -11.9 ± 21.8 | -12.2 ± 18.5 | 0.965 |
| ≥ 10% reduction in HVPG | 15 (55.6) | 9 (69.2) | 0.503 |
| ≥ 20% reduction in HVPG | 8 (29.6) | 4 (30.8) | 1 |
| Decrease to < 12 mmHg | 3 (11.1) | 4 (30.8) | 0.187 |
| Lost to follow-up, n/% | 14 (26.9) | 3 (12.5) | 0.238 |
| Betablocker intolerance | 6 (11.5) | 1 (4.2) | 0.421 |
| Change to carvedilol after second hemodynamic study | 5 (7.7) | ||
| Ceased follow-up | 3 (5.8) | 2 (8.3) | 0.648 |
Serum bilirubin and albumin levels, Child-Pugh class and a history of hepatic encephalopathy were the only variables significantly associated with the risk of decompensation during follow-up in the univariate analysis (Table 4). In a multivariate analysis including the latter two variables (serum bilirubin and albumin were not included since they are part of the Child-Pugh score) together with age and acute hemodynamic response, the only independent predictor of decompensation was a previous bout of overt hepatic encephalopathy (Table 4).
| Variables | Univariable | Multivariable | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age as per year increase | 0.96 (0.91-1.01) | 0.093 | 0.97 (0.92-1.02) | 0.246 |
| Active alcoholism | 2.55 (0.71-9.16) | 0.152 | ||
| Size of varices | 1.71 (0.23-12.90) | 0.602 | ||
| Red signs | 1.89 (0.62-5.73) | 0.262 | ||
| MELD as per 1 point increase1 | 1.12 (0.99-1.26) | 0.072 | ||
| Child class | 0.039 | 0.071 | ||
| B vs A | 2.66 (1.03-6.87) | 0.044 | 2.39 (0.90-6.36) | 0.081 |
| C vs A | 5.87 (1.20-28.63) | 0.029 | 6.00 (1.09-32.97) | 0.039 |
| Platelets as per 1 × 106 | 1.00 (0.99-1.01) | 0.641 | ||
| Creatinine as per 1 mg/dL increase | 0.17 (0.01-2.74) | 0.209 | ||
| Bilirubin as per 1 mg/dL increase2 | 1.21 (1.09-1.35) | < 0.001 | ||
| Albumin as per 1 g/L increase2 | 0.43 (0.20-0.94) | 0.035 | ||
| INR as per 1 point increase | 1.16 (0.20-6.56) | 0.871 | ||
| HVGP as per 1 mmHg increase | 1.07 (0.96-1.20) | 0.209 | ||
| MAP as per 1 mmHg increase | 0.98 (0.94-1.03) | 0.497 | ||
| Previous ascites2 | 2.42 (0.88-6.65) | 0.088 | ||
| Previous hepatocellular carcinoma | 2.44 (0.55-10.77) | 0.240 | ||
| Previous hepatic encephalopathy | 7.29 (2.78-19.13) | < 0.001 | 8.03 (2.76-23.37) | < 0.001 |
| No previous decompensation1 | 0.42 (0.15-1.16) | 0.093 | ||
| Acute hemodynamic response | 0.70 (0.29-1.71) | 0.434 | 0.74 (0.28-1.95) | 0.545 |
| Chronic hemodynamic response-10% | 0.49 (0.13-1.83) | 0.287 | ||
| Chronic hemodynamic response-20% | 0.24 (0.03-1.89) | 0.174 | ||
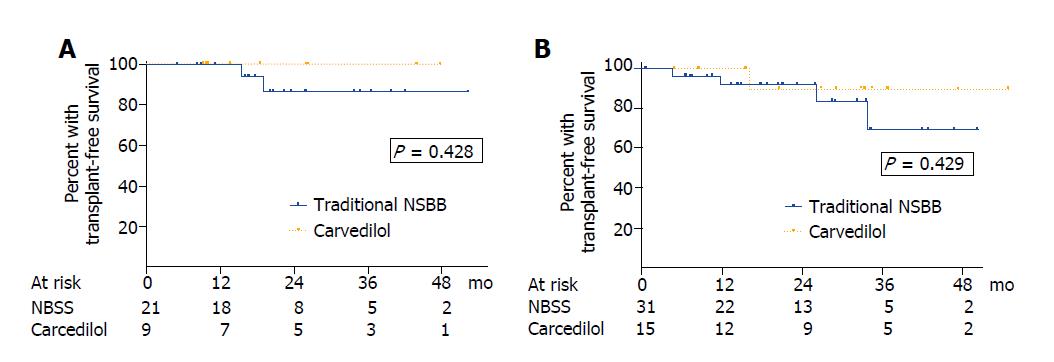
Two patients (3.8%) in the traditional NSBB group and 1 patient (4.2%) in the carvedilol group underwent liver transplantation after 36.6, 16.6 and 4.8 mo of follow up, respectively. Six patients (11.5%) in the traditional NSSB group and one patient (4.2%) in the carvedilol group died during the follow up (P = 0.792). Most of them were liver-related deaths (traditional NSBB: 4 liver-related, 1 hepatocellular carcinoma, 1 no liver-related; carvedilol: 1 liver-related). In patients with compensated cirrhosis, the actuarial probability of mortality at 1, 2 and 3 years was 0%, 13.7% and 13.7% in the traditional NSBB group compared with 0%, 0% and 0% in the carvedilol group (P = 0.428) (Figure 4A). In patients with decompensated liver disease, the actuarial probability of mortality at 1, 2 and 3 years was 7.8%, 7.8% and 30.2% in those receiving traditional NSBB compared with 0%, 10.0% and 10.0% in those receiving carvedilol (P = 0.429) (Figure 4B). No differences in mortality were found either when patients with compensated and decompensated cirrhosis were pooled for analysis (P = 0.505) or when the 6 patients taking statins were excluded from the analysis (P = 0.409). No variables were associated with survival in the univariate analysis (Table 5).
| Variables | Univariable | |
| HR (95%CI) | P value | |
| Age as per year increase | 1.01 (0.93-1.09) | 0.896 |
| Active alcoholism | 0.04 (0.00-2577625.31) | 0.731 |
| Size of varices | 0.28 (0.03-2.55) | 0.259 |
| MELD as per 1 point increase | 0.91 (0.72-1.16) | 0.448 |
| Child score as per 1 point increase | 0.98 (0.59-1.67) | 0.941 |
| Platelets as per 1 × 106 | 0.98 (0.95-1.00) | 0.088 |
| Creatinine as per 1 mg/dL increase | 0.01 (0.00-4.55) | 0.134 |
| Bilirubin as per 1 mg/dL increase | 0.90 (0.59-1.39) | 0.642 |
| Albumin as per 1 g/L increase | 0.65 (0.17-2.43) | 0.521 |
| INR as per 1 point increase | 0.06 (0.00-4.39) | 0.196 |
| HVGP as per 1 mmHg increase | 1.12 (0.94-1.33) | 0.195 |
| MAP as per 1 mmHg increase | 1.01 (0.93-1.10) | 0.791 |
| Previous ascites | 1.73 (0.34-8.96) | 0.511 |
| Previous hepatocellular carcinoma | 3.41 (0.40-29.45) | 0.264 |
| Previous hepatic encephalopathy | 2.72 (0.53-14.08) | 0.233 |
| No previous decompensation | 0.58 (0.11-3.01) | 0.518 |
| Acute hemodynamic response | 2.99 (0.36-24.91) | 0.312 |
| Chronic hemodynamic response-10% | 0.23 (0.02-2.57) | 0.234 |
| Chronic hemodynamic response-20% | 0.02 (0.00-427.79) | 0.455 |
In patients with cirrhosis treated with traditional NSBB, the lack of acute hemodynamic response to i.v. propranolol has been consistently associated with a higher risk of decompensation and death[6,7,12]. Parallelly, beneficial effects of carvedilol have been shown in patients who do not achieve a chronic hemodynamic response with traditional NSBB[20]. None of these studies, however, evaluated the use of the acute hemodynamic response for deciding the initial treatment. In the present study, we evaluated for the first time the clinical impact of an acute hemodynamic response-guided protocol for the primary prophylaxis of variceal bleeding in which acute hemodynamic responders were treated with traditional NSBB and acute nonresponders with carvedilol. Importantly, the risk of decompensation and survival were similar in both groups, regardless of the history or type of decompensation.
The present results suggest that carvedilol improved the prognosis of patients who did not have a positive acute hemodynamic response to propranolol, as we did not find the expected association between the acute hemodynamic response and the risk of decompensation or mortality that has been consistently shown in prior studies. Indeed, the probabilities of decompensation and mortality were similar in acute responders and acute nonresponders regardless of the history of decompensation, and the only independent predictor of new decompensation was a previous bout of overt hepatic encephalopathy. The improved prognosis of acute nonresponders receiving carvedilol is further supported by the comparison of our results with previous studies. Importantly, our patients had similar or worse liver dysfunction compared with the patient population of prior studies, and the risk of decompensation in acute responders was also lower, probably due to the loss of follow-up of some high-risk patients (i.e. five chronic nonresponders to propranolol were changed to carvedilol)[6,7,12]. Despite these considerations, the patients receiving carvedilol in the present study presented a lower risk of decompensation than acute nonresponders treated with propranolol in other studies (2-year risk of variceal bleeding: 16.3% vs 23%-47%; 2-year risk of ascites: 23.8% vs 49%-67%)[6,7,12]. Remarkably, the mortality rate was also substantially lower than the 23% mortality reported by Villanueva et al[6]. Although a control group of acute nonresponders treated with traditional NSBB would be needed for a definitive conclusion, our results together with those of prior studies strongly suggest that carvedilol improved the long-term outcome of acute nonresponders.
The ability of the acute response to i.v. propranolol for identifying a subgroup of patients with a higher risk of decompensation and death is well-established[6,12]. In addition, the test is currently considered the most accurate predictor of the chronic hemodynamic response to traditional NSBB[1,2]. Similar to previous studies, no other clinical, laboratory, or endoscopic variables at baseline were able to predict neither the acute nor the chronic hemodynamic response in our study[6,12]. Of note, we observed an association between a positive acute response and a decrease of MAP. Whether the acute change in MAP could help to identify acute hemodynamic responders would require further investigation, as a decrease in MAP has been observed in some studies[19] but not in others[6]. Based on its unique predictive value, recent studies have proposed using the acute hemodynamic response to i.v. propranolol to guide therapy[13,14]. Such an approach, however, has never been formally evaluated in primary prophylaxis of variceal bleeding. The results of our study provide valuable information in this regard from real clinical practice, indicating that the early identification of acute nonresponders and their subsequent treatment with carvedilol may significantly improve the prognosis of these patients. We did not observe any particular adverse effects, including renal function, in patients treated with carvedilol. In addition to its role for guiding therapy, the inclusion of the acute hemodynamic test in the design of future randomized trials of primary prophylaxis of variceal bleeding would also be important for avoiding selection bias. Contrary to current guidelines that recommend that either of type of beta-blocker can be used[1,2,16], our results suggest that carvedilol should become the beta-blocker of choice in centers with no available hepatic hemodynamic testing until adequate clinical trials are performed.
The high proportion of acute nonresponders (69.2%) that achieved a chronic hemodynamic response with carvedilol and the correlation between the magnitude of HVPG changes in the acute and the chronic hemodynamic responses are other relevant findings from our study that support previous observations[6,18,20]. Accordingly, Reiberger et al[20] recently reported that up to 56% of patients who had no chronic hemodynamic response to propranolol were able to achieve a hemodynamic response after switching to carvedilol, supporting the efficacy of carvedilol in this patient population. The enhanced effects of carvedilol for reducing portal pressure might be responsible for the favorable outcome of acute nonresponders found in our study. The lack of association between the chronic response to NSBB and the risk of decompensation may be related to a low statistical power as well as to the late performance of the second hemodynamic study. Indeed, a late evaluation of the hemodynamic response has been associated with a poorer accuracy in predicting outcome because some chronic nonresponders might benefit from nonhemodynamic effects of NSBB (e.g., reduction of bacterial translocation) leading to a favorable outcome despite such nonresponse[1].
The retrospective and single-center design of our study might account for potential selection bias, but the baseline characteristics of our patients were equally distributed between groups and comparable to those of previous studies[6,12], and they were well followed and studied. Importantly, confounding biases such as alcohol withdrawal, clearance of hepatitis C and relevant concomitant treatments were thoroughly recorded and there were no differences between groups. Noteworthy, excluding 6 patients that received statins, which have been reported to influence portal pressure and decompensations, did not alter the main results[21]. Furthermore, we performed multivariate analyses and compared the risk of decompensation separately in patients with compensated and decompensated cirrhosis to avoid the well-known bias of pooling both groups of patients in portal hypertension research[6,12]. Remarkably, the present study is one of the largest series involving the evaluation of the acute hemodynamic response, and the first to evaluate its usefulness for guiding therapy in real clinical practice. Based on the risk of decompensation of acute and nonacute responders treated with traditional NSBB reported in prior studies[6,7,12], the sample size of our study had enough statistical power to make adequate comparisons of the main endpoint. Indeed, the estimated sample size for patients with compensated cirrhosis, using the arcsin square root transformation, would be of 17 acute responders and 9 acute nonresponders, computing a risk of decompensation at 2 years in acute responders of 20%, a risk ratio of 3, a ratio of acute responders/nonresponders of 2, an alpha error of 0.05 and beta error of 0.20. With similar settings and even a lower risk ratio of 2.5 in patients with decompensated cirrhosis, the required sample size would be of 29 acute responders and 15 nonresponders. It is still possible, however, that the statistical power was limited for some analyses. For instance, the 2-year actuarial probability of variceal bleeding might have been different between groups had the sample size been greater. It should also be recognized that our results may not be generalized to patients with grades of liver dysfunction different from those of our study population.
In conclusion, the early identification of acute nonresponders and their treatment with carvedilol resulted in risks of decompensation and death that were comparable to those of acute responders treated with propranolol. These findings suggest that carvedilol improved the long-term outcome of acute nonresponders, presumably by its greater effects on reducing portal pressure, and should be the preferred choice over NSBB for primary prophylaxis of variceal bleeding when hemodynamic testing is not available.
We wish to thank the nursing team in the Vascular Radiology Department for their technical support in the hemodynamic studies.
Traditional nonselective beta-blockers (NSBBs) (i.e. propranolol and nadolol) and carvedilol are valid first-line treatments in patients starting primary prophylaxis of variceal bleeding. Although no clinical trial has adequately compared their efficacy head-to-head, several randomized controlled trials and a meta-analysis have shown that carvedilol is more effective in reducing portal pressure. NSBB-induced reductions in hepatic venous pressure gradient (HVPG) > 10% from baseline have been associated with a lower risk of decompensation and death. The acute hemodynamic test (i.e. HVPG response after 20 min of the intravenous injection of 0.15 mg/kg propranolol) has been proposed as a valid and more cost-effective alternative to separate HVPG procedures. Supporting this notion, recent studies in patients treated with traditional NSBB showed that the risk of decompensation was lower in those who had an acute response than in those who were acute nonresponders. The acute test also predicted the chronic hemodynamic response.
Since the acute test enables the earlier identification of chronic nonresponders to traditional NSBB and carvedilol has a greater efficacy for reducing portal pressure, this test could guide the type of NSBB to be used in patients starting primary prophylaxis of variceal bleeding.
The primary endpoint was development of first or further decompensation of cirrhosis. Secondary endpoints included death from any cause, association between acute and chronic hemodynamic response, and baseline clinical and laboratory variables related to the acute hemodynamic response.
We retrospectively reviewed all patients starting primary prophylaxis of variceal bleeding following an acute hemodynamic response-guided protocol. Acute or chronic hemodynamic response was defined as a decrease in HVPG to < 12 mmHg or as a ≥ 10% reduction in HVPG from baseline. According to our institutional protocol, 52 acute responders to intravenous propranolol were treated with traditional NSBB (i.e. propranolol or nadolol) and 24 acute nonresponders received carvedilol. A second hemodynamic study was performed in 27 and 13 patients, respectively. Follow-up data (i.e. medical history, laboratory values, imaging tests and treatment compliance) were recorded in each visit (i.e. within 1 mo after the performance of the baseline hemodynamic study, and every 3-6 mo thereafter).
The risk of first or further decompensation was similar in both groups at 1, 2 and 3 years of follow-up. A previous episode of hepatic encephalopathy was the only independent predictor of decompensation. Mortality rates were also similar between groups. No clinical, laboratory, or endoscopic variables at baseline were able to predict neither the acute nor the chronic hemodynamic response. A high proportion of acute nonresponders (69.2%) achieved a chronic hemodynamic response with carvedilol and there was a strong correlation between the acute and chronic changes in HVPG in the traditional NSBB group.
The early identification of acute nonresponders and their treatment with carvedilol resulted in risks of decompensation and death that were comparable to those of acute responders treated with propranolol. These findings suggest that carvedilol improved the long-term outcome of acute nonresponders, presumably by its greater effects on reducing portal pressure, and should be the preferred choice over NSBB for primary prophylaxis of variceal bleeding when hemodynamic testing is not available.
The design of our study cannot definitively conclude that carvedilol should become the beta-blocker of choice in patients starting primary prophylaxis of variceal bleeding. In order to confirm this possibility, a randomized controlled trial with a control group of acute nonresponders treated with traditional NSBB would be needed.
STROBE Statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Furuichi Y, Hashimoto N, Zhuge YZ S- Editor: Ma RY L- Editor: Filipodia E- Editor: Wu YXJ
| 1. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1441] [Article Influence: 180.1] [Reference Citation Analysis (3)] |
| 2. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2293] [Article Influence: 229.3] [Reference Citation Analysis (3)] |
| 3. | D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2133] [Article Influence: 112.3] [Reference Citation Analysis (3)] |
| 4. | Turnes J, Garcia-Pagan JC, Abraldes JG, Hernandez-Guerra M, Dell’Era A, Bosch J. Pharmacological reduction of portal pressure and long-term risk of first variceal bleeding in patients with cirrhosis. Am J Gastroenterol. 2006;101:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Villanueva C, López-Balaguer JM, Aracil C, Kolle L, González B, Miñana J, Soriano G, Guarner C, Balanzó J. Maintenance of hemodynamic response to treatment for portal hypertension and influence on complications of cirrhosis. J Hepatol. 2004;40:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Villanueva C, Aracil C, Colomo A, Hernández-Gea V, López-Balaguer JM, Alvarez-Urturi C, Torras X, Balanzó J, Guarner C. Acute hemodynamic response to beta-blockers and prediction of long-term outcome in primary prophylaxis of variceal bleeding. Gastroenterology. 2009;137:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 7. | Hernández-Gea V, Aracil C, Colomo A, Garupera I, Poca M, Torras X, Miñana J, Guarner C, Villanueva C. Development of ascites in compensated cirrhosis with severe portal hypertension treated with β-blockers. Am J Gastroenterol. 2012;107:418-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 8. | Bañares R, Moitinho E, Matilla A, García-Pagán JC, Lampreave JL, Piera C, Abraldes JG, De Diego A, Albillos A, Bosch J. Randomized comparison of long-term carvedilol and propranolol administration in the treatment of portal hypertension in cirrhosis. Hepatology. 2002;36:1367-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Kim SG, Kim TY, Sohn JH, Um SH, Seo YS, Baik SK, Kim MY, Jang JY, Jeong SW, Lee B. A Randomized, Multi-Center, Open-Label Study to Evaluate the Efficacy of Carvedilol vs. Propranolol to Reduce Portal Pressure in Patients With Liver Cirrhosis. Am J Gastroenterol. 2016;111:1582-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 10. | Sinagra E, Perricone G, D’Amico M, Tinè F, D’Amico G. Systematic review with meta-analysis: the haemodynamic effects of carvedilol compared with propranolol for portal hypertension in cirrhosis. Aliment Pharmacol Ther. 2014;39:557-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | Tripathi D, Ferguson JW, Kochar N, Leithead JA, Therapondos G, McAvoy NC, Stanley AJ, Forrest EH, Hislop WS, Mills PR. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology. 2009;50:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 12. | La Mura V, Abraldes JG, Raffa S, Retto O, Berzigotti A, García-Pagán JC, Bosch J. Prognostic value of acute hemodynamic response to i.v. propranolol in patients with cirrhosis and portal hypertension. J Hepatol. 2009;51:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Villanueva C, Graupera I, Aracil C, Alvarado E, Miñana J, Puente Á, Hernandez-Gea V, Ardevol A, Pavel O, Colomo A, Concepción M, Poca M, Torras X, Reñe JM, Guarner C. A randomized trial to assess whether portal pressure guided therapy to prevent variceal rebleeding improves survival in cirrhosis. Hepatology. 2017;65:1693-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, Bañares R, Morillas R, Poca M, Peñas B, Augustin S, Abraldes JG, Alvarado E, Torres F, Bosch J. Preventing decompensation of cirrhosis with clinically significant portal hypertension and without high-risk varices: a new indication for non-selective beta-blockers (NSBB). J Hepatol. 2017;66:S97-S98. [DOI] [Full Text] |
| 15. | Puente Á, Cabezas J, López Arias MJ, Fortea JI, Arias MT, Estébanez Á, Casafont F, Fábrega E, Crespo J. Influence of sustained viral response on the regression of fibrosis and portal hypertension in cirrhotic HCV patients treated with antiviral triple therapy. Rev Esp Enferm Dig. 2017;109:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1818] [Article Influence: 259.7] [Reference Citation Analysis (2)] |
| 17. | Luca A, García-Pagán JC, Feu F, Lopez-Talavera JC, Fernández M, Bru C, Bosch J, Rodés J. Noninvasive measurement of femoral blood flow and portal pressure response to propranolol in patients with cirrhosis. Hepatology. 1995;21:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 18. | de-Madaria E, Palazón JM, Hernández FT, Sánchez-Paya J, Zapater P, Irurzun J, de España F, Pascual S, Such J, Sempere L. Acute and chronic hemodynamic changes after propranolol in patients with cirrhosis under primary and secondary prophylaxis of variceal bleeding: a pilot study. Eur J Gastroenterol Hepatol. 2010;22:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Feu F, Bordas JM, Luca A, García-Pagán JC, Escorsell A, Bosch J, Rodés J. Reduction of variceal pressure by propranolol: comparison of the effects on portal pressure and azygos blood flow in patients with cirrhosis. Hepatology. 1993;18:1082-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 20. | Reiberger T, Ulbrich G, Ferlitsch A, Payer BA, Schwabl P, Pinter M, Heinisch BB, Trauner M, Kramer L, Peck-Radosavljevic M; Vienna Hepatic Hemodynamic Lab. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non-response to propranolol. Gut. 2013;62:1634-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 21. | Abraldes JG, Villanueva C, Aracil C, Turnes J, Hernandez-Guerra M, Genesca J, Rodriguez M, Castellote J, García-Pagán JC, Torres F. Addition of Simvastatin to Standard Therapy for the Prevention of Variceal Rebleeding Does Not Reduce Rebleeding but Increases Survival in Patients With Cirrhosis. Gastroenterology. 2016;150:1160-1170.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |