Published online Nov 6, 2018. doi: 10.12998/wjcc.v6.i13.600
Peer-review started: July 17, 2018
First decision: August 25, 2018
Revised: September 16, 2018
Accepted: October 16, 2018
Article in press: October 16, 2018
Published online: November 6, 2018
Processing time: 113 Days and 0.7 Hours
To investigate the relationship between levels of iron metabolism markers and hepatitis B virus (HBV)-related chronic liver diseases.
This case-control study with 318 participants included 78 cases of chronic hepatitis B, 85 cases of HBV-related liver cirrhosis, 77 cases of HBV-related hepatocellular carcinoma, and 78 healthy controls. Markers of iron metabolism were detected in participants. Hematological and biochemical parameters and HBV-DNA were assessed. Child-Pugh grade and Barcelona Clinic Liver Cancer stage were determined for each hepatocellular carcinoma patient. Perls’ staining was performed on liver sections. The SPSS program was used for all statistical analyses, and statistical significance was considered if a P-value < 0.05.
Significantly higher serum ferritin and lower serum hepcidin levels were detected in all groups of HBV-infected patients compared with healthy controls. Serum iron, total iron binding capacity, and serum transferrin levels were significantly lower in patients with cirrhosis and hepatocellular carcinoma, whereas the hepcidin level was higher than that in chronic hepatitis B patients. Correlation analysis indicated that serum hepcidin was negatively correlated with HBV-DNA load (P < 0.01). Serum ferritin and transferrin saturation levels increased proportionally to the extent of liver cirrhosis and poorer Child-Pugh scores (P < 0.05). The decreased serum iron and transferrin saturation levels were significantly correlated with a smaller hepatocellular carcinoma tumor burden according to Barcelona Clinic Liver Cancer staging. Liver histology showed a clearly increasing trend in iron deposition in the liver tissues with increased fibrosis, which became prominent at stages 3 (severe liver fibrosis) and 4 (cirrhosis).
Iron metabolism disorders occur in patients with HBV-related liver diseases. The serum markers of iron metabolism disorders vary in different stages of HBV-related liver diseases.
Core tip: The relationship between hepatitis B viruses (HBV) related chronic liver diseases and levels of components in iron metabolism and the corresponding impact on liver disease severity have not been clearly described. In our study, we found that significantly higher serum ferritin and lower serum hepcidin levels were detected in all groups of HBV-infected patients compared with healthy controls. Serum iron, total iron binding capacity, and serum transferrin levels were significantly lower in patients with cirrhosis and hepatocellular carcinoma, whereas the hepcidin level was higher than that in chronic hepatitis B patients. In conclusion, iron metabolism disorders are present in patients with HBV-related liver diseases. The characteristics of iron metabolism disorders in different development stages of HBV-related liver diseases varied.
- Citation: Gao YH, Wang JY, Liu PY, Sun J, Wang XM, Wu RH, He XT, Tu ZK, Wang CG, Xu HQ, Niu JQ. Iron metabolism disorders in patients with hepatitis B-related liver diseases. World J Clin Cases 2018; 6(13): 600-610
- URL: https://www.wjgnet.com/2307-8960/full/v6/i13/600.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i13.600
Chronic hepatitis B virus (HBV) infection affects 240 million people worldwide and can lead to chronic liver diseases including chronic hepatitis B (CHB), cirrhosis, and hepatocellular carcinoma (HCC). An estimated 788000 patients die of HBV-related liver diseases each year[1]. In managing chronic HBV infection, the objective is to detect liver injury early and then stop the progression of liver disease through effective treatment. To achieve this goal, a clear understanding of various factors involved in HBV pathogenesis is required.
Maintenance of iron metabolism within the physiological range is essential to human health. The liver synthesizes several proteins that are required for iron metabolism in addition to iron storage. Chronic liver injury and histological alteration impact iron metabolism, and altered iron metabolism may aggravate liver injury[2-4]. For instance, both experimental and clinical studies have suggested that sustained iron retention in hepatocytes aggravates liver injury and is associated with higher risks of developing fibrosis, cirrhosis, and HCC in CHB patients[5-7]. In 1981, Blumberg et al[8] reported that serum iron indices tend to be higher in CHB patients than in those who had HBV infection cleared. Moderate iron accumulation in liver cells has been observed in patients with nonalcoholic fatty liver disease, and the down-regulation of the iron exporter ferroportin-1 in affected cells was suggested to be responsible for the reduced iron clearance from the liver[9]. Hepcidin, a homeostatic regulator, modulates iron absorption in the intestinal mucosal epithelial cells, iron recycle in macrophages, and iron storage in liver cells. The serum level of hepcidin is decreased in patients with chronic hepatitis C[10,11]. Advanced alcoholic liver disease is associated with abnormally accumulated iron in the liver[12]. Wang et al[13] found that the mean serum hepcidin level was significantly higher in non-cirrhotic CHB patients than in healthy controls. Another study found that successful treatment with lamivudine may lead to a reduction in the serum ferritin level and hepatic siderosis in CHB, implying that hepatic siderosis is secondary to HBV-related liver injury or may contribute to liver injury[14].
The relationship between HBV-related chronic liver diseases and levels of components in iron metabolism and the corresponding impact on liver disease severity have not been clearly described. The aim of this study was to characterize changes in serum iron components in patients with HBV-related diseases and identify correlations between changes in iron metabolism and HBV-related liver injury.
This study included 78 healthy controls, 78 CHB patients including 25 diagnosed by biopsy, 85 patients with HBV-related liver cirrhosis (LC) including 5 diagnosed by biopsy, and 77 with HBV-related HCC. All patients were ethnic Han Chinese recruited between January 2012 and December 2014 in The First Hospital of Jilin University in Changchun, China. The groups were matched for age and sex. CHB, cirrhosis, and HCC were diagnosed based on the criteria outlined in the Guidelines for the Prevention and Treatment of CHB (2010 version adapted at the 10th National Conference on Viral Hepatitis and Hepatology 2011, China). Briefly, CHB was diagnosed when an individual had been HBsAg positive for more than 6 mo and had clinical manifestations of hepatitis and abnormal liver biochemistry or histological evidence of liver injury. The diagnosis of LC was made for cases meting one of the following terms: (1) histological evidence based on liver biopsy; and (2) biochemical/hematological and imaging evidence of liver dysfunction or portal hypertension accompanied by hypersplenism/gastroesophageal varices/hepatic encephalopathy/ascites. LC cases were divided into subgroups according to the Child-Pugh classification[15]. HBV-related cirrhosis was defined as cirrhosis with a history of CHB or chronic HBV infection. Fibrosis in patients with biopsy was staged according to the METAVIR scoring system[16]. HBV-related HCC was diagnosed based on: (1) a solid mass detected by one of the imaging modalities (computed tomography, magnetic resonance imaging, or ultrasonography) or (2) cytological or histological evidence of liver cancer cells appearing in the liver of a chronic HBV-infected patient. All patients were positive for one or multiple HBV serological markers. Patients were excluded if they: (1) were co-infected with hepatitis A, C, or D or had alcoholic liver disease or drug-induced hepatitis; (2) had diseases of the blood system including hereditary hemochromatosis (types I, II, III, and IV) and chronic anemia; or (3) had an iron overload disease.
The healthy controls were negative for hepatitis B surface antigen and hepatitis C antibody and had normal liver transaminase levels and abdominal ultrasound examinations. Individuals who manifested iron metabolism disorders were excluded. Written informed consent was obtained from all participants, and the study was approved by The First Hospital Ethical Committee of Jilin University.
Blood samples were collected from all patients after overnight fasting. Serum iron, ferritin, and total iron binding capacity were analyzed with an automatic clinical chemistry analyzer (Hitachi 7600 D-210, Japan). Transferrin (TF) was determined using an automatic protein analyzer (BN II System, SIEMENS, Germany). HBV serological markers were measured with a ThermoScientific MultiskanGo (Kehua, Shanghai, China). The Elecsys 2010 and Roche COBAS e 411 immunoassay systems (Roche Diagnostics, Grenzach, Germany) were used to quantitate serum HBsAg, and the COBAS AmpliPrep/COBAS TaqMan assay (Roche Molecular Diagnostics, Grenzach, Germany) was used for quantitating HBV DNA in serum, which had a lower limit of detection at 15 U/mL. Biochemical markers were determined with the Synchron LX120 Autoanalyzer (Beckman Coulter, Brea, CA, United States). Hepcidin was measured using an enzyme-linked immuno sorbent assay kit (IBL, Germany), and transferrin saturation was calculated as a percentage [serum iron/total iron binding capacity (TIBC) × 100%].
Liver biopsy was performed with a 16 G Tru-Cut needle guided by color Doppler ultrasound. A biopsy that was 1.5 cm or longer or a liver section that contained at least five portal tracts was considered eligible for diagnosis. The liver sections from each biopsy were stained with hematoxylin and eosin and Perls’ stain, respectively. Liver fibrosis was staged using the METAVIR scoring system, which consists of five stages: S0 (no fibrosis), S1 (portal fibrosis without septa), S2 (portal fibrosis with rare septa), S3 (portal fibrosis with many septa), and S4 (cirrhosis). Two pathologists at the Diagnostic Center of the First Hospital of Jilin University, who were blinded to patients’ clinical data, independently scored sections, and the resultant scores for each section were averaged in the analysis.
Mean ± SEM or median (range) was calculated for all numerical variables. Percentages are used to express categorical data. Differences between groups were determined by analysis of variance and Student’s t-tests (normal distribution), the Kruskal-Wallis and Mann-Whitney U-tests (non-normal distribution), or the χ2-test or Fisher’s exact test for categorical data. Correlations between variables were computed based on Spearman’s correlation coefficients. The identified factors were further subjected to multiple linear regression analysis. The SPSS program (version 18.0, SPSS Inc., Chicago, IL, United States) was used for all statistical analyses, and statistical significance was considered if a P-value < 0.05.
The baseline demographic, clinical and laboratory features of the 318 participants are summarized in Table 1. Among the participants, there were 78 cases of CHB (53 males), 85 cases of LC (54 males), 77 cases of HCC (62 males), and 78 healthy controls (48 males). The median (P25-P75) ages of the CHB, LC, and HCC patients as well as the control participants were 46 years (30-61 years), 49 years (27-65 years), 49 years (22-57 years), and 44 years (22-57 years), respectively, and there was no significant difference in age among the groups (P = 0.137). The proportions of male participants were significantly higher than those of female counterparts among all four groups (P = 0.049). Multiple comparisons showed that the male percentage in the HCC group was significantly higher than those in the CHB and LC groups (both P < 0.05), while there was no significant difference among the CHB, LC, and control groups. Higher alanine transaminase (ALT), aspartate transaminase, and HBV-DNA levels and lower serum albumin level, hemoglobin, and platelets (PLT) were detected in the CHB, LC, and HCC patients compared with controls.
| HC | CHB | LC | HCC | P-value | |
| Characteristic | (n = 78) | (n = 78) | (n = 85) | (n = 77) | |
| Gender (M/F) | 48/30 | 53/25 | 54/31 | 62/15 | 0.05 |
| Age (yr) | 44 (25-67) | 46 (30-61) | 49 (27-65) | 49 (22-57) | 0.137 |
| ALT (U/L) | 14.95 (4.4-564) | 122.9 (7-1906) | 64 (8-855) | 41 (15-450.2) | < 0.01 |
| AST (U/L) | 21.55 (11.0-38.5) | 75.5 (14-1499) | 56 (16-845) | 56 (19-634) | < 0.01 |
| ALB (g/L) | 48.22 ± 2.51 | 38.01 ± 5.82 | 32.10 ± 6.87 | 34.56 ± 6.56 | < 0.01 |
| HGB (g/L) | 147.79 ± 13.80 | 145.41 ± 17.74 | 126.33 ± 22.70 | 133.58 ± 22.5 | < 0.01 |
| PLT (× 109/L) | 217 (117-328) | 152.5 (55-298) | 79 (28-303) | 124 (17-515) | < 0.01 |
| APRI | 0.24 (0.1-0.5) | 1.25 (0.16-68.14) | 2.05 (0.24-42.25) | 1.49 (0.24-9.43) | < 0.01 |
| HBV-DNA (Log10) | 0 (0-0) | 6.64 (1.3-8.91) | 4.96 (1.7-8.35) | 4.61 (1.7-7.88) | < 0.01 |
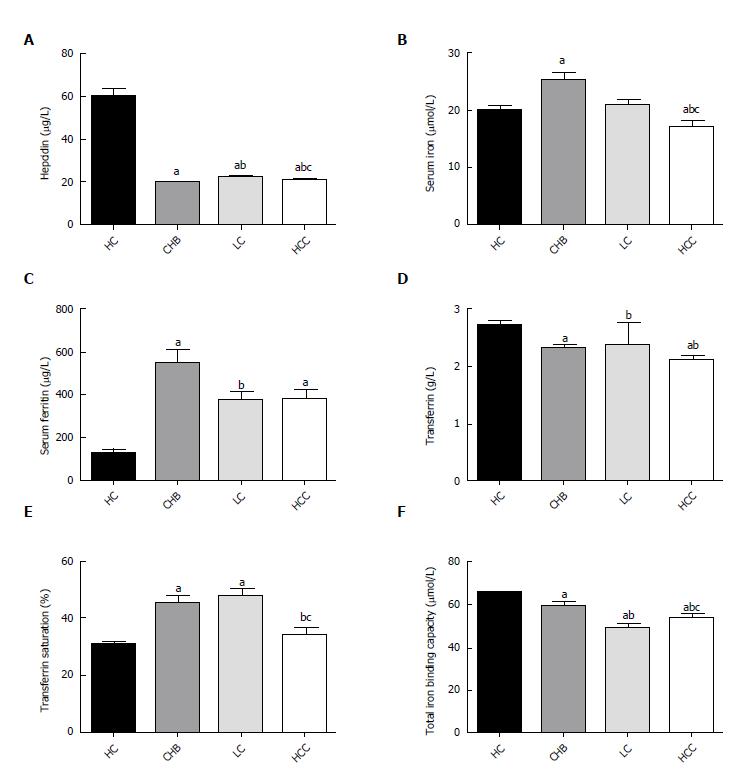
As shown in Figure 1A-F, the serum ferritin levels were significantly higher in the CHB, LC, and HCC groups compared with the control group. The median TIBC level was significantly lower in the CHB group (59.63 ± 15.07), LC (49.29 ± 15.37), and HBV-HCC patients (54.16 ± 12.86) than in healthy controls (65.81 ± 9.18), as was the mean transferrin level in CHB (2.30 ± 0.59), LC (2.37 ± 3.42), and HCC (2.11 ± 0.52) patients relative to the values in controls (2.72 ± 0.47). Among the three liver disease groups, serum iron, total iron binding capacity, and serum transferrin levels were significantly lower, and the hepcidin level was significantly higher in the patients with LC or HCC (P < 0.05) than in the patients with CHB. However, the mean serum iron level in LC patients (20.99 ± 9.51 µmol/L) was comparable to that in the controls (20.05 ± 6.12 µmol/L). Increased transferrin saturation was observed in the CHB and LC groups, but not in HCC patients compared with the control group (P < 0.05 and P = 0.731).
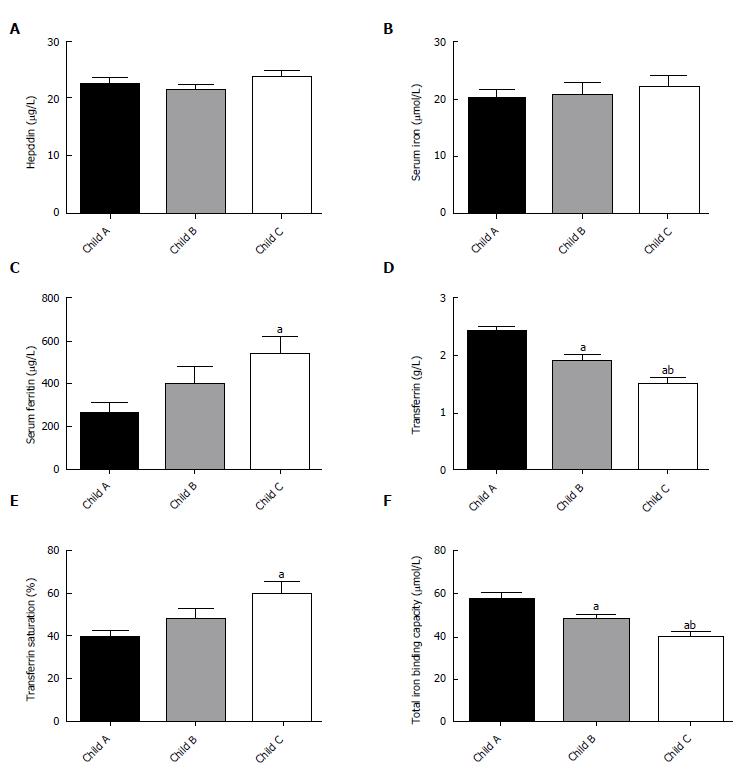
The increases in serum ferritin and transferrin saturation were proportional to the extent of LC and differed significantly among patients with different Child-Pugh scores (P < 0.05; Figure 2C and E). The TIBC and TF levels significantly decreased with increasing Child-Pugh scores (Figure 2F and D). There was no significant difference in serum iron or hepcidin among patients with different Child-Pugh scores (P > 0.05; Figure 2A and B).
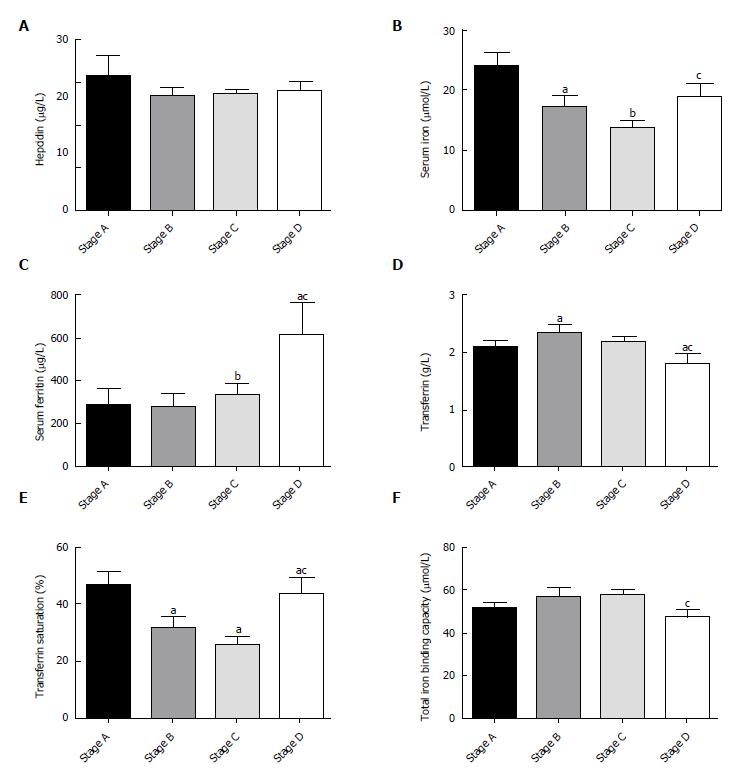
The decreased serum iron and transferrin saturation levels significantly correlated with the smaller tumor burden in the first three stages of HCC by BCLC staging. Unexpectedly, they were significantly higher at stage D compared with stage B or C (Figure 3B and E). However, the mean transferrin level in patients with stage D was significantly lower than that in patients with stage A or B disease (Figure 3D). The serum ferritin level was significantly higher at stage D than during the other three early stages (Figure 3C). There was no significant difference in hepcidin or total iron binding capacity among HCC patients at different BCLC stages (P > 0.05; Figure 3A and F).
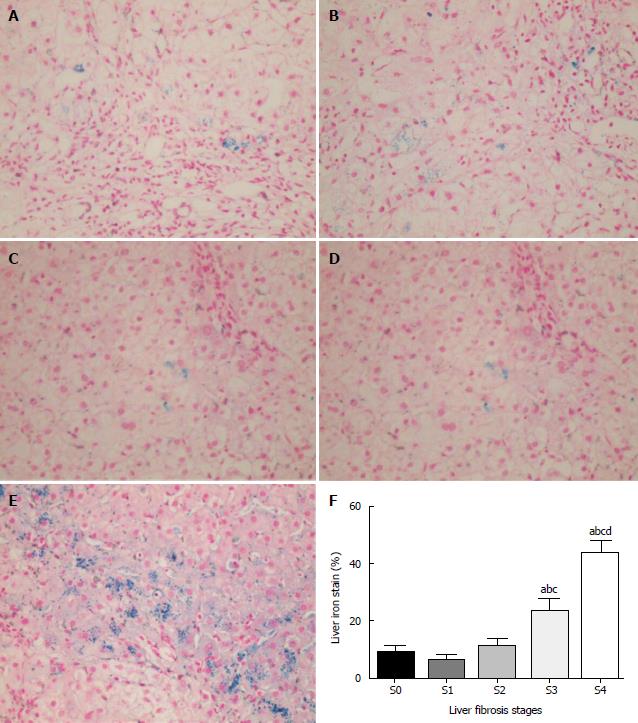
The extent of iron deposition in liver cells was investigated in patients with fibrosis at different stages as indicated by histology. A total of 29 patients were selected for iron staining, including 5 CHB patients (4 males) at S0, 8 (5 males) at S1, 4 (3 males) at S2, 7 (6 males) at S3, and 5 (2 males) at S4. There was no significant difference in the median (P25-P75) ages among the different stage groups (P = 0.122). There was a clear trend of an increase in iron deposition in the liver tissues with increased fibrosis, which became significant at stages 3 and 4 (Figure 4A-F; P < 0.05). The iron level was barely detectable at stage 0 (Figure 4A), while prominent iron retention in hepatocytes was detected at S3 and S4 (Figure 4D and E). Iron deposition in liver cells was observed in 10%–50% of CHB patients. The mean iron level was significantly higher in severe fibrosis (S3: 23.7%) and cirrhosis (S4: 43.6%) compared with that in tissues with no or mild fibrosis (S0: 5.2%; S1: 7.9%; S2: 8.5%).
The relationships of serum hepcidin levels and demographic and clinical findings are summarized in Table 2. Univariate analysis showed that serum hepcidin was significantly correlated with age (rho = 0.3, P < 0.001), total bile acid (TBA; rho = 0.196, P = 0.002), and international normalized ratio (INR; rho = 0.188, P = 0.003). The serum hepcidin level was inversely correlated with HBV-DNA (rho = -0.347, P < 0.001), red blood cell count (RBC; rho = -0.153, P = 0.018), platelet count (PLT; rho = -0.195, P = 0.002), hemoglobin (HGB; rho = -0.158, P = 0.014), and hematocrit (HCT, rho = -0.162, P = 0.012). Subsequently, these factors were subjected to multiple linear regression analysis, which identified age (adjusted effect size = 0.213, P = 0.001), INR (adjusted effect size = 0.198, P ≤ 0.001), and HBV-DNA (adjusted effect size = -0.282, P < 0.001) as independent factors associated with higher serum hepcidin. Notably, HBV-DNA was ranked as the most significant among all independent factors.
| Correlation analysis | Linear regression analysis | |||
| Characteristic | rho | P-value | Effect size | P -value |
| Age (yr) | 0.3 | < 0.01 | 0.213 | 0.001 |
| Gender | 0.01 | 0.986 | - | - |
| ALT (U/L) | -0.095 | 0.141 | - | - |
| AST (U/L) | -0.013 | 0.842 | - | - |
| GGT (U/L) | 0.011 | 0.871 | - | - |
| AKP (U/L) | 0.123 | 0.058 | - | - |
| ALB (g/L) | -0.055 | 0.394 | - | - |
| TBIL (μmol/L) | 0.108 | 0.096 | - | - |
| TBA (μmol/L) | 0.196 | 0.002 | ||
| HBV-DNA (Log10) | -0.374 | < 0.01 | -0.282 | < 0.001 |
| APRI | 0.114 | 0.077 | - | - |
| RBC (× 1012/L) | -0.153 | 0.018 | ||
| WBC (× 109/L) | -0.119 | 0.065 | - | - |
| PLT (× 109/L) | -0.195 | 0.002 | ||
| HGB (g/L) | -0.158 | 0.014 | ||
| MCV (fL) | 0.01 | 0.879 | - | - |
| HCT (L/L) | -0.162 | 0.012 | ||
| INR | 0.188 | 0.003 | 0.198 | < 0.001 |
| Serum iron (μmol/L) | 0.027 | 0.672 | - | - |
| Total iron binding capacity (μmol/L) | -0.073 | 0.261 | - | - |
| Serum ferritin (μg/L) | -0.13 | 0.836 | - | - |
| Transferrin saturation (%) | 0.056 | 0.387 | - | - |
| transferrin (g/L) | 0.002 | 0.98 | - | - |
Our results showed that serum iron level was elevated in CHB but not in LC and HCC. The elevated serum iron level has been described in other chronic liver diseases, including chronic hepatitis C and alcoholic liver disease[17-19]. However, cancer cells consume a large amount of iron to sustain their rapid proliferation[20], resulting in a reduced serum iron level as shown in this study (Figure 1B). In addition, the serum ferritin level was significantly higher in hepatitis B-related liver diseases including LC and HCC (Figure 1C), likely reflecting the increased release of ferritin from hepatocytes that had increased iron deposition and were destroyed as a result of HBV replication and/or iron retention-related injury. This suggestion was supported by our finding that the serum ferritin level was higher in CHB patients than in LC and HCC patients, because a more prominent liver injury, as indicated by ALT level, occurred in CHB[21]. Sebastiani et al[22] reported that serum ferritin is an independent protein associated with hepatic iron deposition, while serum iron and transferrin saturation were not[23].
In the present study, decreased serum transferrin and total iron binding capacity accompanied by increased transferrin saturation was detected in patients with HBV-related liver diseases compared with controls (Figure 1D-F). Similar findings were reported by two studies. One study found that both serum transferrin saturation and ferritin concentration were elevated in patients with HBV-related liver disease[24]. The other showed that 27.1% of the study participants experienced elevated transferrin saturation and 48.7% experienced increased liver iron deposition[25]. However, we found no significant increase in the serum iron level, and in fact, the serum iron was reduced in HCC patients. Similar findings to ours were also reported. For instance, previous studies also found there was no significant change in the serum iron level[26] and no reduction of the serum iron level in patients with LC and HCC[27]. The diverse stages of liver diseases among different studies may have contributed to the discrepancies in the serum iron level.
Intracellular iron deposition may trigger the cellular oxidative stress that may lead to liver injury. Liver iron accumulation significantly correlated with the severity of fibrosis based on METAVIR classification and cirrhosis[28]. Our results also suggested the following relationship: Higher levels of serum ferritin and transferrin saturation were proportional to poorer Child-Pugh scores (Figure 2C and E). The extent of the intracellular iron staining was associated with the degree of liver fibrosis (Figure 4). We found cellular iron accumulation in more than 20% of patients with severe fibrosis and cirrhosis (P < 0.05), which was in line with the report that intracellular iron deposition was detected in chronic hepatitis C patients. Hepatic iron density was an independent predictor of advanced fibrosis in CHC[29]. We did not observe coincident changes among different BCLC stages in the HCC group. The relatively small population in the HCC group may hinder more definitive results. Still, whether subgroups of HCC patients have coincidently changed iron remains to be determined by further studies.
In addition to ferritin and transferrin, hepatocytes also produce and secret hepcidin, an acute phase reactant protein that may negatively regulate the endogenous iron level and reduce the release of iron from cells by interacting with the cellular iron exporter ferroportin that leads to subsequent internalization and degradation[30,31]. Our study further indicates that the major factors independently associated with the altered hepcidin levels were age, HBV-DNA, and INR in patients with HBV-related diseases. The cross-sectional study design limited the ability of the study to clarify causality between HBV-DNA load and altered hepcidin levels. Wang et al[32] indicated that hepcidin expression was regulated by iron and inflammatory factors in HBV-infected patients, and that the virus accumulation in infected hepatocytes can affect hepcidin production. However, the molecular basis by which hepcidin may alter HBV replication is unknown. Interestingly, iron-induced hepcidin expression altered HCV replication in cultured cells[33]. Of note, other studies found no link between HBV infection and hepcidin production[34].
This study does have some limitations. We were unable to determine the cause-effect relationship between the increased iron accumulation and liver injury in HBV-infected patients because of the nature of this cross-sectional study. The relatively small size of the study population may render insufficient statistical power in the analysis. Our findings should be verified in future studies.
In conclusion, iron metabolism disorders can occur in patients with HBV-related liver disease. The serum markers of iron metabolism disorders vary in different stages of HBV-related liver diseases.
Chronic hepatitis B virus (HBV) infection affects 240 million people worldwide and can lead to chronic liver diseases including chronic hepatitis B (CHB), cirrhosis, and hepatocellular carcinoma (HCC). In managing chronic HBV infection, the objective is to detect liver injury early and then stop the progression of liver disease through effective treatment. To achieve this goal, a clear understanding of various factors involved in HBV pathogenesis is required. The relationship between HBV-related chronic liver diseases and levels of serum markers of iron metabolism and their impact on liver disease severity have not been well investigated. The aim of this study was to characterize changes in serum iron markers in patients with HBV-related diseases and to assess correlations between changes in iron metabolism and HBV-related liver injury.
In managing chronic HBV infection, the objective is to detect liver injury early and then stop the progression of liver disease through effective treatment. To achieve this goal, a clear understanding of various factors involved in HBV pathogenesis is required.
The aim of this study was to characterize changes in serum iron markers in patients with HBV-related diseases and assess correlations between changes in iron metabolism and HBV-related liver injury.
This was a case-control study, which included 78 healthy controls, 78 CHB patients including 25 diagnosed by biopsy, 85 patients with HBV-related liver cirrhosis including 5 diagnosed by biopsy, and 77 with HBV-related HCC. Blood samples were collected from all patients after overnight fasting. Serum iron metabolism markers, HBV serological markers and HBV-DNA, and biochemical markers were determined with autoanalyzers. Liver biopsy was performed with a 16 G Tru-Cut needle guided by color Doppler ultrasound. A biopsy that was 1.5 cm or longer or a liver section that contained at least five portal tracts was considered eligible for diagnosis. The liver sections from each biopsy were stained with hematoxylin and eosin and Perls, respectively. Liver fibrosis was staged using the METAVIR scoring system. The SPSS program was used for all statistical analyses, and statistical significance was considered if a P-value < 0.05.
The serum ferritin levels were significantly higher in CHB, liver cirrhosis, and HCC groups compared with the control group. The median TIBC level was significantly lower in the CHB group, liver cirrhosis, and HBV-HCC patients than in healthy controls, as was the mean transferrin level in CHB, liver cirrhosis, and HCC patients relative to the values in controls. Among the three liver disease groups, serum iron, total iron binding capacity, and serum transferrin levels were significantly lower, and the hepcidin level was significantly higher in the patients with cirrhosis or HCC (P < 0.05) than in the patients with CHB. Serum ferritin and transferrin saturation levels increased proportionally to the extent of liver cirrhosis and poorer Child-Pugh scores (P < 0.05). The decreased serum iron and transferrin saturation levels significantly correlated with a smaller hepatocellular carcinoma tumor burden according to Barcelona Clinic Liver Cancer staging. There was a clear trend of an increase in iron deposition in the liver tissues with increased fibrosis, which became prominent at stages 3 and 4 (Figure 4A-F; P < 0.05). The iron level was barely detectable at stage 0 (Figure 4A), while prominent iron retention in hepatocytes was detected at S3 and S4 (Figure 4D and E). Iron deposition in liver cells was observed in 10%–50% of CHB patients. The mean iron level was significantly higher in severe fibrosis and cirrhosis compared with that in tissues with no or mild fibrosis. We also analyzed the relationships between serum hepcidin levels and demographic factors. Age, international normalized ratio, and HBV-DNA were independent factors associated with higher serum hepcidin. The cross-sectional study design limited the ability of this study to clarify causality between HBV-DNA load and altered hepcidin levels.
Iron metabolism disorders occur in patients with HBV-related liver disease. The iron metabolism disorders vary in different stages of HBV-related liver diseases.
The mechanism of iron metabolism disorders present in patients of HBV-related liver disease should be further studied in the future.
We appreciate Li-Shan Su for assistance in revising this manuscript.
STROBE Statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Shimizu Y, Trinder D S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Tan WW
| 1. | Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, Abu-Raddad LJ, Assadi R, Bhala N, Cowie B. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388:1081-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 988] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 2. | Hino K, Nishina S, Hara Y. Iron metabolic disorder in chronic hepatitis C: mechanisms and relevance to hepatocarcinogenesis. J Gastroenterol Hepatol. 2013;28 Suppl 4:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Wei Y, Ye W, Zhao W. Serum Iron Levels Decreased in Patients with HBV-Related Hepatocellular Carcinoma, as a Risk Factor for the Prognosis of HBV-Related HCC. Front Physiol. 2018;9:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Zou DM, Sun WL. Relationship between Hepatitis C Virus Infection and Iron Overload. Chin Med J (Engl). 2017;130:866-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 872] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 6. | Powell LW, Seckington RC, Deugnier Y. Haemochromatosis. Lancet. 2016;388:706-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 193] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 7. | Mao W, Hu Y, Lou Y, Chen Y, Zhang J. Abnormal serum iron markers in chronic hepatitis B virus infection may be because of liver injury. Eur J Gastroenterol Hepatol. 2015;27:130-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Blumberg BS, Lustbader ED, Whitford PL. Changes in serum iron levels due to infection with hepatitis B virus. Proc Natl Acad Sci USA. 1981;78:3222-3224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Lecube A, Hernández C, Simó R. Glucose abnormalities in non-alcoholic fatty liver disease and chronic hepatitis C virus infection: the role of iron overload. Diabetes Metab Res Rev. 2009;25:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Metwally MA, Zein CO, Zein NN. Clinical significance of hepatic iron deposition and serum iron values in patients with chronic hepatitis C infection. Am J Gastroenterol. 2004;99:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Nishina S, Hino K, Korenaga M, Vecchi C, Pietrangelo A, Mizukami Y, Furutani T, Sakai A, Okuda M, Hidaka I. Hepatitis C virus-induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology. 2008;134:226-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 12. | Varghese J, James JV, Sagi S, Chakraborty S, Sukumaran A, Ramakrishna B, Jacob M. Decreased hepatic iron in response to alcohol may contribute to alcohol-induced suppression of hepcidin. Br J Nutr. 2016;115:1978-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Wang J, Dong A, Liu G, Anderson GJ, Hu TY, Shi J, Hu Y, Nie G. Correlation of serum hepcidin levels with disease progression in hepatitis B virus-related disease assessed by nanopore film based assay. Sci Rep. 2016;6:34252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Ohkoshi S, Yoshimura A, Yamamoto S, Yano M, Kurita S, Yamazaki K, Aoki YH, Yamagiwa S, Wakabayashi H, Sugiyama M. Successful treatment with lamivudine may correlate with reduction of serum ferritin levels in the patients with chronic hepatitis and liver cirrhosis type B. Hepatol Int. 2008;2:382-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Suk KT, Baik SK, Yoon JH, Cheong JY, Paik YH, Lee CH, Kim YS, Lee JW, Kim DJ, Cho SW. Revision and update on clinical practice guideline for liver cirrhosis. Korean J Hepatol. 2012;18:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Rozario R, Ramakrishna B. Histopathological study of chronic hepatitis B and C: a comparison of two scoring systems. J Hepatol. 2003;38:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Di Bisceglie AM, Axiotis CA, Hoofnagle JH, Bacon BR. Measurements of iron status in patients with chronic hepatitis. Gastroenterology. 1992;102:2108-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 272] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Datz C, Müller E, Aigner E. Iron overload and non-alcoholic fatty liver disease. Minerva Endocrinol. 2017;42:173-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Britton LJ, Subramaniam VN, Crawford DH. Iron and non-alcoholic fatty liver disease. World J Gastroenterol. 2016;22:8112-8122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 91] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (4)] |
| 20. | Ba Q, Hao M, Huang H, Hou J, Ge S, Zhang Z, Yin J, Chu R, Jiang H, Wang F. Iron deprivation suppresses hepatocellular carcinoma growth in experimental studies. Clin Cancer Res. 2011;17:7625-7633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Lustbader ED, Hann HW, Blumberg BS. Serum ferritin as a predictor of host response to hepatitis B virus infection. Science. 1983;220:423-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Sebastiani G, Tempesta D, Alberti A. Hepatic iron overload is common in chronic hepatitis B and is more severe in patients coinfected with hepatitis D virus. J Viral Hepat. 2012;19:e170-e176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505-3516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 814] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 24. | Yonal O, Akyuz F, Demir K, Ciftci S, Keskin F, Pinarbasi B, Uyanikoglu A, Issever H, Ozdil S, Boztas G. Decreased prohepcidin levels in patients with HBV-related liver disease: relation with ferritin levels. Dig Dis Sci. 2010;55:3548-3551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Martinelli AL, Filho AB, Franco RF, Tavella MH, Ramalho LN, Zucoloto S, Rodrigues SS, Zago MA. Liver iron deposits in hepatitis B patients: association with severity of liver disease but not with hemochromatosis gene mutations. J Gastroenterol Hepatol. 2004;19:1036-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Boige V, Castéra L, de Roux N, Ganne-Carrié N, Ducot B, Pelletier G, Beaugrand M, Buffet C. Lack of association between HFE gene mutations and hepatocellular carcinoma in patients with cirrhosis. Gut. 2003;52:1178-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Büyükaşik NS, Nadır I, Akin FE, Cakal B, Kav T, Ersoy O, Büyükaşik Y. Serum iron parameters in cirrhosis and chronic hepatitis: detailed description. Turk J Gastroenterol. 2011;22:606-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Hézode C, Cazeneuve C, Coué O, Roudot-Thoraval F, Lonjon I, Bastie A, Duvoux C, Pawlotsky JM, Zafrani ES, Amselem S. Liver iron accumulation in patients with chronic active hepatitis C: prevalence and role of hemochromatosis gene mutations and relationship with hepatic histological lesions. J Hepatol. 1999;31:979-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Missiha SB, Ostrowski M, Heathcote EJ. Disease progression in chronic hepatitis C: modifiable and nonmodifiable factors. Gastroenterology. 2008;134:1699-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 30. | Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3537] [Article Influence: 168.4] [Reference Citation Analysis (0)] |
| 31. | Singh B, Arora S, Agrawal P, Gupta SK. Hepcidin: a novel peptide hormone regulating iron metabolism. Clin Chim Acta. 2011;412:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Wang XH, Cheng PP, Jiang F, Jiao XY. The effect of hepatitis B virus infection on hepcidin expression in hepatitis B patients. Ann Clin Lab Sci. 2013;43:126-134. [PubMed] |
| 33. | Bartolomei G, Cevik RE, Marcello A. Modulation of hepatitis C virus replication by iron and hepcidin in Huh7 hepatocytes. J Gen Virol. 2011;92:2072-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Olmez OF, Gurel S, Yilmaz Y. Plasma prohepcidin levels in patients with chronic viral hepatitis: relationship with liver fibrosis. Eur J Gastroenterol Hepatol. 2010;22:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |