Published online Oct 26, 2018. doi: 10.12998/wjcc.v6.i12.570
Peer-review started: July 2, 2018
First decision: July 29, 2018
Revised: August 21, 2018
Accepted: August 28, 2018
Article in press: August 28, 2018
Published online: October 26, 2018
Processing time: 116 Days and 23.4 Hours
The mutation in CNKSR2 leads to a broad spectrum of phenotypic variability and manifests as an X-linked intellectual disability. However, we reported that the male patient in this study not only had intellectual disability but also epileptic seizures. In addition, there were progressive language impairment, attention deficit hyperactivity disorder and autism. Electroencephalograms showed continuous spike-and-wave during sleep. Genetic testing revealed a de novo mutation of the CNKSR2 gene (c.2185C>T, p.Arg729Ter) in the child that was not detected in the parents. Therefore, the child was diagnosed with X-linked epilepsy aphasia syndrome. Deletion of the CNKSR2 gene has been rarely reported in epilepsy aphasia syndrome, but no de novo mutation has been found in this gene. This report not only adds to the spectrum of epilepsy aphasia syndrome but also helps clinicians in diagnosis and genetic counseling.
Core tip: Patient with epileptic seizures and progressive language impairment. Genetic testing revealed a de novo mutation of the CNKSR2 gene in the child and was not detected in the parents. Therefore, the gene may lead to X-linked epilepsy aphasia syndrome.
- Citation: Sun Y, Liu YD, Xu ZF, Kong QX, Wang YL. CNKSR2 mutation causes the X-linked epilepsy-aphasia syndrome: A case report and review of literature. World J Clin Cases 2018; 6(12): 570-576
- URL: https://www.wjgnet.com/2307-8960/full/v6/i12/570.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i12.570
Atypical epilepsy-aphasia syndrome is caused by Landau-Kleffner syndrome (LKS) and epileptic encephalopathy, with a continuous spike-and-wave pattern during sleep[1]. The synapse is the core component of brain operations and executive functions, and its function plays an important role in brain neuron function[2]. CNKSR2 is located on the X chromosome, and as a synaptic protein, it is involved in RAS/MAPK signal transduction[3]. It is highly expressed in the brain[4], and its mutation or deletion causes a wide range of neurodevelopmental defects[5]. Currently, CNKSR2 deletion or mutation has been shown to induce symptoms that are part of the EAS spectrum[6-9]. In this paper, we review clinical data and genetic test results of a child with epilepsy and aphasia and have identified a de novo mutation: CNKSR2 (c.2185C>T, p.Arg729Ter). We reviewed the literature and analyzed the clinical features of X-linked epilepsy-aphasia syndrome in order to assist clinicians in their diagnosis of this condition and to help provide genetic counseling.
An 8-year and 8-mo-old boy from China was admitted to the hospital due to paroxysmal unconsciousness for more than 6 years. The performance of paroxysmal loss of consciousness was associated with brief jerks of the limbs, eye staring, lip bruising, spitting foam from the mouth, and sometimes with urinary incontinence; however, fever was absent. Each episode lasted 2-3 min and resolved on its own. The episodes of epileptic seizures occurred in varying lengths of times; sometimes, the episodes occurred once every six months, and sometimes only once a month. The form of each seizure was similar.
During the clinical examination, the child did not have any abnormal physical signs. There was no abnormal language expression before epileptic episodes. After the onset of a seizure, he gradually showed signs of poor language expression, repeated speech, unanswerable questions, uncooperative actions, and attention deficit hyperactivity disorder (ADHD). The child had intellectual disability, childish behavior, poor communication skills with the outside world, and autism performance. Parents were healthy and involved in a nonconsanguineous marriage, and family history was not significantly abnormal. Maternal pregnancy was normal, and perinatal history of hypoxia and asphyxia was denied. The Apgar score was unknown.
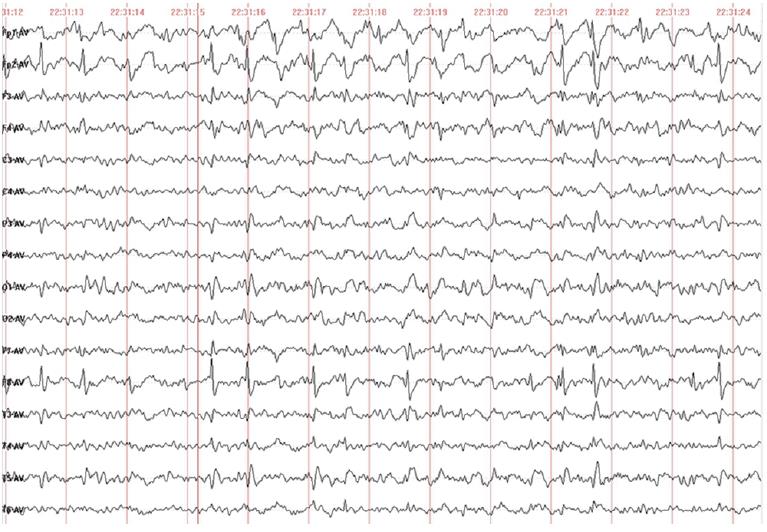
Auxiliary check: There were no abnormalities in routine laboratory examinations, ultrasonography or craniocerebral MRI. His video-electroencephalogram (EEG) (Figure 1) results showed an abnormal EEG and supported the diagnosis of epilepsy.
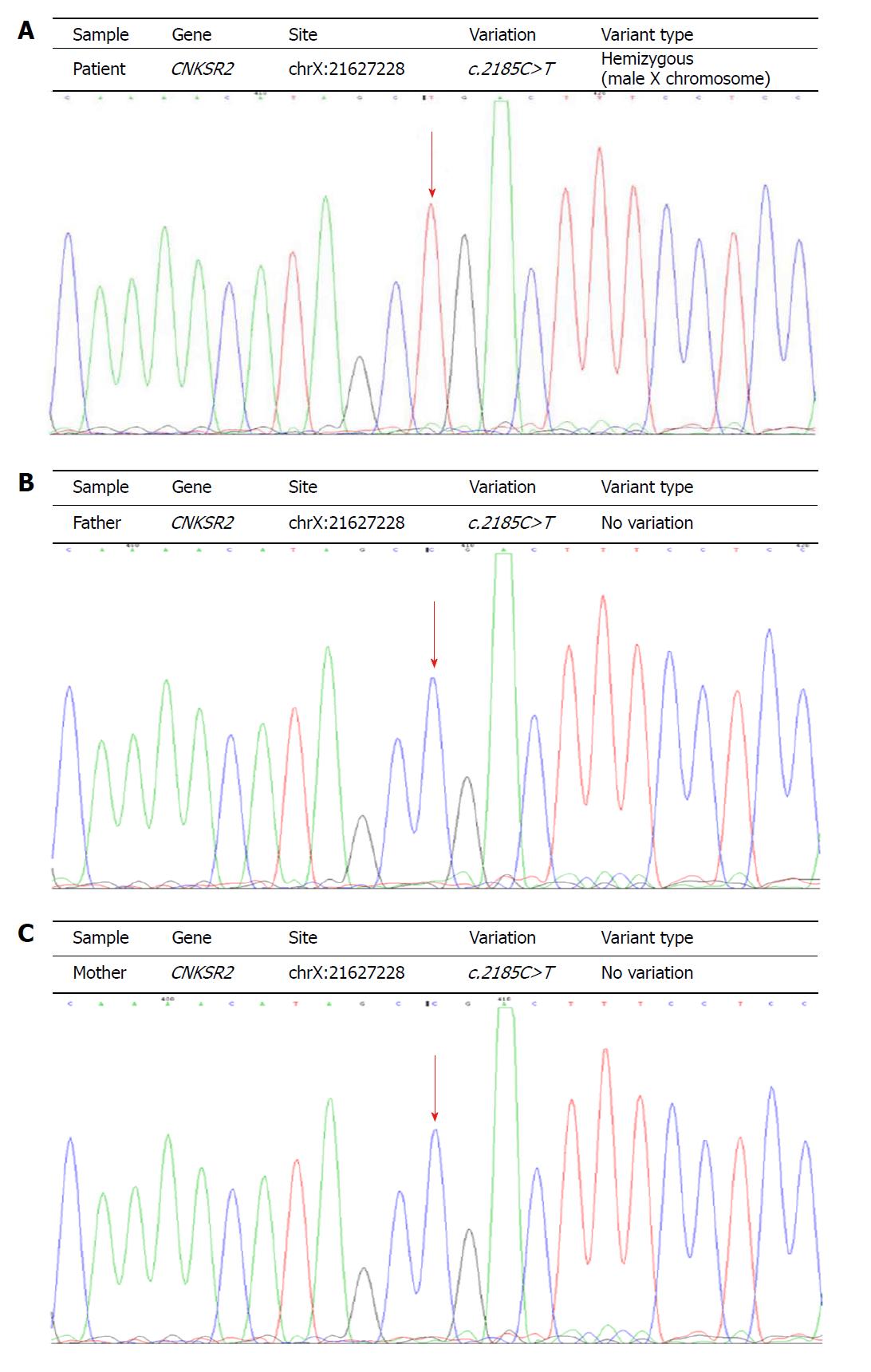
Two milliliter of peripheral venous blood was extracted from the patient and her parents. Genomic DNA from the patient was extracted from blood using standard methods for whole exome sequencing. Mutation of CNKSR2 gene was found in the children. Primers were designed based on the gene tested (chrX:21627228). The parents used Sanger sequencing after PCR to analyze the coding exons and flanking introns of the CNKSR2 gene (NM_014927). The established variant was sequenced in both forward (AGTCCCCAAGCCCAAGCTAC) and reverse directions (ACTGGCTGTCTTGCGAATGG). A nucleotide variation of c.2185C>T (code no. 2185 nucleotides changed from C to T) was identified in the patient’s CNKSR2 gene. The mutation altered the codon sequence of the amino acid Arg into a termination codon (p.Arg729Ter). No abnormalities were identified at this site in the parents. The mutation was de novo (Figure 2). The child was eventually diagnosed with X-Linked EAS, and was treated with hormone and anti-epileptic drugs (Sodium valproate, Levetiracetan). After these treatments, his seizures had eased.
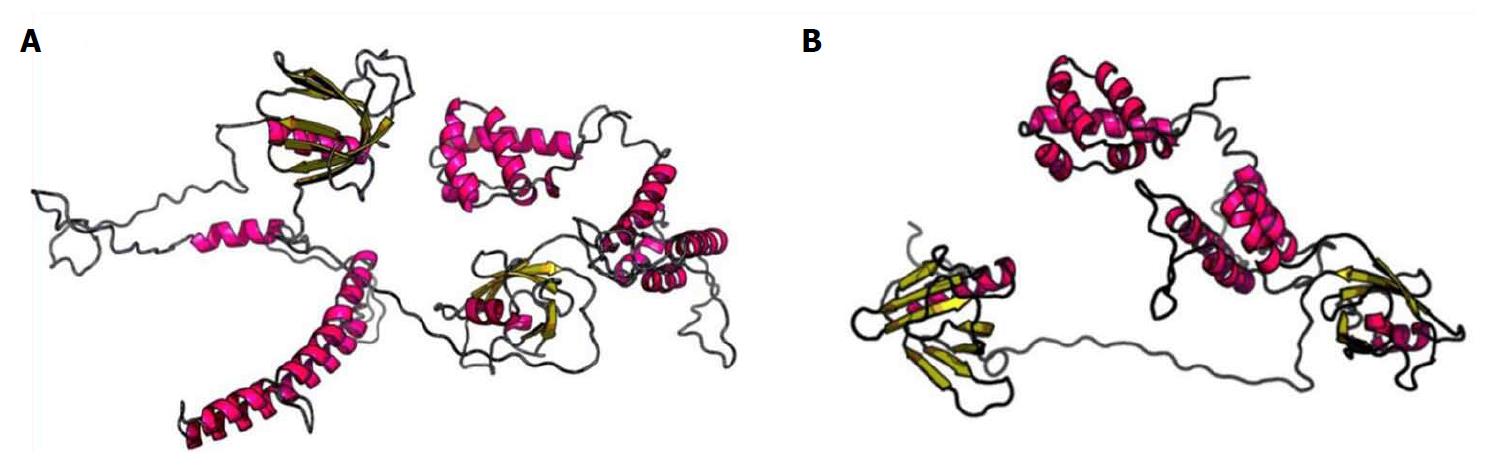
To highlight changes in the secondary structure of the CNKSR2 gene, we used the more popular PSIPRED (http://bioinf.cs.ucl.ac.uk/psipred/)[10] for structural prediction. The codon of no. 729 amino acid Arg was altered into a termination codon (p.Arg729Ter), resulting in the inability to express the 729-1034 sequence of amino acids (Figure 3). RaptorX (http://raptorx.uchicago.edu)[11-13] can predict protein tertiary structures. After inputting the sequence, the 3D structure of the protein sequence can be predicted from the protein database (PDB) (Figure 4). Compared with the wild type, the patient’s CNKSR2 gene did not fold completely in its spatial structure, thus affecting protein function.
CNKSR2 (also known as CNK2, KSR2, MAGUIN)[8] interacts with synaptic scaffold molecules (S-SCAM) and the postsynaptic density (PSD)-95/synaptic-associated protein (SAP) 90 to form a complex[14]. The complex is involved in RAS/MAPK signaling and mediates neuronal proliferation, migration, differentiation and death, as well as RAS-mediated synapse formation[5-9]. It also connects N-methyl-D-aspartate (NMDA) receptors to neuronal cell adhesion molecules[14]. The NMDA subunit encoded by GRIN2A is the first gene associated with EAS[2]. GRIN2A mutations reduce NMDA receptor trafficking and agonist potency–molecular profiling as well as functional rescue[15]. GRIN2A gene is a rare causative gene in Chinese patients with EAS, suggesting the possibility of other genes being involved in the pathogenesis[16]. Hence, we speculate that a mutation or deletion of CNKSR2 may result in changes to the NMDA receptor activity and might affect downstream signaling cascades. Abnormal NMDA receptor will potentially damage the cortical thalamus network during sleep[17]. CNKSR2 is highly expressed in the brain (especially in the hippocampus, amygdala, and cerebellum), and mutations result in loss of specificity and might also affect brain function[7], leading to seizures and neurodevelopmental disorders that especially affect the patient’s speech expression[2]. CNKSR2 is a gene located on the X chromosome, and its mutations or deletions lead to X linkage intelligence disorder (XLID)[8]. The main features of XLID are: (1) intellectual disability; (2) highly restrictive speech (especially expression of language); (3) ADHD; (4) transient childhood epilepsy; and (5) epilepsy with continuous spike waves of slow-wave sleep (CSWS) in early childhood[5].
Before experiencing seizures, our patient suffered from developmental delays and ADHD, which is consistent with the performance of X-linked intellectual disability. After seizure occurrence, the patients’ speech expression gradually decreased, the EEG continued to show abnormal wave patterns during sleep, and a de novo mutation of the CNKSR2 gene was identified. Therefore, we diagnosed this patient as X-linked epilepsy-aphasia syndrome. After definite diagnosis, patients were given immunoglobulin (400-500 mg/kg per day, 3-5 d for 1 course) and oral prednisone (from 1-3 mg/kg per day, and after one month, changed to 1 mg/kg per day), with a total course of 6 to 12 mo. Meanwhile, lamotrigine (75 mg/qd) and sodium valproate oral solution (6 mL/bid) were continued for antiepileptic treatment. At telephone follow-up one year later, the child had fewer epileptic seizures than before as well as partial improvement in verbal ability and an ability to repeat speech; however, the patient had no improvement in intelligence. The disease duration was more than 6 years. If diagnosed early and actively treated, the patient's intelligence, seizures, and language may have been better mitigated.
The underlying mechanism for EAS disorders occ urrence remains unknown, although environmental factors such as thalamic injury[18] and immunity disorders[19], with evidence of onconeural antibodies that can cause the EEG phenotype, have been reported. Studies have shown that the antibodies of brain endothelial cells and nuclei in children were elevated[20]. Additionally, inflammatory markers of children with electrical status epilepticus in sleep (ESES) may be increased[21]. Some researchers have proposed a potential autoimmune reaction secondary to blood-brain-barrier disruption from a thalamocortical uncoupling secondary to the spike-wave activation seen in slow-wave sleep[22]. Furthermore, few genetic causes of ESESS/CSWSS/epilepsy aphasia spectrum have been reported, where the common underlying pathway is channelopathy[23]. The different forms of seizures in EAS include partial seizure, generalized tonic-clonic seizure, atypical absence seizure, myoclonic seizure, atonic seizure, etc. Aphasia can occur before or after epilepsy. Moreover, 70% of patients with epilepsy-aphasia syndrome have epileptic seizures with EEG features that reveal spike-and-wave patterns in the unilateral or bitemporal lobes during the waking period. Generalized continuous spike-and-wave is seen in all leads during sleep, and bilateral synchronous discharge accounts for more than 85% of stage in abnormal discharge[1]. The other 30% of children do not seizures, but show EEG abnormalities (which does not meet the EEG standards of CSWS). Their EEG’s showed the following during sleep: Induced focal epileptiform discharges were identified mainly at the center (but may also might be involved in other areas); or there was no bilateral synchronous activity; or synchronous activity accounted for less than 85% of NREM (non-rapid eye movement) sleep. These cases are all called intermediate epilepsy aphasia (IEADs). The speech recovery ability of IEADs patients is better than that of EAS.
Patients with epilepsy and speech disorders should be advised to undergo EEG monitoring and genetic testing to confirm the diagnosis. Currently, there are no specific medications for the treatment of X-linked epilepsy-aphasia syndrome. The early diagnosis and early use of antiepileptic drugs as well as hormone therapy can recover speech comprehension to different degrees and improve abnormal discharge. Therefore, the overall prognosis of patients is good. Clinical seizures should be treated with antiseizure drugs, and barbiturates, carbamazepine, and phenytoin should be avoided as they can potentiate spike wave discharges during sleep[24,25]. Although there is evidence that mutations or deletions of CNKSR2 lead to neurological development defects, such as epilepsy and intellectual disability, the pathogenesis remains unclear. Therefore, the next step is to screen a large number of epileptic encephalopathy individuals to delineate the phenotypic spectrum of the CNKSR2 mutation. Second-generation gene sequencing can assist in the identification of hereditary etiology and discovery of new mutations while expanding on the early epilepsy encephalopathy clinical phenotype and genetic spectrum. Simultaneously, the pathogenesis of X-linked epilepsy-aphasia syndrome should be studied to assist clinicians in diagnosis and genetic counseling.
Before experiencing seizures, our patient suffered from developmental delays and attention deficit hyperactivity disorder, which is consistent with the performance of X-linked intellectual disability. After seizure occurrence, the patients’ speech expression gradually decreased, the electroencephalogram (EEG) continued to show abnormal wave patterns during sleep, and a de novo mutation of the CNKSR2 gene was identified.
X-linked epilepsy-aphasia syndrome.
Hysteria and childhood autism.
A de novo mutation of the CNKSR2 gene.
EEG continued to show abnormal wave patterns during sleep.
Immunoglobulin, oral prednisone, lamotrigine and sodium valproate oral solution.
Frequency of CNKSR2 mutation in the X-linked epilepsy-aphasia spectrum has been reported in the journal of Epilepsia.
Epileptic encephalopathy with continuous spike-and-wave during sleep.
This case will contribute to improvements in our understanding of X-linked epilepsy-aphasia syndrome. Patients with epilepsy and speech disorders should be advised to undergo EEG monitoring and genetic testing to confirm the diagnosis. The early diagnosis and early use of antiepileptic drugs as well as hormone therapy can recover speech comprehension to different degrees and improve abnormal discharge.
We are grateful to the patient for allowing us to publish this information.
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Garg RK, Kai K, Razek AAKA, Takahashi H S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
| 1. | Tsai MH, Vears DF, Turner SJ, Smith RL, Berkovic SF, Sadleir LG, Scheffer IE. Clinical genetic study of the epilepsy-aphasia spectrum. Epilepsia. 2013;54:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Damiano JA, Burgess R, Kivity S, Lerman-Sagie T, Afawi Z, Scheffer IE, Berkovic SF, Hildebrand MS. Frequency of CNKSR2 mutation in the X-linked epilepsy-aphasia spectrum. Epilepsia. 2017;58:e40-e43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Therrien M, Wong AM, Rubin GM. CNK, a RAF-binding multidomain protein required for RAS signaling. Cell. 1998;95:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 140] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Nagase T, Ishikawa K, Suyama M, Kikuno R, Hirosawa M, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O. Prediction of the coding sequences of unidentified human genes. XII. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 1998;5:355-364. [PubMed] |
| 5. | Vaags AK, Bowdin S, Smith ML, Gilbert-Dussardier B, Brocke-Holmefjord KS, Sinopoli K, Gilles C, Haaland TB, Vincent-Delorme C, Lagrue E. Absent CNKSR2 causes seizures and intellectual, attention, and language deficits. Ann Neurol. 2014;76:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Aypar U, Wirrell EC, Hoppman NL. CNKSR2 deletions: a novel cause of X-linked intellectual disability and seizures. Am J Med Genet A. 2015;167:1668-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Houge G, Rasmussen IH, Hovland R. Loss-of-Function CNKSR2 Mutation Is a Likely Cause of Non-Syndromic X-Linked Intellectual Disability. Mol Syndromol. 2012;2:60-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Hu H, Haas SA, Chelly J, Van Esch H, Raynaud M, de Brouwer AP, Weinert S, Froyen G, Frints SG, Laumonnier F. X-exome sequencing of 405 unresolved families identifies seven novel intellectual disability genes. Mol Psychiatry. 2016;21:133-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 227] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 9. | Piton A, Redin C, Mandel JL. XLID-causing mutations and associated genes challenged in light of data from large-scale human exome sequencing. Am J Hum Genet. 2013;93:368-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 204] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 10. | Buchan DW, Minneci F, Nugent TC, Bryson K, Jones DT. Scalable web services for the PSIPRED Protein Analysis Workbench. Nucleic Acids Res. 2013;41:W349-W357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1112] [Cited by in RCA: 1054] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 11. | Källberg M, Wang H, Wang S, Peng J, Wang Z, Lu H, Xu J. Template-based protein structure modeling using the RaptorX web server. Nat Protoc. 2012;7:1511-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1211] [Article Influence: 93.2] [Reference Citation Analysis (0)] |
| 12. | Ma J, Wang S, Zhao F, Xu J. Protein threading using context-specific alignment potential. Bioinformatics. 2013;29:i257-i265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Peng J, Xu J. RaptorX: exploiting structure information for protein alignment by statistical inference. Proteins. 2011;79 Suppl 10:161-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 251] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 14. | Yao I, Ohtsuka T, Kawabe H, Matsuura Y, Takai Y, Hata Y. Association of membrane-associated guanylate kinase-interacting protein-1 with Raf-1. Biochem Biophys Res Commun. 2000;270:538-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Addis L, Virdee JK, Vidler LR, Collier DA, Pal DK, Ursu D. Epilepsy-associated GRIN2A mutations reduce NMDA receptor trafficking and agonist potency - molecular profiling and functional rescue. Sci Rep. 2017;7:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Yang X, Qian P, Xu X, Liu X, Wu X, Zhang Y, Yang Z. GRIN2A mutations in epilepsy-aphasia spectrum disorders. Brain Dev. 2018;40:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Carvill GL, Regan BM, Yendle SC, O’Roak BJ, Lozovaya N, Bruneau N, Burnashev N, Khan A, Cook J, Geraghty E. GRIN2A mutations cause epilepsy-aphasia spectrum disorders. Nat Genet. 2013;45:1073-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 283] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 18. | Kelemen A, Barsi P, Gyorsok Z, Sarac J, Szucs A, Halász P. Thalamic lesion and epilepsy with generalized seizures, ESES and spike-wave paroxysms--report of three cases. Seizure. 2006;15:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Hu LY, Shi XY, Feng C, Wang JW, Yang G, Lammers SH, Yang XF, Ebrahimi-Fakhari D, Zou LP. An 8-year old boy with continuous spikes and waves during slow sleep presenting with positive onconeuronal antibodies. Eur J Paediatr Neurol. 2015;19:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Connolly AM, Chez MG, Pestronk A, Arnold ST, Mehta S, Deuel RK. Serum autoantibodies to brain in Landau-Kleffner variant, autism, and other neurologic disorders. J Pediatr. 1999;134:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 139] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | van den Munckhof B, de Vries EE, Braun KP, Boss HM, Willemsen MA, van Royen-Kerkhof A, de Jager W, Jansen FE. Serum inflammatory mediators correlate with disease activity in electrical status epilepticus in sleep (ESES) syndrome. Epilepsia. 2016;57:e45-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Stephani U, Carlsson G. The spectrum from BCECTS to LKS: The Rolandic EEG trait-impact on cognition. Epilepsia. 2006;47 Suppl 2:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Kessi M, Peng J, Yang L, Xiong J, Duan H, Pang N, Yin F. Genetic etiologies of the electrical status epilepticus during slow wave sleep: systematic review. BMC Genet. 2018;19:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Fine AL, Wong-Kisiel LC, Wirrell EC. A Novel Phenotype in a Previously Described Epilepsy-Aphasia Disorder. Semin Pediatr Neurol. 2018;26:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Striano P, Capovilla G. Epileptic encephalopathy with continuous spikes and waves during sleep. Curr Neurol Neurosci Rep. 2013;13:360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |