Published online Oct 6, 2018. doi: 10.12998/wjcc.v6.i11.472
Peer-review started: April 27, 2018
First decision: June 20, 2018
Revised: July 26, 2018
Accepted: August 19, 2018
Article in press: August 20, 2018
Published online: October 6, 2018
Processing time: 154 Days and 20 Hours
The descending duodenum is rarely involved in Schistosoma japonicum (S. japonicum) infection. Here, we report a case of acute Schistosoma infection, which presented with abdominal pain, abdominal distension and irregular fever. Tumor-like lesions were observed in the descending duodenum. Simultaneously, heterogeneity in hepatic perfusion was demonstrated by dynamic computed tomography scanning. Biopsy of the descending duodenum showed the deposition of Schistosoma eggs. Following administration of the antihelminthic drug praziquantel, the patient showed rapid clinical improvement. In conclusion, we report a patient with acute S. japonicum infection presenting as tumor-like lesions in the descending duodenum and heterogeneity of blood perfusion in liver parenchyma.
Core tip:Schistosoma japonicum (S. japonicum) is primarily found in the mesenteric veins and tends to involve the colon, rectum and liver. Here, we report a case of acute S. japonicum infection in a patient presenting with tumor-like lesions in the descending duodenum and heterogeneity of blood perfusion in liver parenchyma.
- Citation: Xiao ZL, Xu KS, Song YH. Unusual cause of lesions in the descending duodenum and liver: A case report and review of literature. World J Clin Cases 2018; 6(11): 472-476
- URL: https://www.wjgnet.com/2307-8960/full/v6/i11/472.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i11.472
Schistosomiasis is a disease caused by parasitic flatworms called schistosomes. The three major species of human schistosome are Schistosoma mansoni, Schistosoma haematobium, and Schistosoma japonicum (S. japonicum)[1]. S. japonicum is widespread in East Asia and the southwest Pacific region. It is primarily found in the mesenteric veins and tends to involve the colon, rectum and liver, and occasionally the duodenum. In this study, we report S. japonicum infection involving the descending duodenum presenting as tumor-like lesions. In addition, heterogeneity of blood perfusion in liver parenchyma was observed in the patient. This is the first report to describe patchy infiltration of S. japonicum in liver parenchyma.
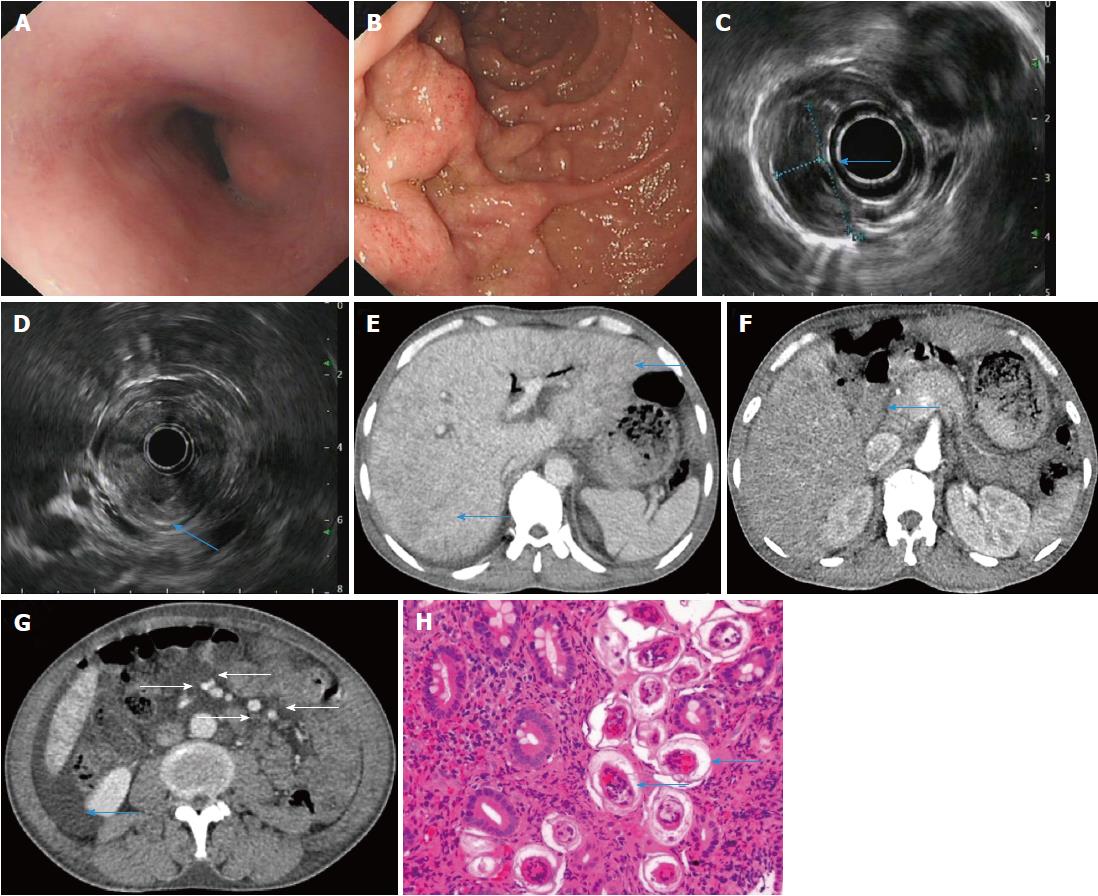
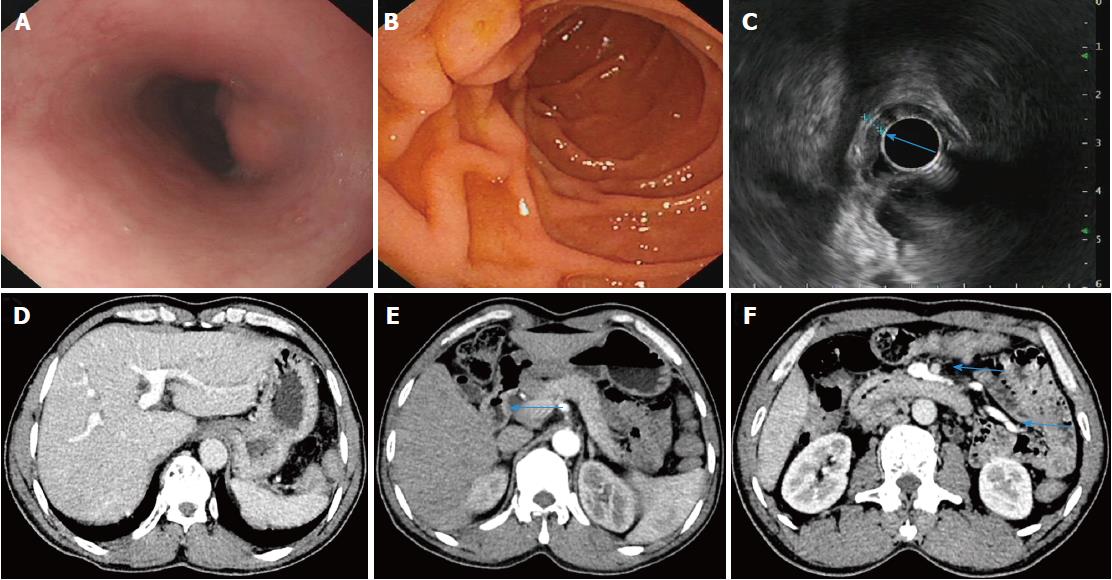
A 47-year-old male farmer presented to our hospital with a 3 wk history of persistent abdominal pain, abdominal distension and irregular low fever. Abdominal examination revealed tenderness in the right upper quadrant with no rebound tenderness or guarding. The results of laboratory tests were as follows: leukocytes, 15450/mm3; eosinophils, 28.7%; erythrocytes, 2.84 × 1012/L; hemoglobin, 86 g/L. Liver function test findings were as follows: albumin, 24.6 g/L; alkaline phosphatase (ALP), 341 U/L (40150 U/L); γglutamyl transferase (γ-GT), 94 U/L (1753 U/L). The hepatitis B results were as follows: hepatitis B surface antigen, positive; hepatitis B virus (HBV) e antibody, positive; hepatitis B core antibody, positive. HBV DNA was undetectable, and hepatitis C virus antibody was negative. Erythrocyte sedimentation rate was 96 mm/h; autoantibodies were negative; immunoglobulins were within the normal range. Neither aerobic nor anaerobic bacteria were detected in blood culture. Tumor markers (carcinoembryonic antigen, alpha fetoprotein, carbohydrate antigen 199, carbohydrate antigen 72-4, prostate specific antigen, squamous cell carcinoma antigen) were within the normal range. The results of ascitic fluid examination were as follows: total protein, 26.0 g/L; albumin, 12.0 g/L; serum-ascites albumin gradient, 12.6 g/L; cell count, 450/mm3; leukocytes, 254/mm3; polymorphonuclear, 46/mm3. A panel of ascitic tumor markers was within the normal range, and the remaining blood tests were normal. Upper gastrointestinal (GI) endoscopy indicated protrusive lesions in the esophagus (Figure 1A), swollen mucosa and protrusive lesions in the descending duodenum (Figure 1B). Endoscopic ultrasonography (EUS) revealed a hypoechoic mass in the muscularis mucosa of the esophagus (Figure 1C, arrow), thickening of the descending duodenal wall, destruction of the descending duodenal wall (Figure 1D, arrow), and ascites (not shown). Dynamic abdominal computed tomography (CT) scanning showed heterogeneous hypointensity in the liver (portal phase) (Figure 1E, arrow), thickening of the descending duodenal wall (Figure 1F, arrow), swollen mesentery around the arteries (regional increase in mesenteric fat density as a result of edema) (Figure 1G, arrowhead), and ascites (Figure 1G, arrow). Serological tests for anti-Schistosoma antibody (ELISA) were positive; a biopsy of the descending duodenum showed the deposition of Schistosoma eggs and infiltration of eosinophils (Figure 1H). The patient received the antihelminthic drug praziquantel. Two months later, the patient underwent a complete checkup, and his symptoms of abdominal pain, abdominal distension and fever had resolved. The results of laboratory tests were as follows: leukocytes, 9280/mm3; eosinophils, 17.60%; erythrocytes, 4.6 × 1012/L; hemoglobin, 142 g/L; albumin, 49.6 g/L; ALP, 169 U/L; γGT, 78 U/L. Upper GI endoscopy demonstrated protrusive lesions in the esophagus (Figure 2A) and normal mucosa (Figure 2B) in the descending duodenum. EUS showed a hypoechoic mass in the muscularis mucosa of the esophagus (no change, not shown), slight thickening and normal layer of the descending duodenal wall (Figure 2C, arrow). Dynamic abdominal CT scanning showed homogeneous hepatic perfusion on the portal phase (Figure 2D), slight thickening of the descending wall (Figure 2E, arrow), shrinkage of swollen mesentery to normal size (Figure 2F, arrowhead), and disappearance of ascites.
S. japonicum, which is widespread in China, is primarily found in the mesenteric veins and involves the colon, rectum and liver. Occasionally, the duodenum is involved in S. japonicum infection and is manifested as erosion, ulcer, bleeding and granular changes in the mucous membrane[2-5]. The patient in our study presented with tumor-like lesions in the descending duodenum, which has not been reported in the literature. EUS and CT revealed thickening and destruction of the descending duodenal wall, which resembled tumor-like lesions. Pathological examination demonstrated S. japonicum infection in the descending duodenum. The diagnosis of GI Schistosomiasis is established by histological evidences in clinical practice. Thus, endoscopic examination identifies the lesion of GI parasites, and pahological evidence from endoscopic biopsies define the diagnosis of parasites infection[6]. In addition, ELISA tests for anti-Schistosoma antibody demonstrated the infection of S. japonicum because S. japonicum is the only human blood fluke that occurs in China. Importantly, rapid improvement of the descending duodenal lesions was observed following administration of praziquantel. The clinical manifestations, pathological changes (uncalcified eggs) and the increase in eosinophils confirmed the diagnosis of acute S. japonicum infection in this patient.
In addition, heterogeneous hypointensity in liver parenchyma on the portal phase of dynamic CT was observed in the patient, which demonstrated a difference in blood perfusion of liver parenchyma. Patchy liver enhancement on the portal phase of dynamic CT was observed in the areas of liver parenchyma, which received better blood perfusion[7-9]. Patchy hepatic infiltration of S. japonicum gave rise to the heterogeneity of blood perfusion in liver parenchyma. Liver biopsy can provide direct evidence of S. japonicum infection in patients; however, liver biopsy was not performed in our patient due to extensive ascites. Following treatment with praziquantel, dynamic abdominal CT showed homogeneous hepatic perfusion on the portal phase. These findings showed that patchy infiltration of S. japonicum resulted in the heterogeneity of blood perfusion in the liver. Portal hypertension resulted from massive deposition of S. japonicum eggs in portal branches of the liver, which might give rise to ascites and heterogeneity in hepatic perfusion.
In conclusion, we report a patient with acute S. japonicum infection presenting as tumor-like lesions in the descending duodenum and heterogeneity of blood perfusion in liver parenchyma. Thus, in areas affected by Schistosomiasis epidemics, S. japonicum infection should be considered when patients have tumor-like lesions in the duodenum or hepatic heterogeneous hypointensity on dynamic CT. In addition, duodenal involvement in patients with S. japonicum infection should receive long-term follow-up to prevent the development of malignant lesions.
A 47-year-old male farmer presented with persistent abdominal pain, abdominal distension and irregular low fever.
The diagnosis of parasitic disease was made by serological test or histological examinations.
Differential diagnosis with malignant lesions due to the thickening and the destruction of the descending duodenal wall.
Serological tests for anti-Schistosoma antibody (ELISA) were positive.
Dynamic abdominal computed tomography (CT) scanning showed heterogeneous hypointensity in the liver, thickening of the descending duodenal wall, swollen mesentery around the arteries, and ascites.
Pathologic examination of the descending duodenum showed the deposition of Schistosoma eggs and infiltration of eosinophils.
The patient received the antihelminthic drug praziquantel.
The infection of Schistosoma japonicum (S. japonicum) is primarily found in the mesenteric veins and tends to involve the colon, rectum and liver, and occasionally the duodenum.
S. japonicum infection should be considered when patients have tumor-like lesions in the duodenum or hepatic heterogeneous hypointensity on dynamic CT.
We would like to thank Professor Liangru Zhu (Division of Gastroenterology, Union Hospital, Tongji Medical College) for providing the EUS images, Professor Xin Li (Department of Radiology, Union Hospital, Tongji Medical College) for providing radiologic images and Dr. You Zhou for providing pathological data.
CARE Checklist (2013) statement: Guidelines of the CARE Checklist (2013) have been adopted while writing this manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gassler N, Ikura Y, Shimizu Y S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Song H
| 1. | McManus DP, Gray DJ, Li Y, Feng Z, Williams GM, Stewart D, Rey-Ladino J, Ross AG. Schistosomiasis in the People’s Republic of China: the era of the Three Gorges Dam. Clin Microbiol Rev. 2010;23:442-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 2. | Contractor QQ, Benson L, Schulz TB, Contractor TQ, Kasturi N. Duodenal involvement in Schistosoma mansoni infection. Gut. 1988;29:1011-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | el Shiekh Mohamed AR, al Karawi MA, Yasawy MI. Organ involvement in hepato-intestinal schistosomiasis. Hepatogastroenterology. 1994;41:370-376. [PubMed] |
| 4. | Madácsy L, Molnár T, Nagy I, Tiszlavicz L, Lonovics J. Recurrent nonvariceal upper gastrointestinal bleeding in a patient with gastroduodenal schistosomiasis. Endoscopy. 2003;35:230-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Witham RR, Mosser RS. An unusual presentation of schistosomiasis duodenitis. Gastroenterology. 1979;77:1316-1318. [PubMed] |
| 6. | Mohamed AE, Ghandour ZM, Al-Karawi MA, Yasawy MI, Sammak B. Gastrointestinal parasites presentations and histological diagnosis from endoscopic biopsies and surgical specimens. Saudi Med J. 2000;21:629-634. [PubMed] |
| 7. | Kan X, Ye J, Rong X, Lu Z, Li X, Wang Y, Yang L, Xu K, Song Y, Hou X. Diagnostic performance of Contrast-enhanced CT in Pyrrolizidine Alkaloids-induced Hepatic Sinusoidal Obstructive Syndrome. Sci Rep. 2016;6:37998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Li X, Yang X, Xu D, Li Q, Kong X, Lu Z, Bai T, Xu K, Ye J, Song Y. Magnetic Resonance Imaging Findings in Patients With Pyrrolizidine Alkaloid-Induced Hepatic Sinusoidal Obstruction Syndrome. Clin Gastroenterol Hepatol. 2017;15:955-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Liu F, Cao X, Ye J, Pan X, Kan X, Song Y. Oxaliplatin-induced hepatic sinusoidal obstruction syndrome in a patient with gastric cancer: A case report. Mol Clin Oncol. 2018;8:453-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |