Published online Oct 6, 2018. doi: 10.12998/wjcc.v6.i11.455
Peer-review started: June 5, 2018
First decision: July 3, 2018
Revised: July 17, 2018
Accepted: August 11, 2018
Article in press: August 11, 2018
Published online: October 6, 2018
Processing time: 116 Days and 1 Hours
Isolated neurofibromas that affect the gastrointestinal tract are rare and almost always manifest as neurofibromatosis type 1 or multiple endocrine neoplasia type 2b. In this paper, we present a case of a 24-year-old female with abdominal pain who discharged a neurofibroma in her stool without any blood on it. A colonoscopy showed multiple small polyps in the sigmoid colon and a nodule in the ileocecus. The pathology results and the immunohistochemical stains of the removed neoplasm from the ileocecus confirmed the diagnosis was a bowel neurofibroma. We report a rare case of ileocecal neurofibroma due to the patient’s affected gastrointestinal tract, without any associated systemic syndrome other than a neurofibroma discharged in the stool.
Core tip: Neurofibromas of the gastrointestinal tract are rare, and various types have been previously reported. However, to our knowledge, this is the first report of a neurofibroma discharged from the patient’s intestine with stool without any other associated systemic syndromes.
- Citation: Miao Y, Wang JJ, Chen ZM, Zhu JL, Wang MB, Cai SQ. Neurofibroma discharged from the anus with stool: A case report and review of literature. World J Clin Cases 2018; 6(11): 455-458
- URL: https://www.wjgnet.com/2307-8960/full/v6/i11/455.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i11.455
Neurofibroma of the bowel can occur with multiple symptoms and has several different names, including intestinal neurofibromatosis, ganglioneuromatosis, diffuse plexiform neurofibromatosis, neuronal intestinal dysplasia, and diffuse colonic ganglioneuromatous polyposis. About a quarter of neurofibromatosis type-1 (NF1) and multiple endocrine-neoplasia type-2b (MEN2b) cases have been reported to be associated with gastrointestinal neurofibromatosis[1], while the occurrence of gastrointestinal neurofibromatosis alone was reported to be extremely rare[2,3]. Currently, whether neurofibromatosis of the bowel tract without any systemic syndrome is a distinct condition or simply a phenotypic manifestation of NF1 or MEN2b remains an open question.
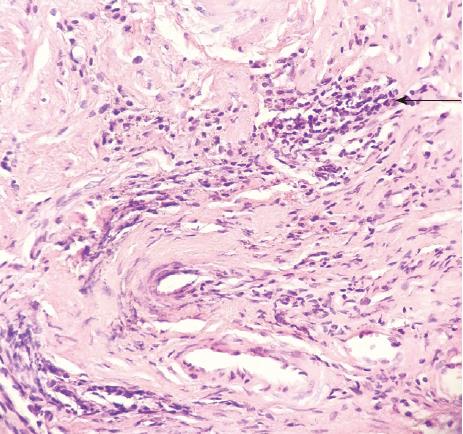
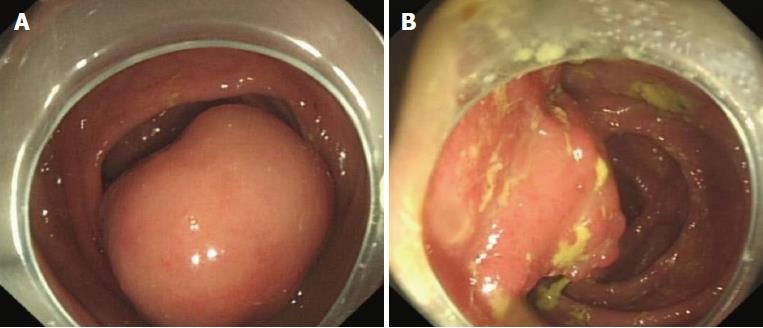
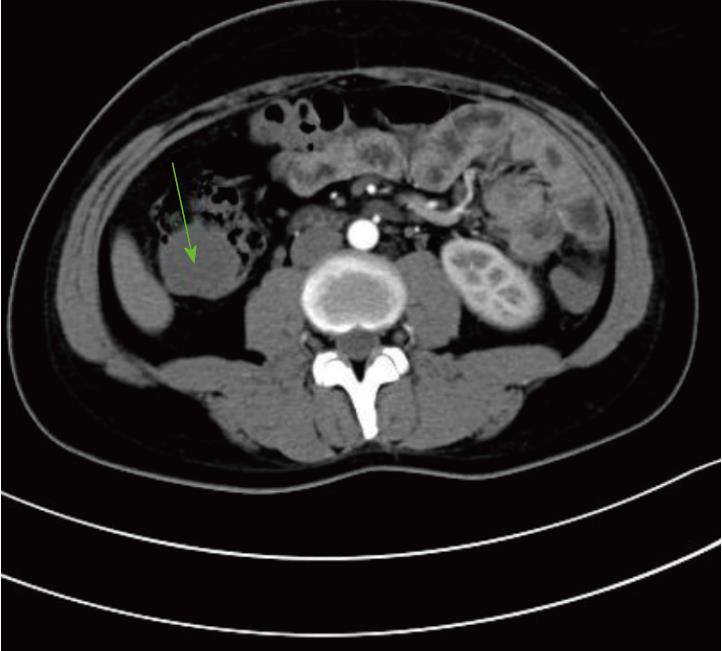
A previously healthy 24-year-old female came to our hospital complaining of a month-long history of abdominal pain after meals. She stated that she had never developed dysphagia, diarrhea, nausea, vomiting, or fever during the month of abdominal pain. Forty days prior to her symptoms, she found an approximately 8 cm × 5 cm × 5 cm lump in her stool without any blood on it (Figure 1). The lump was sent to another hospital for biopsy, and the results showed a submucosal spindle-cell tumor with surface-tissue necrosis that was inclined toward leiomyoma (Figure 2). Slices of the lump were taken to our institution for immunohistochemical analysis, which indicated that it came from a submucosal neurofibroma. Three days later, the female was given a colonoscopy, which showed a neoplasm with a smooth surface at the ileocecus (Figure 3A) with polyps at the sigmoid colon (Figure 3B). After admitting the female as an inpatient, a computed tomography (CT) scan of the abdomen was performed, revealing a hypoattenuating tumor of the ascending colon (Figure 4).
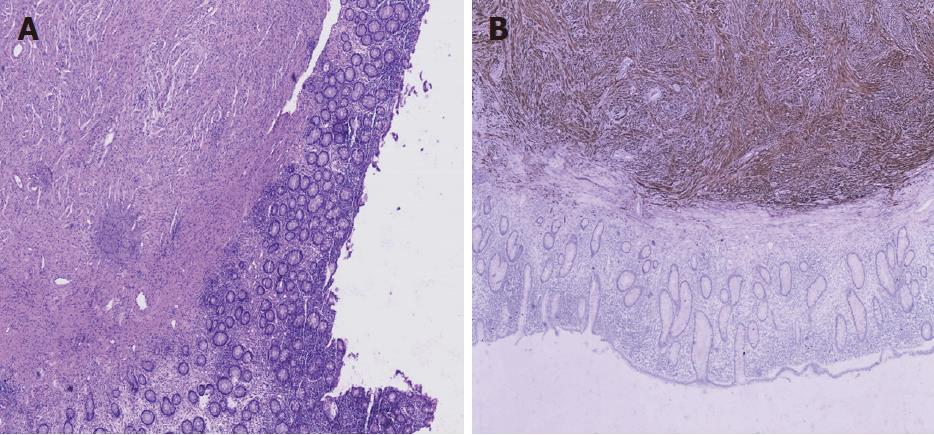
The patient then underwent an exploratory laparotomy, with primary anastomosis, after optimization for removing the tumor. We found the 5 cm × 6 cm tumor on the ileocecus at surgery but did not find anything else on the affected bowel. Pathologic examination of the resected specimen revealed it was a submucosal spindle-cell tumor of the ileocecus (Figure 5A). Immunohistochemical stains of the resected specimen showed that it was CD117(-), CD34(-), Ki67(+) 1%, Actin(-), S100(+++), Desmin(-), CD10(-), and Dog(-) (Figure 5B). The pathology results confirmed the tumor to be a neurofibroma. The patient did well initially; however, on the 10th postoperative day, the female had an anastomotic fistula (Clavien-Dindo Class I), and finally recovered well.
Isolated colonic neurofibromatosis is a benign neural tumor of the lower gastrointestinal tract. It can originate from the plexus of Meissner, the plexus of Auerbach, or even the serosa[4]. It can also be the onset manifestation of generalized systemic NF1 or MEN2b. Histologically, although isolated colonic neurofibromatosis manifests as a single or multiple high-degree of histologic differentiation of neoplasms or as a diffuse neuronal hyperplasia, it is commonly termed ganglioneuromatosis. In this case, the histology reports alone cannot specify whether it is NF1 or MEN2b because these conditions share some identical features[5].
The differing clinical symptoms found in neurofibromatosis of the hindgut tract depend on the lesion characteristics, such as the location, motility, and adjacent structures of the affected tract. Clinical presentation of the lesions can be abdominal pain[4], gut obstruction[6,7], palpable masses[8], constipation[9], or diarrhea[10].
In MEN2b, the development of medullary thyroid carcinoma, pheochromocytoma, and medullary carcinoma[11] is a clinical indicator besides what can be seen on the histological exam. In NF1, the development of classic dermal neurofibromas, café-au-lait macules or Lisch nodules[12] is an additional clinical indicator. In the current case, our patient did not show any of the additional clinical features.
The diffuse form of colonic neurofibromatosis and Crohn’s disease can mimic each other radiographically. Both colonic neurofibromatosis and Crohn’s disease can appear as single or multiple thickened portions of the gastrointestinal tract on CT scans. In one report, a patient was clinically suspected of having Crohn’s disease based on a CT scan, but the diagnosis was corrected to neurofibromatosis after histological exam of the resected bowel[9].
The etiology of isolated neurofibromatosis is still not clarified, but circulating nerve factors[13] and a neurofibromatosis gene mutation[14] were reported to be involved in hyperplasia of the nerve plexus. The primary treatment of isolated neurofibromatosis is surgical removal. Whether further therapy is required depends on the endoscopic findings and the histological exam.
In summary, we report a case of isolated neurofibromatosis with the onset of a lump in the patient’s stool and without any other additional clinical features. Despite its rarity, the neurofibromatosis is the only clinical indicator in this case. We suspect that part or all of the neurofibroma underwent necrosis and fell into the stool.
The unique character and only clinical symptom of this particular case is that the patient presented with a month-long history of abdominal pain after meals and a lump discharged from intestine with stool without any blood on it.
Ileocecal neoplasia.
Appendicitis, cholecystitis, gastroenteritis, colon cancer, and bowel obstruction.
Ileocecal neoplasia.
Ileocecal neurofibroma.
Ileocecectomy with primary anastomosis.
CARE Checklist (2013) statement: The manuscript was revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Wang W S- Editor: Wang JL L- Editor: Filipodia E- Editor: Song H
| 1. | Hughes MS, Feliberti E, Perry RR, Vinik A. Multiple Endocrine Neoplasia Type 2A (including Familial Medullary Carcinoma) and Type 2B. Endotext. South Dartmouth: MDText.com, Inc 2000; . [PubMed] |
| 2. | Carter JE, Laurini JA. Isolated intestinal neurofibromatous proliferations in the absence of associated systemic syndromes. World J Gastroenterol. 2008;14:6569-6571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Hochberg FH, Dasilva AB, Galdabini J, Richardson EP Jr. Gastrointestinal involvement in von Recklinghausen’s neurofibromatosis. Neurology. 1974;24:1144-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 120] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Boldorini R, Tosoni A, Leutner M, Ribaldone R, Surico N, Comello E, Min KW. Multiple small intestinal stromal tumours in a patient with previously unrecognised neurofibromatosis type 1: immunohistochemical and ultrastructural evaluation. Pathology. 2001;33:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Mendelsohn G, Diamond MP. Familial ganglioneuromatous polyposis of the large bowel. Report of a family with associated juvenile polyposis. Am J Surg Pathol. 1984;8:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Urschel JD, Berendt RC, Anselmo JE. Surgical treatment of colonic ganglioneuromatosis in neurofibromatosis. Can J Surg. 1991;34:271-276. [PubMed] |
| 7. | Bakker JR, Haber MM, Garcia FU. Gastrointestinal neurofibromatosis: an unusual cause of gastric outlet obstruction. Am Surg. 2005;71:100-105. [PubMed] |
| 8. | Hirata K, Kitahara K, Momosaka Y, Kouho H, Nagata N, Hashimoto H, Itoh H. Diffuse ganglioneuromatosis with plexiform neurofibromas limited to the gastrointestinal tract involving a large segment of small intestine. J Gastroenterol. 1996;31:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Charagundla SR, Levine MS, Torigian DA, Campbell MS, Furth EE, Rombeau J. Diffuse intestinal ganglioneuromatosis mimicking Crohn’s disease. AJR Am J Roentgenol. 2004;182:1166-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Siderits R, Hanna I, Baig Z, Godyn JJ. Sporadic ganglioneuromatosis of esophagogastric junction in a patient with gastro-esophageal reflux disorder and intestinal metaplasia. World J Gastroenterol. 2006;12:7874-7877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Cuthbert JA, Gallagher ND, Turtle JR. Colonic and oesophageal disturbance in a patient with multiple endocrine neoplasia, type 2b. Aust N Z J Med. 1978;8:518-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Gutmann DH, Ferner RE, Listernick RH, Korf BR, Wolters PL, Johnson KJ. Neurofibromatosis type 1. Nat Rev Dis Primers. 2017;3:17004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 499] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 13. | DeSchryver-Kecskemeti K, Clouse RE, Goldstein MN, Gersell D, O’Neal L. Intestinal ganglioneuromatosis. A manifestation of overproduction of nerve growth factor? N Engl J Med. 1983;308:635-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | d’Amore ES, Manivel JC, Pettinato G, Niehans GA, Snover DC. Intestinal ganglioneuromatosis: mucosal and transmural types. A clinicopathologic and immunohistochemical study of six cases. Hum Pathol. 1991;22:276-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 47] [Article Influence: 1.4] [Reference Citation Analysis (0)] |